Abstract
Glyphosate (GLY) is a well-known herbicide with significant applications in both agriculture and non-agriculture. However, GLY overuse in recent years has resulted in detection of GLY residues in many crops, endangering human health and food safety. Our aim is to investigate the relationship between urinary GLY and mortality, as well as its influencing factors. The National Health and Nutrition Examination Survey (NHANES) data from 4740 American adults were examined. Fitted smooth curves, generalized summation models, and multiple logistic regression models were used to investigate the relationship between urinary GLY and mortality. To investigate potential regulatory elements between the two effects, perform subgroup analysis. During a median follow-up of 4.03 years, there were a total of 238 all-cause deaths, 75 cardiovascular disease (CVD) deaths and 52 cancer deaths. The urinary GLY is positively correlated with all-cause mortality. Each 1 ng/ml increase in urinary GLY was associated with a 40% increased risk of all-cause mortality (Hazard ratio (HR) 1.40, 95% confidence interval (CI) 1.09–1.80), and an 50% increased risk of all-cause mortality in High group compared with Low group (HR 1.50, 95% CI 1.05–2.14). In subgroup analysis, the association between urinary GLY and all-cause mortality was significantly modified by gender (P for interaction = 0.03), and the association between urinary GLY and cancer mortality was significantly modified by hypertension (P for interaction = 0.022). Higher urinary GLY seems to be associated with more all-cause death, and gender may affect this association. Furthermore, urine GLY may have a higher effect on cancer mortality in people without hypertension.
Similar content being viewed by others
Introduction
Glyphosate (GLY) is the most widely used herbicide in the US agricultural sector1. It inhibits the 5-enolacetone shikimic acid-3-phosphate synthase (EPSPS), which interferes with the formation of aromatic amino acids and ultimately causes plant death in plants by acting on the shikimic acid pathway in plants2,3. Currently, farms, orchards, and gardens utilize it primarily for weeding1,4. However, because of the exponential increase in GLY use over the past century, its presence has been found in a wide range of foods3,5,6,7,8,9. As its use continues to rise, the public and scientific community are paying more and more attention to GLY’s possible effects on human health. Several investigations have revealed that GLY is not as safe and innocuous as first thought. GLY can strongly irritate human skin, eyes, and respiratory tracts during acute exposure, resulting in discomfort sensations10,11,12. Even more concerning are the long-term health concerns associated with exposure, which can disrupt the endocrine system, alter hormone balance, and negatively impact vital physiological functions including development and reproduction13,14. GLY may be carcinogenic, as evidenced by some research that have connected it to an increased risk of cancer15,16. Furthermore, GLY may harm the cardiovascular system, immune system, the nervous system, the liver, kidneys, et17,18,19,20. In 2015, GLY was categorized as a chemical that was “potentially carcinogenic to humans” by the World Health Organization’s International Agency for Research on Cancer (IARC)1,21,22. Furthermore, extensive and prolonged usage of GLY may have detrimental effects on aquatic and soil species23,24,25,26. Moreover, GLY usage over an extended period of time may cause weed resistance27,28. Higher doses of GLY or other herbicides are required to suppress weeds that have developed resistance to GLY, which increases agricultural production costs and strains the environment29. On the other hand, GLY is not expected to cause cancer, according to the findings of the Joint Meeting on Pesticide Residues between the Food and Agriculture Organization (FAO) and the World Health Organization (WHO)30. The U.S. EPA came to the conclusion that there was “inadequate information to assess carcinogenic potential,” “carcinogenic to humans,” or “likely to be carcinogenic to humans,” based on the weight of the evidence and available data31,32. In a similar vein, the Glyphosate Assessment Group of the European Union declared that, when used in accordance with recommended usage, GLY is safe for all purposes and suggested declassifying it as carcinogenic33. Therefore, opinions on GLY’s safety are currently divided. Nonetheless, it is easy to conclude from the first two NHANES cycles’ data that GLY exposure is common among Americans and that most people’s urine contains GLY34,35.
Here, we examined the relationship between urine GLY and adult mortality in America by extracting data on urinary GLY and all-cause and cardiovascular mortality from the National Health and Nutrition Examination Surveys (NHANES) conducted from 2013 to 2018.
Methods
Study design and population
National Health and Nutrition Examination Survey (NHANES) is a cross-sectional study conducted by the U.S. Centers for Disease Control and Prevention (CDC). NHANES utilizes a complex, multistage sampling design designed to be representative of the nation’s diverse population groups. The data collected include physical examinations, interviews, and laboratory tests that help researchers analyze health trends and inform public health policy. Ethical approval for the study was obtained from the National Center for Health Statistics (NCHS) Ethics Review Board, and all NHANES participants provided written informed consent. The data collected and related documents are publicly available. For more information, please visit the official NHANES website. In addition to the core survey, NHANES links participant data to the National Death Index (NDI) to track mortality outcomes. NDI mortality data are available at https://www.cdc.gov/nchs/data-linkage/mortality.htm.
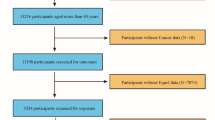
We conducted a secondary analysis using data from three independent NHANES cycles between 2013 and 2018 to investigate the association between GLY exposure and all-cause and cardiovascular mortality. A total of 8,507 participants with available urinary GLY data were included for the study. Due to the vulnerability of children and adolescents, we excluded 3,109 participants under the age of 18. Next, we excluded 17 participants with missing mortality data and 641 participants with missing urinary GLY data. In the end, 4,740 eligible individuals were included in the final analysis (Fig. 1).
Urinary GLY
In the NHANES, urinary GLY measurements are part of the environmental exposure data collected to assess the levels of this widely used herbicide in the U.S. population. The eligible sample consisted of all examined participants aged 3 to 5 years and one-third of the examined participants aged 6 years and older. NHANES’s official website provides information about urinary GLY’s range and handling options. The median GLY level in the population’s urine serves as the grouping concentration for the independent variables. Urinary GLY was measured by using 200 µl of urine and was based on 2D-on-line ion chromatography coupled with tandem mass spectrometry (IC-MS/MS) and isotope dilution quantification36. The analytical measurements were conducted following strict quality control/quality assurance CLIA guidelines. Along with the study samples, each analytical run included high- and low-concentration quality control materials (QCMs) and reagent blanks to assure the accuracy and reliability of the data. The concentrations of the high-concentration QCMs and the low-concentration QCMs, averaged to obtain one measurement of high-concentration QCM and low-concentration QCM for each run, were evaluated using standard statistical probability rules37.
All-cause, cardiovascular mortality and cancer mortality
In this study, the outcome variables included all-cause mortality, cardiovascular mortality and cancer mortality. These mortality data were obtained through linkage with the NDI38. Specifically, participants without a recorded death were considered alive during the follow-up period, which extended from the time of their participation in the survey until December 31, 2019. All-cause mortality encompasses deaths from any cause. Cardiovascular mortality was defined using the International Classification of Diseases, 10th Revision (ICD-10) codes: I00-I09, I11, I13, I20-I51, and I60-I69, which represent a range of cardiovascular-related conditions, including heart disease, hypertensive heart disease, and cerebrovascular disease. Cancer mortality include any death brought on by cancer.
Potential covariates
The covariates in this study were pre-selected based on prior research identifying risk factors for all-cause mortality. After variable screening, the final multivariable logistic regression analysis included the following covariates: Continuous variables include age, poverty-to-income ratio (PIR), body mass index (BMI, kg/m²), alanine aminotransferase (ALT, U/L), serum creatinine (SCR, µmol/L), blood urea nitrogen (BUN, mg/dL), estimated glomerular filtration rate (eGFR, mL/min/1.73 m²), uric acid (UA, µmol/L), fasting blood glucose (FBG, mmol/L), glycated hemoglobin (HbA1c, %), total cholesterol (TC, mmol/L), and triglycerides (TG, mmol/L); Categorical variables include sex, ethnicity, education level, smoking and drinking status, physical activity, presence of hypertension, and presence of diabetes. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: eGFR = 141 × min(SCR/κ, 1)α × max(SCR/κ, 1)-1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], κ is 0.7 for females and 0.9 for males, α is -0.329 for females and − 0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 139. Hypertension was defined as either a self-reported diagnosis from a healthcare professional, a systolic blood pressure ≥ 140 mmHg, and/or diastolic blood pressure ≥ 90 mmHg40. Diabetes was defined as a self-reported diagnosis, a fasting blood glucose (FBG) ≥ 7 mmol/L, or HbA1c > 6.5%41.
Statistical analysis
All statistical analyses in this study were conducted following the CDC guidelines for NHANES data analysis (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx), with each NHANES participant assigned a sampling weight to ensure the representativeness of the data42. To compare baseline characteristics across tertiles of urinary GLY levels, continuous variables were analyzed using weighted linear regression models and presented as means with 95% confidence intervals (CI), while categorical variables were analyzed using weighted chi-squared tests and reported as counts and percentages. The relationship between urinary GLY and mortality (both all-cause and cardiovascular) was evaluated using univariate and multivariate Cox proportional hazards regression models. Three models were constructed: model 1: Unadjusted, model 2: Adjusted for sociodemographic factors, and model 3: Adjusted for all covariates listed in Table 1. To account for potential nonlinear relationships between urinary GLY levels and mortality, cubic spline functions and smooth curve fitting (penalized spline method) were applied in Cox regression models. The Kaplan-Meier method was employed to plot survival curves, comparing mortality rates across different GLY exposure groups. The Log-rank test was used to assess statistical differences between the survival curves. In addition to the main analyses, stratified analyses and interaction tests were conducted to assess the potential modifying effects of various covariates on the relationship between urinary GLY levels and mortality. The following variables were included for stratification: age (< 60 vs. ≥60 years), sex (man vs. woman), race (Mexican American vs. Other Hispanic vs. Non-Hispanic White vs. Non-Hispanic Black vs. other race), educational attainment (< 9th grade vs. 9-11th grade vs. high school vs. college vs. graduate and above), BMI (< 25 vs. ≥25 kg/m2), smoking status (never vs. quit vs. current), alcohol consumption (never vs. 1 ~ 5 drinks/month vs. 5 ~ 10 drinks/month vs. >10 drinks/month vs. unknown), and eGFR (< 60 vs. ≥60 mL/min/1.73 m2), hypertension (yes vs. no), and diabetes (yes vs. no).
To maximize statistical power and reduce potential bias from excluding observations with missing covariate data, we employed a robust approach to handle missing values. Multiple imputation was used for continuous variables, ensuring that the missing data were replaced with plausible values based on other available information. For categorical variables, dummy variables were introduced to account for missing data. All statistical analyses were conducted using R software (version 4.2.2) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A two-sided P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
Overall, this study included 4740 individuals (median follow-up time: 4.03 years) (Fig. 1). The social demographic characteristics and other covariates of the selected participants are shown in Table 1. The ranges of urinary GLY content for the two groups are < 0.33 and 0.33-8.21ng/mL, respectively. Except for education level, physical activity, smoking status, ALT, UA and Triglyceride, there were significant statistical differences between both groups for all included characteristics. Compared to the low group, the high group participants are more likely to be male, older, non-Hispanic white and black and have a history of hypertension and diabetes, have lower levels of PIR, eGFR, TC and HDL-C; and have a higher level of more than 10 drinks per month, BMI, SCR, BUN, FBG and HbA1c.
Association of urinary GLY with all-cause, cardiovascular mortality and cancer mortality
As indicated by Table 2 and Table S1, there were 238 deaths from all causes, 75 deaths from cardiovascular disease and 52 deaths from cancer during the follow-up period of 19,092.33 person-years. We constructed three models to analyze the independent role of urinary GLY in all-cause, cardiovascular mortality and cancer mortality. Overall, regardless of adjusting for confounding factors, the urinary GLY of all participants was significantly positively correlated with all-cause mortality. In the unadjusted model, the all-cause mortality increased by 49% with each 1 ng/mL increase in the urinary GLY (HR 1.49, 95% CI 1.24–1.79). This positive relationship in Model 2 (HR 1.31, 95% CI 0.99–1.71) and Model 3 (HR 1.40, 95% CI 1.09–1.80) remained robust after adjusting for the confounding factors. All-cause mortality was significantly higher in the High group as compared to the Low group. In Model 3, the corresponding HRs for all-cause mortality was 1.50 (95% CI, 1.05–2.14). Moreover, in Model 1, mortality from cardiovascular disease was positively correlated with urinary GLY, with corresponding mortality HRs of 1.44 (95% CI, 1.21–1.71). While the effect values in Models 2 and 3 were still more prominent and the association’s direction remained constant across all models, the relationships in those models did not attain statistical significance. Adjusting for additional factors may have diminished statistical significance, but generally the association between cardiovascular mortality and urinary GLY appears to be robust. Cancer mortality and urine GLY had a neutral correlation in Model 1, with corresponding mortality HRs of 1.07 (95% CI, 0.67–1.72) 0.775. Although not statistically significant, there was a negative connection between cancer mortality and urine GLY in Models 2 and 3, with corresponding mortality HRs of 0.66 (95% CI, 0.37–1.18) and 0.77 (95% CI, 0.42–1.44). We reanalyzed the relationship between urinary GLY and mortality using post-interpolation data, and the results were not qualitatively different (Table 3 and Table S2). The fully adjusted smooth curve fitting results also support the prior findings (Fig. 2 and Figure S1). Additionally, we investigated urinary GLY’s threshold effect analysis on all-cause mortality, cardiovascular mortality and cancer mortality (Table 4 and Table S3). To fit the relationship between urinary GLY and mortality, we employed the Cox proportional hazards model and the 2-segment Cox proportional hazards model, respectively. Table 4 and Table S3 demonstrates a linear correlation between urinary GLY and all-cause mortality, but not with urinary GLY and cardiovascular mortality and cancer mortality (P < 0.05 for the log likelihood ratio test). We discovered that urinary GLY has an inflection point of 0.38 ng/mL. When urinary GLY < 0.38 ng/mL, an increase in urinary GLY is substantially associated with an increased risk of cardiovascular mortality (HR = 73.08; 95% CI, 2.12-2523.35; P = 0.018). Conversely, when urinary GLY > 0.38 ng/mL, there is no statistically significant association between increased urinary GLY and cardiovascular mortality. Furthermore, the results of the unadjusted Kaplan Meier curve indicated that the High group had a higher risk of all-cause, cardiovascular mortality and cancer mortality as compared to the Low group (Fig. 3 and Figure S2).
Association between glyphosate and all-cause and cardiovascular mortality. (A) All-cause mortality, (B) Cardiovascular mortality. The solid and dotted lines represent the estimated values and their corresponding 95% confidence intervals, respectively. Adjustment factors included sex, age, race, education status, PIR, physical activity, smoking and drinking status, BMI, hypertension and diabetes, ALT, SCR, BUN, eGFR, UA, TC, TG, HDL-C, FBG, and HbA1c. PIR poverty income ratio, BMI body mass index, ALT alanine aminotransferase, SCR Serum creatinine, BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, UA uric acid, TC total cholesterol, TG triglycerideHDL-C high density lipoprotein cholesterol, FBG fasting blood glucose, HbA1c hemoglobin A1c.
Subgroup analyses
We stratified the primary covariates and performed subgroup analysis to further confirm the results’ reliability in the presence of confounding variables and to determine whether there are any factors that could change the connection between urinary GLY and mortality. Other than gender and hypertension, no other covariates—such as age, PIR, diabetes, BMI, and eGFR—had a statistically significant effect on the association between GLY and mortality (all p interactions > 0.05) (Fig. 4 and Figure S3). The significant interaction (p-value of 0.03 for the interaction) was found between all-cause mortality and sex, suggesting that sex may be an important factor influencing the effects of urinary GLY, with female detriment the most (HR 1.59, 95% CI 1.20–2.10). Cancer mortality and hypertension have a significant interaction (p-value of 0.022 for the interaction), indicating that urine GLY may have a greater impact on cancer mortality in persons without hypertension (HR 2.31, 95% CI 0.87–6.13). The urinary GLY effects, nevertheless, were highly consistent for other important factors, such as age, PIR, diabetes, BMI, and eGFR subgroups; none of the interaction effects, however, reached statistical significance (P for all interactions > 0.05).
Discussion
In this large prospective analysis, we discovered a strong positive connection between urinary GLY and all-cause mortality, which persisted even after controlling for confounding variables. Furthermore, we discovered that the relationship between urinary GLY and all-cause mortality may vary depending on a person’s sex in the stratification and interaction study. The findings demonstrated a substantial positive correlation between the urinary GLY by female individuals and all-cause mortality, but not a significant correlation between the urinary GLY by male individuals and all-cause mortality.
GLY is an efficient and broad-spectrum herbicide29. It works well in fields with a variety of crops, including cotton, corn, soybeans, etc5,43,44. Broad-leaved weeds like amaranth, quinoa, and purslane, as well as annual and perennial weeds like barnyard grass, sagebrush, cowweed, and horseweed, can all be successfully prevented and controlled by it45,46,47,48. GLY can be used before to planting or during crop growth to enhance crop quality and production by establishing a conducive growing environment49. Additionally, GLY works well against some harmful plants that are hard to eradicate, such reeds, tiny flying awns, etc50,51. Furthermore, GLY finds extensive use in horticulture, forestry, and other domains52,53. However, GLY has been abused precisely because of its potent weed control action. Extensive and prolonged use of GLY may alter the diversity and activity of soil microbes, diminish soil fertility, and harm soil structure54,55,56. GLY may be dangerous to aquatic life if it gets into a body of water57,58. Elevated levels of GLY have the potential to impede the development of aquatic vegetation and impact the equilibrium of aquatic environments59,60. Fish and shellfish are examples of aquatic species that may be toxically affected by GLY61,62,63. Furthermore, while gathering nectar and pollen, insects like bees and butterflies may come into contact with GLY and become poisoned as a result64,65,66,67. In northwestern Germany, Liebing et al. discovered that two-thirds of the examined samples for Phasianus colchicus hens that were free-ranged showed positive results for GLY68. A retrospective analysis of all suspected cases of livestock poisoning revealed that GLY was the cause in cases involving dogs, cats, horses, goats, and sheep69. Moreover, it is important to consider how GLY affects aquatic organisms, as we have previously mentioned. Agbohesi et al. discovered that GLY damages the liver of Clarias gariepinus, an African catfish70. According to Ames et al., GLY can harm zebrafish embryonic larvae, resulting in teratogenicity, heart defects, and even death71. When adult zebrafish exposed to GLY, Sulukan et al. observed that the progeny had decreased blood flow and heart rate, delayed hatching, increased physical malformations, and a worse survival rate72,73. GLY was discovered by Lu et al. to have an impact on zebrafish body length shortening, improper hatching, and embryo death74. Zebrafish embryos exposed to GLY, on the other hand, showed cardiac anomalies such as ventricular dilatation, ventricular wall thinning, and irregular rhythms74. A study by Pompermaier et al. indicated that exposure to GLY lowers the survival rates of zebrafish, causes hyperactivity and anti-anxiety behavior, affects larval anti-predation behavior negatively, and raises acetylcholinesterase activity75. In zebrafish larvae, Lanzarin et al. observed that GLY triggers oxidative stress, inflammation, and cell death76. Moreover, embryos exposed to high concentrations of GLY showed lower heart rate and hatching rate, as well as increased mortality and number of abnormalities76. On the other hand, exposure to amounts that do not result in teratogenic effects caused a dose-dependent drop in heart rate without significantly altering development77. Nonetheless, the larvae exposed to these quantities did not exhibit any alterations in histology77. According to DíazMartín et al., zebrafish embryos exposed to GLY for a brief period of time may develop skeletal, craniofacial, and motor disorders78. Bridi et al. showed that GLY shortens the zebrafish larvae’s eye distance and decreases the adult zebrafish’s walking distance, average speed, and crossing line79. It was discovered by Roy et al. that GLY causes neurotoxicity in zebrafish80. Research by Flach et al. showed that GLY has an impact on the Xenopus laevis embryos’ development of the heart and nervous system81. Bullfrogs (Lithobates catesbeiana) tadpoles’ metabolic processes and behavioral performance are affected by GLY exposure, according to research done by Costa et al.82. Furthermore, research has shown that GLY’s lethal and sublethal effects on non-target plants may contribute to the decline of biodiversity in natural forest remnants submerged in agricultural settings83. All of these instances suggest that GLY has infiltrated the ecosystem and might stay there, potentially endangering the ecosystem’s stability and equilibrium.
In this work, we first examined the association between urine GLY and mortality using representative large sample prospective data. We have made some significant new discoveries. First off, there is a strong link between the urine GLY and all-cause mortality according to our research. Additionally, the Kaplan Meier curve supports our findings. People with high urine GLY have comparatively greater rates of all-cause and cardiovascular mortality when compared to those with low urinary GLY. GLY has been shown to be harmful to the heart in numerous investigations. According to a retrospective study, patients who are exposed to GLY poisoning may have certain arrhythmia, such as I degree atrioventricular block, intraventricular conduction delay, and prolonging of the QTc interval84. Case reports from recent years have shown demonstrated that ingesting GLY can harm the cardiovascular system to varied degrees85,86,87,88,89. The majority of patients received gastric lavage, norepinephrine and vasopressin infusion, intubation and mechanical breathing, and substantial intravenous fluid replacement, despite the fact that the quantity of GLY ingested varied. Per Calderon et al., prolonged exposure to the GLY environment may have an impact on women’s development of cardiovascular disease90. On a cardiac organoid model produced from human pluripotent stem cells, Sun et al. discovered that GLY had developmental toxicity91. In the study by Maia et al., GLY can lead to atherosclerosis regardless of exposure route and concentration92. However, Printemps et al. contend that Roundup ® A herbicide formulated with GLY and adjuvants can induce severe cardiac toxicity by blocking the CaV1.2 channel, leading to worsening cardiac contractility and arrhythmia, which cannot be attributed to GLY93. Lee et al. discovered that infusion of isopropylamine (IPA) salt (IPAG) containing GLY can alter the hemodynamics of piglets and cause piglet death, while GLY has no such impact94. Maybe as a result of the active ingredient GLY’s interaction with other ingredients in herbicide formulations, or maybe because of their increased cytotoxic activity. In order to determine the true health concerns associated with occupational and environmental exposure, more formulation research is necessary when creating herbicides based on GLY. Notably, this research shows for the first time the unique biological significance of urinary GLY concentration in the low threshold range, meaning that a small increase in urinary GLY concentration is significantly positively correlated with the risk of cardiovascular mortality when the concentration is below 0.38 ng/mL. This finding challenges the conventional toxicology’s linear “dose-response” cognitive framework and raises the prospect of non-monotonic dose-response relationships or critical threshold effects at very low exposure levels. Based on our research data and analysis process, urinary GLY concentration is very likely to become a potential biomarker for cardiovascular mortality risk which may be more significant in the low concentration range, even though there are currently no epidemiological studies reporting similar phenomena. The finding offers fresh concepts and avenues for investigation in the area of risk assessment for cardiovascular disease. Further investigation into the inherent connection between variations in low-level urine GLY concentration and the risk of cardiovascular mortality may be feasible in the future, thereby providing more valuable information for the prevention and management of cardiovascular disease.
Several investigations have indicated that GLY may have carcinogenic properties. Dal’Bó et al. discovered that while GLY can have a major cytotoxic effect on cells, it can also have a major proliferative effect, particularly on papillary thyroid cancer cells95. An increased risk of breast cancer may be linked to exposure to aminomethyl phosphonic acid (AMPA), the primary metabolite of GLY, according to a prospective study by Franke et al.96. Herbicides based on GLY have been shown by Silva et al. to decrease autophagy and enhance energy metabolism in C6 glioma cell lines97. Martínez et al. established that exposure to GLY in the environment is problematic by demonstrating how GLY and AMPA cause oxidative stress, development, and cell death in human neuroblastoma cell line SH-SY5Y neurons via the pathways of necrosis, autophagy, and apoptosis98. Malatesta et al. showed that hepatic cancer tissue culture (HTC) cells’ metabolic pathways can be disrupted by low doses of GLY99. Conversely, Parajuli and colleagues showed that the combination of methoxyacetic acid (MAA) and AMPA can induce apoptosis in prostate cancer cells, indicating its potential as a prostate cancer treatment medication100. The main source of the debate on GLY’s carcinogenicity is an IARC assessment. GLY was categorized as a “possible human carcinogen” (Class 2 A) by the IARC in 2015. This classification is based on findings from in vitro research, animal trials, and epidemiological studies, some of which have linked GLY to non-Hodgkin’s lymphoma (NHL). But it’s important to keep in mind that the IARC’s designation of GLY as “possibly carcinogenic” does not imply that it causes cancer; rather, it suggests that there is enough evidence to support the possibility that it may cause cancer, but the findings are conflicting or the evidence is insufficient. The degree and mode of exposure must also be taken into account when assessing GLY’s carcinogenic consequences. The amounts that people who are directly exposed to GLY, such farmers, gardeners, and nearby residents, receive from GLY exposure or spraying are significantly higher than those that they consume through food. These populations may therefore be at greater risk for health problems. However, there isn’t any solid proof that GLY causes cancer directly, even in these populations. It’s also important to note that GLY may be present in trace amounts in plants themselves. This does not imply that all plants are GLY-contaminated; rather, it indicates that because GLY is so pervasive in the environment, plants may absorb traces of the chemical while they are growing. Whether these small levels of GLY are harmful to human health is still up for debate, though. The results of our research indicated that, even after controlling for confounding variables, there was a negative correlation between urine GLY content and cancer mortality; however, this relationship was not statistically significant. Furthermore, the protective effect of GLY against cancer is not yet supported by any trustworthy scientific data. We consider that the incredibly complicated influencing factors of cancer mortality may be the cause of this outcome. Despite our best efforts to account for known confounding variables, the results may still be imprecise due to unmeasured or insufficiently controlled confounding variables. To further confirm whether GLY has carcinogenic effects or its possible health impacts, more carefully planned and sizable sample investigations are therefore still required in the future, particularly long-term tracking and large-scale population research.
There is mounting evidence that GLY may also harm different organs to differing degrees. According to a recently released study, there is a positive linear link between non-alcoholic fatty liver disease (NAFLD) and GLY exposure101. Another prospective study discovered that early exposure to GLY and AMPA during childhood may raise the risk of metabolic diseases related to the liver and heart in early adulthood102. Tang et al. showed that GLY significantly harmed rats’ livers and resulted in an imbalance in the concentration of different mineral elements in the rats’ various organs103. Liu et al. demonstrated that GLY can worsen liver toxicity by blocking the Nrf2/GSH/GPX4 axis, which causes iron death in the liver cells104. The work of Gasnier et al. discovered that GLY can harm liver cell lines intracellularly at various levels; however, human cell lines can be somewhat shielded from this pollution by a combination of Dig1 medicinal plant extract105. Urine KIM-1 is the most effective early biomarker for kidney injury, according to research by Wunnapuk et al. who also showed that GLY-induced nephrotoxicity can occur106. Furthermore, GLY appears to have some effect on the neurological system. Oliveira et al. reported that GLY has an age- and tissue-specific impact on the hypothalamus pituitary thyroid axis107. Adewale et al. observed that in the brains of Wistar rats, GLY can trigger markers of oxidative stress, inflammation, and cell death108. Winstone et al. showed that GLY penetrates the brain, causes a dose-dependent disruption of the transcriptome, and raises the expression of TNF α and soluble A β109. Cattani noticed that GLY may cause extracellular glutamate levels to become too high, which would then cause oxidative stress and glutamate excitotoxicity in the rat hippocampal tissues110. According to Gui et al., Parkinson’s disease may be linked to exposure to commonly used GLY111. Numerous studies have offered sufficient data to demonstrate the possible risk of urinary GLY, despite the fact that there is still considerable debate and doubt about the connection between this chemical and damage to human organs. Thus, given the possible health hazards associated with GLY, we need to take proactive preventive actions, such as tightening laws governing the use of pesticides, increasing public awareness of safety issues, lowering exposure outside of the workplace, and promoting the adoption of greener substitutes. In order to lessen or even reverse the harm that GLY does to organs, people that are already impacted by it must receive prompt medical monitoring and intervention. In summary, the application of GLY needs to be prudent and careful in order to protect human health and safety while preserving agricultural output efficiency. To strengthen the scientific foundation for protecting human health, additional comprehensive and long-term studies are required in the future to elucidate the precise mechanism and extent of urinary GLY’s impact on organ damage.
It’s crucial to keep in mind that GLY has also been linked to some degree of reproductive system impairment. According to Chianese et al., GLY can activate estrogen receptors and cause cell death in prostate cells. It also functions as a heteroestrogen. Hormonal changes that follow could reduce fertility112. Lu et al. discovered that GLY, which may be harmful to reproduction, stimulates iron death and suppresses testosterone synthesis via ferritin autophagy mediated by NCOA4113. Long-term dietary exposure to GLY in chickens has been demonstrated by Estienne et al. to cause the accumulation of GLY in egg yolks, which causes severe early embryo mortality and delayed embryo development in survivors114. These negative effects go away after two weeks of GLY exposure114. Ganesan et al. revealed that ovarian mitochondria and oxidative stress proteins are changed in female C57BL6 mice exposed to GLY115,116. Cai et al. determined that even very low concentrations (0.9 ppm) of GLY are detrimental to pre-implantation development in cattle [117]. They also reported that exposure to agricultural recommended dosages of GLY can result in stunted in vitro growth and fast loss of bovine embryos117. Additionally, Cavalli et colleagues demonstrated that GLY may affect male fertility118. GLY has been shown by Razi et al. to impact spermatogenesis, motility of sperm, and anomalies in rat testicular tissue, all of which may result in infertility119. Benachour et al. reported that in human umbilical cord, embryonic, and placental cells, GLY causes necrosis and apoptosis120. Dallegrave and colleagues found that GLY causes embryonic bone development to be delayed and is hazardous to the mother of Wistar rats121. In conclusion, it is impossible to overlook the possible harm that GLY could cause to the developing reproductive system. Numerous studies have alerted us to the danger, even though there is still some debate on its precise impact. It is additionally essential to keep in consideration that the majority of GLY research is based on animal studies, and that the dosage of GLY in animal studies may differ significantly from the amounts of exposure in humans. This necessitates analyzing research findings with caution and a scientific mindset. To investigate the possible toxicity of GLY under high exposure, animal tests are usually carried out in harsh environments. The results of animal trials cannot be directly applicable to humans because these concentrations are frequently far greater than the amounts that people may encounter in their daily lives. Additionally, GLY exposure in humans typically occurs through a variety of pathways, including the food chain, air, and water, whereas in animals, trials are typically carried out in highly controlled situations and the sources of GLY that animals come into contact with are single and obvious. It is challenging to determine the precise amount of GLY that humans are exposed to due to the intricacy of these exposure pathways. Therefore, in order to have a more thorough picture of the actual state of human exposure to GLY, we must rely on epidemiological studies and environmental monitoring data. Moreover, species differences may also play a role. Different animal species may react, tolerate, and metabolize GLY quite differently than humans do. Certain animals may have a high resistance to GLY and will not react similarly to people, even at high doses, while other animals may be more sensitive to the chemical and need higher doses to have effects comparable to those seen in humans. Therefore, the amount and concentration of GLY in animal studies cannot be directly compared to human exposure. Thus, we should completely take into account variables like exposure pathways, dosages, length, and individual characteristics when describing the distinctions between exposure in humans and animal study. In order to more precisely evaluate the possible effects of GLY on human health, we need also keep an eye out for fresh research findings. In conclusion, even though the dosages used in animal tests and human exposure differ, these studies nevertheless give us important insights into how to use GLY more safely and shield people from any potential risks.
Secondly, we note that sex and hypertension may affect the relationship between urinary GLY and mortality. In subgroup analysis of sex, we observed that female individuals had lower rates of all-cause mortality. It is true that there is a correlation between gender and mortality, but this correlation is highly complex and influenced by a variety of factors, such as work, environment, behavior and lifestyle, and heredity. Furthermore, we observed that the fraction of low concentration urine GLY in females is larger than in males, which could be one of the causes of the lower all-cause death rate in females (Table 1). Additionally, GLY may have a higher effect on cancer mortality in patients without hypertension, according to our subgroup study. We speculate that it might be because the toxic effects of GLY are more likely to appear in non-hypertensive patients with relatively simple health status, whereas the numerous illness loads and medication interference of hypertensive patients may obscure the impact of GLY. It is important to remember that these conclusions are merely theories, and additional clinical data will be needed to validate our research findings. As of yet, there is no known cure for GLY poisoning, and the only available treatment is prompt systemic support. More research could lead to the development of GLY inhibitors that are more potent in the future. However, the primary way to solve the issue is to stop the misuse of herbicides like GLY. Therefore, we should strengthen policy supervision and regulatory enforcement, strictly formulate and implement usage norms, enhance agricultural technology guidance and training, and raise public environmental awareness, in order to bring the minimum negative impact while maximizing the benefits of GLY.
We must recognize that this study has significant drawbacks even though it is a huge sample size and thoroughly illustrates the intricate stratified sampling methodology of NHANES. First off, despite our best efforts to account for confounding variables, lifestyle, medication, occupational characteristics, and other factors that may be known or unmeasured confounding factors cannot be completely ruled out as potential sources of bias in the research findings. In addition, there may be complex interactions between confounding variables and between confounding variables and research factors, which can make the impact of confounding factors more complex. There may be a synergistic effect between GLY exposure and lifestyle factors such as smoking and alcohol consumption, which collectively affect human health. However, it is difficult to accurately separate and effectively control the effects of this interaction separately in statistical analysis. Moreover, in studying the effects of long-term GLY exposure on human health, confounding variables such as dietary habits and living environment of research subjects may change during the study period. For instance, an individual’s water intake can have a big impact on how diluted their urine is, which can change the target substance’s detection concentration. The accuracy of detection results may be hampered by diets that contain compounds that physically resemble urine GLY or that alter the body’s metabolism of GLY. Furthermore, statistical analysis can often only be controlled based on data from a specific time point or limited time period, making it difficult to consider these dynamic changes in a real-time and comprehensive manner. Second, the majority of our findings are derived from questionnaire surveys, and self-reporting by participants could contain bias. It is noteworthy, however, that questionnaire surveys constitute a significant part of the National Health and Nutrition Survey, and that a great deal of research has been done using the data from these surveys. Thirdly, results should not be extended to other nations or age groups because the population we covered consisted of adult Americans who are 18 years of age or older. In conclusion, before the aforementioned results are applied in clinical settings, more clinical research is required to confirm these results.
Conclusion
Overall, this study investigated the relationship between urinary GLY and mortality, and discovered that there might be an association between urinary GLY and all-cause mortality, which we observed that this correlation is more pronounced in female populations. Furthermore, urine GLY may have a higher effect on cancer mortality in people without hypertension.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Csete, J. et al. Public health and international drug policy. Lancet 387(10026), 1427–1480 (2016).
Khurshid, C. et al. Pesticide residues in European sediments: A significant concern for the aquatic systems? Environ. Res. 261, 119754 (2024).
Colopi, A. et al. Dietary exposure to pesticide and veterinary drug residues and their effects on human fertility and embryo development: A global overview. Int. J. Mol. Sci. 25(16) (2024).
Meddeb-Limem, S., Ben, A. & Fredj Computational study of the dimerization of glyphosate: mechanism and effect of solvent. RSC Adv. 14(32), 23184–23203 (2024).
Mortensen, D. A., Ryan, M. R. & Smith, R. G. Another step on the transgene-facilitated herbicide treadmill. Pest Manag Sci. 80(9), 4145–4149 (2024).
Albini, B. et al. Food safety issues in the Oltrepò Pavese area: A SERS sensing perspective. Sensors (Basel) 23(22) (2023).
Ferreira, M. F. et al. Glyphosate affects the susceptibility of non-target native plant species according to their stage of development and degree of exposure in the landscape. Sci. Total Environ. 865, 161091 (2023).
Nunes, R. F. N. et al. Glyphosate contamination of drinking water and the occurrence of oxidative stress: exposure assessment to rural Brazilian populations. Environ. Toxicol. Pharmacol. 108, 104476 (2024).
van Larebeke, N. et al. Organic food and internal exposure to pollutants among Flemish adolescents. Food Addit. Contam. Part. Chem. Anal. Control Expo Risk Assess. 1–22 (2024).
Acquavella, J. F. et al. Human ocular effects from self-reported exposures to roundup herbicides. Hum. Exp. Toxicol. 18(8), 479–486 (1999).
Lindberg, T. et al. An integrated transcriptomic- and proteomic-based approach to evaluate the human skin sensitization potential of glyphosate and its commercial agrochemical formulations. J. Proteom. 217, 103647 (2020).
Sidthilaw, S. et al. Effects of exposure to glyphosate on oxidative stress, inflammation, and lung function in maize farmers, Northern Thailand. BMC Public. Health. 22(1), 1343 (2022).
Tajai, P. et al. Effects of glyphosate-based herbicides and glyphosate exposure on sex hormones and the reproductive system: from epidemiological evidence to mechanistic insights. Environ. Toxicol. Pharmacol. 102, 104252 (2023).
Franco, G. A. et al. Enviromental endocrine disruptor risks in the central nervous system: neurotoxic effects of PFOS and glyphosate. Environ. Toxicol. Pharmacol. 109, 104496 (2024).
Panis, C. et al. Exposure to pesticides and breast cancer in an agricultural region in Brazil. Environ. Sci. Technol. 58(24), 10470–10481 (2024).
Schluter, H. M., Bariami, H. & Park, H. L. Potential role of glyphosate, Glyphosate-Based herbicides, and AMPA in breast cancer development: A review of human and human Cell-Based studies. Int. J. Environ. Res. Public. Health 21(8) (2024).
Lu, J. et al. Glyphosate causes vascular toxicity through cellular senescence and lipid accumulation. Chem. Res. Toxicol. 36(7), 1151–1161 (2023).
Chávez-Reyes, J. et al. Neurotoxicity of glyphosate: focus on molecular mechanisms probably associated with alterations in cognition and behavior. Environ. Toxicol. Pharmacol. 106, 104381 (2024).
Leblanc, P. O. et al. The impact of the herbicide glyphosate and its metabolites AMPA and MPA on the metabolism and functions of human blood neutrophils and their sex-dependent effects on reactive oxygen species and CXCL8/IL-8 production. Environ. Res. 252(Pt 1), 118831 (2024).
Mazuryk, J. et al. Glyphosate: hepatotoxicity, nephrotoxicity, hemotoxicity, carcinogenicity, and clinical cases of endocrine, reproductive, cardiovascular, and pulmonary system intoxication. ACS Pharmacol. Transl Sci. 7(5), 1205–1236 (2024).
Martinek, R. et al. Comprehensive investigation of the mutagenic potential of six pesticides classified by IARC as probably carcinogenic to humans. Chemosphere 362, 142700 (2024).
Guyton, K. Z. et al. Carcinogenicity of Tetrachlorvinphos, parathion, malathion, Diazinon, and glyphosate. Lancet Oncol. 16(5), 490–491 (2015).
Mohy-Ud-Din, W. et al. Glyphosate in the environment: interactions and fate in complex soil and water settings, and (phyto) remediation strategies. Int. J. Phytorem. 26(6), 816–837 (2024).
Pagano, A. D. et al. Assessing reproductive effects and epigenetic responses in austrolebias Charrua exposed to roundup Transorb®: insights from MiRNA profiling and molecular interaction analysis. Environ. Toxicol. Pharmacol. 110, 104539 (2024).
Morozov, A. & Yurchenko, V. Glyphosate and Aminomethylphosphonic Acid Impact on Redox Status and Biotransformation in Fish and the Mitigating Effects of Diet Supplementation(Vet Res Commun, 2024).
Moller, S. R. et al. Persistence and pathway of glyphosate degradation in the coastal wetland soil of central Delaware. J. Hazard. Mater. 477, 135238 (2024).
Faleco, F. A. et al. Resistance To Protoporphyrinogen Oxidase Inhibitors in Giant Ragweed (Ambrosia trifida)(Pest Manag Sci, 2024).
Varah, A. et al. Acting pre-emptively reduces the long-term costs of managing herbicide resistance. Sci. Rep. 14(1), 6201 (2024).
Singh, R. et al. Systemic analysis of glyphosate impact on environment and human health. ACS Omega. 9(6), 6165–6183 (2024).
Portier, C. J. et al. Differences in the carcinogenic evaluation of glyphosate between the international agency for research on cancer (IARC) and the European food safety authority (EFSA). J. Epidemiol. Community Health. 70(8), 741–745 (2016).
Vandenberg, L. N. et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J. Epidemiol. Community Health. 71(6), 613–618 (2017).
Bradley, P. M. et al. Expanded Target-Chemical analysis reveals extensive Mixed-Organic-Contaminant exposure in U.S. Streams. Environ. Sci. Technol. 51(9), 4792–4802 (2017).
Bjørnåvold, A. et al. To tax or to ban? A discrete choice experiment to elicit public preferences for phasing out glyphosate use in agriculture. PLoS One. 18(3), e0283131 (2023).
Hakme, E., Poulsen, M. E. & Lassen, A. D. A comprehensive review on pesticide residues in human urine. J. Agric. Food Chem. 72(32), 17706–17729 (2024).
Guzman-Torres, H. et al. Frequency of urinary pesticides in children: a scoping review. Front. Public. Health. 11, 1227337 (2023).
Schütze, A. et al. Quantification of glyphosate and other organophosphorus compounds in human urine via ion chromatography isotope Dilution tandem mass spectrometry. Chemosphere 274, 129427 (2021).
Caudill, S. P. Characterizing populations of individuals using pooled samples. J. Expo Sci. Environ. Epidemiol. 20(1), 29–37 (2010).
Rogot, E. et al. On the feasibility of linking census samples to the National death index for epidemiologic studies: a progress report. Am. J. Public. Health. 73(11), 1265–1269 (1983).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Vidal-Petiot, E. Thresholds for hypertension definition, treatment initiation, and treatment targets: recent guidelines at a glance. Circulation 146(11), 805–807 (2022).
Classification and diagnosis of diabetes: standards of medical care in Diabetes-2021. Diabetes Care 44(Suppl 1), S15–S33 (2021).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 161, 1–24 (2013).
Kalsing, A. et al. The population genomics of Conyza spp. In soybean macroregions suggest the spread of herbicide resistance through Intraspecific and Interspecific gene flow. Sci. Rep. 14(1), 19536 (2024).
Reed, K. B. et al. Evolving dual-trait EPSP synthase variants using a synthetic yeast selection system. Proc. Natl. Acad. Sci. U S A. 121(35), e2317027121 (2024).
Sandhu, P. K. et al. Global Metabolome of Palmer Amaranth (Amaranthus palmeri) Populations Highlights the Specificity and Inducibility of Phytochemical Responses To Abiotic Stress(J Agric Food Chem, 2023).
García-Ruiz, E. et al. Weeds and ground-dwelling predators’ response to two different weed management systems in glyphosate-tolerant cotton: A farm-scale study. PLoS One. 13(1), e0191408 (2018).
Hoagland, R. E., Boyette, C. D. & Stetina, K. C. Bioherbicidal activity of albifimbria verrucaria (Formerly myrothecium verrucaria) on Glyphosate-Resistant Conyza Canadensis. J. Fungi (Basel) 9(7) (2023).
Liu, J. et al. Dynamic seed emission, dispersion, and deposition from horseweed (Conyza canadensis (L.) Cronquist). Plants (Basel) 11(9) (2022).
Shi, G. et al. Effects of Biochar and compost on microbial community assembly and metabolic processes in glyphosate, Imidacloprid and pyraclostrobin polluted soil under freezethaw cycles. J. Hazard. Mater. 471, 134397 (2024).
Robichaud, C. D. & Rooney, R. C. Title: low concentrations of glyphosate in water and sediment after direct over-water application to control an invasive aquatic plant. Water Res. 188, 116573 (2021).
Schütte, G. et al. Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 29(1), 5 (2017).
Elhawat, N. et al. Living mulch enhances soil enzyme activities, nitrogen pools and water retention in giant Reed (Arundo Donax L.) plantations. Sci. Rep. 14(1), 1704 (2024).
Popradit, A. et al. Soil degradation and herbicide pollution by repeated cassava monoculture within Thailand’s conservation region. PLoS One. 19(8), e0308284 (2024).
Ferreira, N. G. C. et al. Hotspots of soil pollution: possible glyphosate and aminomethylphosphonic acid risks on terrestrial ecosystems and human health. Environ. Int. 179, 108135 (2023).
Zhang, Q. et al. Glyphosate disorders soil enchytraeid gut microbiota and increases its antibiotic resistance risk. J. Agric. Food Chem. 72(4), 2089–2099 (2024).
Charbonneau, A. et al. Fertilisation of agricultural soils with municipal biosolids: glyphosate and aminomethylphosphonic acid inputs to Québec field crop soils. Sci. Total Environ. 922, 171290 (2024).
Lima, I. B. et al. Glyphosate pollution of surface runoff, stream water, and drinking water resources in Southeast Brazil. Environ. Sci. Pollut Res. Int. 30(10), 27030–27040 (2023).
Delprat, N. et al. User-friendly one-step disposable signal-on bioassay for glyphosate detection in water samples. Biosens. Bioelectron. 241, 115689 (2023).
Lin, J. F., Chang, F. C. & Sheen, J. F. Determination of glyphosate, aminomethylphosphonic acid, and glufosinate in river water and sediments using microwave-assisted rapid derivatization and LC-MS/MS. Environ. Sci. Pollut Res. Int. 29(30), 46282–46292 (2022).
Hénault-Ethier, L. et al. Potential efficiency of grassy or shrub Willow buffer strips against nutrient runoff from soybean and corn fields in Southern Quebec, Canada. J. Environ. Qual. 48(2), 352–361 (2019).
Wang, W. et al. Glyphosate induces lymphocyte cell dysfunction and apoptosis via regulation of miR-203 targeting of PIK3R1 in common carp (Cyprinus Carpio L). Fish. Shellfish Immunol. 101, 51–57 (2020).
Matozzo, V. et al. Effects of aminomethylphosphonic acid, the main breakdown product of glyphosate, on cellular and biochemical parameters of the mussel mytilus galloprovincialis. Fish. Shellfish Immunol. 83, 321–329 (2018).
Geret, F. et al. Effects of low-dose exposure to pesticide mixture on physiological responses of the Pacific oyster, Crassostrea gigas. Environ. Toxicol. 28(12), 689–699 (2013).
Ferreira, L. M. N. et al. Climatic fluctuations alter the preference of stingless bees (Apidae, Meliponini) towards food contaminated with acephate and glyphosate. Sci. Total Environ. 952, 175892 (2024).
Vázquez, D. E., Verellen, F. & Farina, W. M. Early exposure to glyphosate during larval development induces late behavioural effects on adult honey bees. Environ. Pollut. 360, 124674 (2024).
Mallick, B., Rana, S. & Ghosh, T. S. Role of herbicides in the decline of butterfly population and diversity. J. Exp. Zool. Ecol. Integr. Physiol. 339(4), 346–356 (2023).
Thompson, L. J. et al. Bumblebees can be exposed to the herbicide glyphosate when foraging. Environ. Toxicol. Chem. 41(10), 2603–2612 (2022).
Liebing, J. et al. Health status of free-ranging ring-necked pheasant chicks (Phasianus colchicus) in North-Western Germany. PLoS One. 15(6), e0234044 (2020).
Caloni, F. et al. Suspected poisoning of domestic animals by pesticides. Sci. Total Environ. 539, 331–336 (2016).
Agbohessi, P. et al. Evaluation of acute toxicity and histology effect on liver of glyphosate and atrazine in the African catfish Clarias Gariepinus (Burchell 1822). J. Environ. Sci. Health B. 58(1), 21–30 (2023).
Ames, J. et al. Glyphosate-based herbicide (GBH) causes damage in embryo-larval stages of zebrafish (Danio rerio). Neurotoxicol Teratol. 95, 107147 (2023).
Sulukan, E. et al. Global warming and glyphosate toxicity (I): adult zebrafish modelling with behavioural, immunohistochemical and metabolomic approaches. Sci. Total Environ. 858(Pt 3), 160086 (2023).
Sulukan, E. et al. Global warming and glyphosate toxicity (II): offspring zebrafish modelling with behavioral, morphological and immunohistochemical approaches. Sci. Total Environ. 856(Pt 1), 158903 (2023).
Lu, J. et al. Characterization of glyphosate-induced cardiovascular toxicity and apoptosis in zebrafish. Sci. Total Environ. 851(Pt 2), 158308 (2022).
Pompermaier, A. et al. Impaired initial development and behavior in zebrafish exposed to environmentally relevant concentrations of widely used pesticides. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 257, 109328 (2022).
Lanzarin, G. A. B. et al. Evaluation of the developmental effects of a glyphosate-based herbicide complexed with copper, zinc, and manganese metals in zebrafish. Chemosphere 308(Pt 2), 136430 (2022).
Lanzarin, G. A. B. et al. Dose-dependent effects of a glyphosate commercial formulation - Roundup(®) UltraMax - on the early zebrafish embryogenesis. Chemosphere 223, 514–522 (2019).
Díaz-Martín, R. D. et al. Short exposure to glyphosate induces locomotor, craniofacial, and bone disorders in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 87, 103700 (2021).
Bridi, D. et al. Glyphosate and Roundup(®) alter morphology and behavior in zebrafish. Toxicology 392, 32–39 (2017).
Roy, N. M., Carneiro, B. & Ochs, J. Glyphosate induces neurotoxicity in zebrafish. Environ. Toxicol. Pharmacol. 42, 45–54 (2016).
Flach, H. et al. Impact of glyphosate-based herbicide on early embryonic development of the amphibian xenopus laevis. Aquat. Toxicol. 244, 106081 (2022).
Costa, M. J. et al. Oxidative stress biomarkers and heart function in bullfrog tadpoles exposed to roundup original. Ecotoxicology 17(3), 153–163 (2008).
Florencia, F. M. et al. Effects of the herbicide glyphosate on non-target plant native species from Chaco forest (Argentina). Ecotoxicol. Environ. Saf. 144, 360–368 (2017).
Kim, Y. H. et al. Heart rate-corrected QT interval predicts mortality in glyphosate-surfactant herbicide-poisoned patients. Am. J. Emerg. Med. 32(3), 203–207 (2014).
Ge, X., Yang, Z. & Cai, Q. The capillary-leakage syndrome caused by glyphosate poisoning: a case report. Ann. Med. Surg. (Lond). 85(4), 1180–1183 (2023).
Tao, Y. et al. Treatment of Gastrointestinal corrosive burns caused by highly lethal glyphosate solution poisoning. J. Coll. Physicians Surg. Pak. 32(12), pSs131–ss133 (2022).
Yokoyama, S. et al. Transient glyphosate encephalopathy due to a suicide attempt. Neuropsychopharmacol. Rep. 41(3), 444–447 (2021).
Kunapareddy, T. & Kalisetty, S. Glyphosate poisoning - a case report. J. Postgrad. Med. 67(1), 36–38 (2021).
Ghosh, S. et al. Cardiogenic shock with first-degree heart block in a patient with glyphosate-surfactant poisoning. Trop. Doct. 51(2), 244–246 (2021).
Calderon, L. et al. Residential proximity to agricultural pesticide use and cardiovascular disease risk factors among adult Latina women in California’s Salinas Valley. Am. J. Epidemiol. (2024).
Sun, H. et al. Polyoxyethylene tallow amine and glyphosate exert different developmental toxicities on human pluripotent stem cells-derived heart organoid model. Sci. Total Environ. 918, 170675 (2024).
Maia, F. C. C. et al. Cardiovascular damage associated with subchronic exposure to the glyphosate herbicide in Wistar rats. Toxicol. Ind. Health. 37(4), 210–218 (2021).
Printemps, R. et al. The cardiotoxic effect of Roundup® is not induced by glyphosate: A Non-specific Blockade of human Ca(V)1.2 channels. Cardiovasc. Toxicol. 22(7), 676–688 (2022).
Lee, H. L. et al. Comparative effects of the formulation of glyphosate-surfactant herbicides on hemodynamics in swine. Clin. Toxicol. (Phila). 47(7), 651–658 (2009).
Dal’ Bó, I. F. et al. Alternation between toxic and proliferative effects of Roundup® on human thyroid cells at different concentrations. Front. Endocrinol. (Lausanne). 13, 904437 (2022).
Franke, A. A. et al. Pilot study on the urinary excretion of the glyphosate metabolite aminomethylphosphonic acid and breast cancer risk: the multiethnic cohort study. Environ. Pollut. 277, 116848 (2021).
Silva, L. C. M., Daam, M. A. & Gusmao, F. Acclimation alters glyphosate temperature-dependent toxicity: implications for risk assessment under climate change. J. Hazard. Mater. 385, 121512 (2020).
Martínez, M. A. et al. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 135, 105414 (2020).
Malatesta, M. et al. Hepatoma tissue culture (HTC) cells as a model for investigating the effects of low concentrations of herbicide on cell structure and function. Toxicol. Vitro. 22(8), 1853–1860 (2008).
Parajuli, K. R. et al. Aminomethylphosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 16(5), 11750–11765 (2015).
Han, K. et al. Analysis of the association between urinary glyphosate exposure and fatty liver index: a study for US adults. BMC Public. Health. 24(1), 703 (2024).
Eskenazi, B. et al. Association of lifetime exposure to glyphosate and aminomethylphosphonic acid (AMPA) with liver inflammation and metabolic syndrome at young adulthood: findings from the CHAMACOS study. Environ. Health Perspect. 131(3), 37001 (2023).
Tang, J. et al. Ion imbalance is involved in the mechanisms of liver oxidative damage in rats exposed to glyphosate. Front. Physiol. 8, 1083 (2017).
Liu, J., Yang, G. & Zhang, H. Glyphosate-triggered hepatocyte ferroptosis via suppressing Nrf2/GSH/GPX4 axis exacerbates hepatotoxicity. Sci. Total Environ. 862, 160839 (2023).
Gasnier, C. et al. Dig1 protects against cell death provoked by glyphosate-based herbicides in human liver cell lines. J. Occup. Med. Toxicol. 5, 29 (2010).
Wunnapuk, K. et al. Use of a glyphosate-based herbicide-induced nephrotoxicity model to investigate a panel of kidney injury biomarkers. Toxicol. Lett. 225(1), 192–200 (2014).
Oliveira, J. M. et al. The effects of glyphosate-based herbicide on the hypothalamic-pituitary thyroid axis are tissue-specific and dependent on age exposure. Environ. Pollut. 334, 122216 (2023).
Adewale, O. O. et al. Xylopia aethiopica suppresses markers of oxidative stress, inflammation, and cell death in the brain of Wistar rats exposed to glyphosate. Environ. Sci. Pollut Res. Int. 30(21), 60946–60957 (2023).
Winstone, J. K. et al. Glyphosate infiltrates the brain and increases pro-inflammatory cytokine TNFα: implications for neurodegenerative disorders. J. Neuroinflammation. 19(1), 193 (2022).
Cattani, D. et al. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology 320, 34–45 (2014).
Gui, Y. X. et al. Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicol Teratol. 34(3), 344–349 (2012).
Chianese, T. et al. Glyphosate exposure induces cytotoxicity, mitochondrial dysfunction and activation of ERα and ERβ Estrogen receptors in human prostate PNT1A cells. Int. J. Mol. Sci. 25(13) (2024).
Lu, L. et al. Glyphosate drives autophagy-dependent ferroptosis to inhibit testosterone synthesis in mouse Leydig cells. Sci. Total Environ. 914, 169927 (2024).
Estienne, A. et al. Chronic dietary exposure to a glyphosate-based herbicide results in reversible increase early embryo mortality in chicken. Ecotoxicol. Environ. Saf. 241, 113741 (2022).
Ganesan, S., McGuire, B. C. & Keating, A. F. Absence of glyphosate-induced effects on ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 96, 156–164 (2020).
Ganesan, S. & Keating, A. F. Ovarian mitochondrial and oxidative stress proteins are altered by glyphosate exposure in mice. Toxicol. Appl. Pharmacol. 402, 115116 (2020).
Cai, W. et al. Low-dose roundup induces developmental toxicity in bovine preimplantation embryos in vitro. Environ. Sci. Pollut Res. Int. 27(14), 16451–16459 (2020).
de Cavalli, L. O. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic Biol. Med. 65, 335–346 (2013).
Razi, M. et al. Histological and histochemical effects of Gly-phosate on testicular tissue and function. Iran. J. Reprod. Med. 10(3), 181–192 (2012).
Benachour, N. & Séralini, G. E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 22(1), 97–105 (2009).
Dallegrave, E. et al. The teratogenic potential of the herbicide glyphosate-Roundup in Wistar rats. Toxicol. Lett. 142(1–2), 45–52 (2003).
Acknowledgements
We would like to give special thanks to the supporters, workers and participants of NHANES for their contribution to the completion of this study.
Funding
This work was supported by the National Natural Science Foundation of China (81660085) and the Key Science and Technology Innovation Projects of Jiangxi Provincial Health Commission (2024ZD007).
Author information
Authors and Affiliations
Contributions
YC and ZW conceived and designed the study; YC drafted the manuscript and participated in the literature search, data analysis, and interpretation. ZW collected the data and contributed to the statistical analysis. All authors contributed to the review/editing of key intellectual content of the manuscript. ML and YW provided key revisions. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by Ethics Review Committee of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Wu, Z., Li, M. et al. The relationship between urinary glyphosate and all-cause and specific-cause mortality: a prospective study. Sci Rep 15, 10759 (2025). https://doi.org/10.1038/s41598-025-95139-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95139-y