Abstract
In urological surgery, the Da Vinci Xi robotic system has many advantages, but it also has limitations such as high equipment costs and the need for professional training. Kangduo is a laparoscopic surgical robot developed in China, and SR2000 is its newly approved four-arm laparoscopic surgical robot. This study compares the short-term outcomes of the two in radical cystectomy. Data from a centre-based randomised non-inferiority trial were used and patients between March 2023 and June 2024 were enrolled and randomised according to criteria. The primary outcome was surgical success, and secondary outcomes included intraoperative variables, surgical outcomes, pathological outcomes, and postoperative outcomes. A total of 34 patients, 16 in the KD group and 18 in the DV group, had no intermediate open or conventional laparoscopic surgery, and the surgical success rate was 100%, with no significant differences between the two groups in many aspects. The effectiveness and safety of the KangDuo SR2000 system were demonstrated. Experienced surgeons confirmed that performing radical cystectomy using the KangDuo SR2000 system yielded results comparable to those achieved using the Da Vinci robotic system.
Similar content being viewed by others
Introduction
Bladder cancer (BC) is one of the most common malignancies of the urinary system. Globally, BC is the tenth most common cancer and the sixth most common cancer in men1,2; therefore, it is a significant threat to human health3,4,5. Urothelial carcinoma (UC) is the most common histological type of BC6. Based on the extent of invasion, BC is classified into non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)7,8,9.Radical cystectomy (RC) is the standard treatment for MIBC, which includes complete removal of the bladder and surrounding tissues, thereby achieving a radical cure by eradicating the primary tumor and potential local metastases10,11. Postoperative urinary diversion methods include ureterostomy, ileal conduit placement, and neobladder reconstruction. Furthermore, the surgical approaches include open, laparoscopic, and robot-assisted surgeries12,13.
RC is a complex procedure that requires a multidisciplinary collaboration. Surgical success depends on the complete removal of the tumor and the patient’s overall health and quality of life. Advances in medical technology have significantly improved the safety and efficacy of RC. From traditional open surgery to laparoscopic surgery and now robotic surgery, advanced surgical techniques and equipment have provided greater benefits to patients. Laparoscopic radical cystectomy (LRC) has been widely adopted in clinical practice because of its advantages of minimal surgical trauma, reduced blood loss, and a shorter recovery time; however, laparoscopic surgery has certain limitations such as unclear visualization, need for suturing skills, suboptimal ergonomics, and operator fatigue after prolonged use. Developing and introducing robotic surgical systems has helped advance minimally invasive surgery.
Robot-assisted radical cystectomy (RARC) is a modern surgical technique that combines robotic technology with minimally invasive surgery14,15. RARC uses robotic systems, such as the Da Vinci surgical or KangDuo robotic systems. Surgeons operate the robotic arms remotely through consoles to perform precise surgical operations16,17.
RARC offers an outstanding three-dimensional (3D) high-definition view. The surgeon can see the surgical field magnified 10–15 times through the console, significantly enhancing the identification of subtle anatomical structures18. This clear view allows more precise identification and excision of the tumor tissue while protecting the surrounding critical structures, such as the nerve and vascular bundles. This may improve the radicality of surgery and patients’ functional recovery, especially regarding urinary control and sexual function19. In addition, the flexibility of robotic systems is a significant advantage. The robotic arms can rotate by nearly 360° within a narrow pelvic space, enabling the surgeon to reach angles that are difficult to achieve in traditional open or laparoscopic surgery. This advantage is particularly evident during delicate procedures, such as lymph node dissection and nerve preservation. Additionally, the robotic system can eliminate natural tremors of the human hand, improving surgical precision20.
RARC may also result in reduced blood loss and faster postoperative recovery. A clear view and precise control during surgery allow more effective blood loss management. Smaller incisions and reduced tissue damage indicate that patients may experience less postoperative pain, which promotes early mobilization and faster recovery21. Many studies suggest that patients who undergo RARC may have shorter hospital stays compared to those who undergo open surgery21,22.
From the surgeon’s perspective, RARC offers a better ergonomic experience. Traditional laparoscopic surgery often requires the surgeon to maintain awkward positions for extended periods. In contrast, RARC allows the surgeon to operate in a comfortable, seated position, thereby reducing fatigue, particularly during long surgeries. This comfort may indirectly improve the quality and safety of the surgery23.
It is important to note that RARC has several challenges. The primary challenge is the steep learning curve, as this technique requires specialized training and practice. In addition, the initial investment in equipment is expensive, which may increase healthcare costs. Nevertheless, these challenges are gradually being overcome with continuous technological advancements and accumulated experience.
Overall, RARC represents a significant advancement in urology. It combines the advantages of minimally invasive surgery with high-tech assistance, providing patients with potentially better surgical outcomes and experience. However, multiple factors must be considered when selecting this surgical approach, including the medical team’s experience, the hospital’s equipment capabilities, and patient’s circumstances. With more long-term follow-up data, we can comprehensively evaluate the value and role of RARC in treating BC.
In 2000, the United States Food and Drug Administration (FDA) approved the Da Vinci system for laparoscopic surgery, marking its official introduction into clinical practice and the beginning of its transformative journey in surgery. Over the years, the range of applications of the Da Vinci system has expanded rapidly, from prostatectomy to cardiac surgery, and by 2005, it was also being used in gynecological surgeries. This rapid expansion reflects the medical community’s recognition and demand for innovative technologies. Continuous technological upgrades have been the key to maintaining the leading position of the Da Vinci system, which was introduced in 2006. For example, the addition of a high-definition (HD) 3D vision system significantly improved surgical precision. The Si system, launched in 2009, introduced dual consoles, making surgeon training more convenient. In 2014, the Xi system, with more flexible robotic arms and a broader range of surgical indications, further expanded the system’s applications24.
The development of the Da Vinci system has been a challenge. The high cost of equipment and maintenance has been a major obstacle to the widespread adoption of this system. Recently, China domestically developed the KangDuo robotic system, which is a significant breakthrough in the field of high-end medical equipment25,26. The KangDuo 1.0 robotic system has since been widely applied in clinical practice, particularly in urology, where it has demonstrated unique advantages and value27,28. With the continuous development of technology, the new-generation KangDuo 2.0 robot marks another important milestone in the development of surgical robotics in China, representing continued innovation and progress in the field of domestic high-end medical equipment29. The upgraded surgical robotic system integrates advanced technologies and functions to provide surgeons with more precise and intelligent surgical assistance.Liu Y, Wang Y, et al. have cited the Kangduo robot to perform a considerable number of surgeries in the field of colorectal cancer surgery with satisfactory results30.
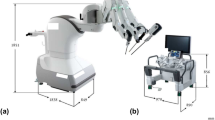
KangDuo 2.0 has made breakthroughs in several areas (Fig. 1). The visual system uses high-resolution 3D imaging technology to provide surgeons with a clearer and more detailed surgical view. This not only improves surgical precision but also reduces visual fatigue. Additionally, the flexibility and precision of the new generation of robotic arms, which mimic the complex movements of human hands, have been improved. This allows surgeons to perform delicate surgeries in small spaces. This intelligent and user-friendly design will enable surgeons to master system operations more quickly and naturally, thus shortening the learning curve and reducing operational errors during surgery31.
The KangDuo 2.0 robotic system is still in the clinical trial phase and has not yet been widely applied in clinical practice. This study is part of the ongoing research at our center evaluating the short-term surgical outcomes of the KangDuo 2.0 robotic system compared to the Da Vinci Xi system in patients undergoing RARC.
Methods
Study population
This study included 36 patients who underwent RARC at our hospital between March 2023 and June 2024, only ileal conduits and ureterostomy were taken into consideration. This study uses a web-based central randomisation system (IWRS) for subject randomisation. This trial uses block group randomisation, where enrolled subjects will be randomly assigned to the test and control groups in a 1:1 ratio.The urinary diversion methods after total cystectomy included ureterostomy in 25 patients, ileal conduit in nine, and neobladder reconstruction in two. After excluding two patients who underwent neobladder reconstruction, 34 were finally enrolled in the study. Among them, 16 patients underwent surgery using the KangDuo 2.0 robotic system (KD group), and 18 underwent surgery using the Da Vinci Xi robotic system (DV group). All surgeries were performed by three experienced chief surgeons with the same seniority level.(Fig. 2).
The inclusion criteria were as follows:
-
1.
Pathologically confirmed UC of the bladder.
-
2.
Age between 18 and 80 years.
-
3.
Imaging revealed no infiltration of the surrounding organs or tissues, pelvic lymph node metastasis, or distant metastasis.
-
4.
American Society of Anesthesiologists (ASA) classification I–II.
-
5.
Consent to undergo RARC and voluntary participation in the trial were obtained using a signed informed consent form.
The exclusion criteria were as follows:
-
1.
Preoperative pathological findings were incompatible with study inclusion.
-
2.
Inability to tolerate anesthesia or laparoscopic surgery.
-
3.
Body mass index (BMI) > 30 kg/m2.
-
4.
Presence of urinary system stones, renal pelvis or ureteral tumors, or history of severe systemic diseases or abdominal surgery.
-
5.
Pregnant or breastfeeding women.
-
6.
Participation in other ongoing clinical trials involving drugs or medical devices.
The withdrawal criteria were as follows:
-
1.
Withdrawal of the patient’s informed consent to treatment.
-
2.
Patients deemed unsuitable to continue participation by the researchers (e.g., disease progression requiring urgent surgery, worsening of underlying conditions affecting surgery, or discrepancies between surgical exploration and imaging findings).
-
3.
Death of the patient.
-
4.
Patient lost to follow-up.
-
5.
Termination of the trial, as requested by the sponsor.
This study was a part of a research project titled “Study on the Application of Laparoscopic Surgery Systems in Urological Surgeries” (protocol number IITGC-3866-20231117). The study was approved by the Institutional Review Board and Ethics Committee of all participating centers (approval number 2023-301-QX-IIT). Written informed consent was obtained from all patients. This trial was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The KangDuo SR2000 laparoscopic surgical system used in this study is a new product developed by Harbin Sizerui Intelligent Medical Equipment Co., Ltd., which is an iteration of the KangDuo SR1000 laparoscopic surgical system developed by Suzhou KangDuo Robotics Co., Ltd., a wholly owned subsidiary of Sizerui. The KD group in this study used the KangDuo SR2000 system.
Procedures
The surgeons participating in this study were highly experienced chief surgeons who had independently completed over 100 urological robotic surgeries. Prior to the trial, KangDuo Robotics ensured that the surgeons had sufficient time to learn and use the equipment. The surgeons practiced extensively on the simulators provided by the company and were certified to perform robotic surgeries.
All patients underwent preoperative evaluations, including ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and cystoscopy. Biopsies were performed during cystoscopy to assess the local tumor invasion and preoperative staging. Eligible patients were randomly assigned to either the KD group, which used the KangDuo SR2000 robotic system, or the DV group, which used the Da Vinci Xi robotic system. Both systems followed similar operating procedures and only ileal conduits and ureterostomy were taken into consideration.
Perioperative management was performed according to the standards of our centre, with no statistically significant differences between the two groups. RC followed standard urological principles, including the extent of surgery, including the bladder, surrounding fatty tissues, distal ureters, and lymph node dissection. In male patients, the prostate and seminal vesicles were also removed, whereas in female patients, the uterus and part of the anterior vaginal wall were resected. Urinary diversion methods include ureterostomy, ileal conduit placement, and neobladder reconstruction, with the final decision made based on the patient’s condition and personal or family preferences. The surgeon made other intraoperative decisions, such as frozen section analysis, ureteral-ileal conduit anastomosis techniques, and conversion to open surgery, based on the intraoperative situation. The surgical videos and specimen photographs were retained for post-operative review. (Fig. 3).
We kept detailed records of the preoperative patient demographics, imaging data, and intrao-perative information, such as the operative time, docking time, robotic arm surgical time, and intra-operative blood loss. Postoperative recovery was monitored, including the time to first flatus, first feeding, first mobilization, first defecation, and the total hospital stay. The main postoperative assessments included blood tests on the day before surgery and on postoperative days 1 and 3, and week 4, including the white blood cell count, hemoglobin, alanine transaminase (ALT), and creatinine levels. Postoperative complications were graded using the Clavien–Dindo classification. Additionally, the safety and efficacy of the KangDuo SR2000 robotic system in RC were evaluated. Surgeons also assessed the operability of the surgical equipment to confirm the reasonability of its structural design and the usability of its supporting instruments.
Outcomes
Efficacy evaluation
The primary efficacy indicator was the success rate of the surgery, which was defined as the completion of robot-assisted surgery as planned without transition to other surgical methods. Secondary efficacy evaluations included the time to first flatus, time to first feeding, time to first mobilization, time to first bowel movement, total hospital stay, and surgeon satisfaction with the robotic system. The satisfaction evaluations included the following:
-
1.
Assessment of task load during equipment docking was measured using the NASA task load index (NASA-TLX) scale.
-
2.
Intraoperative surgical sensation rating.
Safety evaluation
Safety evaluations included operative time, blood loss, and adverse events. The instrument-related operational times were as follows:
-
1.
Docking time: Time taken to move the surgical arm system to the operating table and dock the last sleeve of the corresponding robotic arm.
-
2.
Console time: Time from the surgeon’s first intervention to the end of the operation.
Statistical methods
All statistical analyses were performed using IBM SPSS Statistics for Windows 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) or as median (interquartile range (Q1–Q3)). Categorical data were counted and expressed as percentages. Continuous variables were compared using non-parametric tests and t-tests, while categorical variables were compared using the chi-square (χ2) test or Fisher’s exact test. Differences were considered statistically significant at p < 0.05. All quantitative data were tested for normality, with variables that conformed to normal distribution described by means plus or minus standard deviation and using parametric tests, and variables that did not conform to normal distribution described by medians and quartiles and using non-parametric tests.
Results
The baseline characteristics of the two groups were similar, and no statistically significant differences were observed (Table 1). All three surgeons performed surgeries using both the KangDuo and Da Vinci robotic systems, as well as urinary diversion techniques, ileal conduit, and ureterostomy. The KD group included 16 patients, of whom five underwent an ileal conduit and 11 underwent ureterostomy. The DV group included 18 patients, of whom four underwent an ileal conduit and 14 underwent ureterostomy. The incidence of Clavien–Dindo grade II or higher complications was 18.75% in the KD group and 11.12% in the DV group. The rate of adverse events in both groups was 0% (Table 2).
Efficacy
The surgical success rate for both the KD and DV groups was 100%, with no cases of failure or conversion to other surgical methods. The time to first bowel movement was similar between the KD and DV groups (6.94 ± 1.35 days vs. 6.94 ± 2.29 days, p = 0.9916) (Table 2). When the NASA-TLX task load evaluation scores and intraoperative surgical sensation ratings were compared (Table 3), no statistically significant differences were observed between the KD and DV groups.
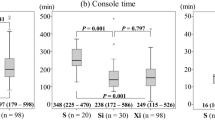
Safety
Overall, the median operative time in the KD and DV groups was 262.50 (205.00–315.00) min and 272.50 (217.50–357.50) min, with no statistically significant difference between the groups (p = 0.4261). Additionally, there were no significant differences in the docking time (p = 0.8440) or robotic arm operation time (p = 0.5881). Furthermore, the differences in intraoperative blood loss were not statistically significant between groups, and no intraoperative complications were observed in either group (Table 2).Table 2 also shows the occurrence of postoperative adverse events and Clavien-Dindo grade I complications. Only five patients had intraoperative or postoperative complications, all of which were Clavien-Dindo grade I complications. Five surgery-related adverse events occurred (one case each of severe postoperative abdominal distension, wound bleeding, incision bleeding, trocar site bleeding, and gastroparesis and indigestion); there was no statistically significant difference between the groups (p = 0.4721). After treatment during hospitalization, the symptoms resolved, and the patients were discharged without any adverse symptoms. A follow-up CT scan 1-month post-surgery showed no significant abnormalities. In addition, the laboratory test results indicated no statistically significant differences in blood parameters before and after surgery between the two groups (Fig. 4).
The subgroup analysis (Table 4) showed that for patients who underwent ileal conduit urinary diversion, there were no statistically significant differences between the DV and KD groups in terms of operative time (392.50 ± 74.67 min vs. 438.20 ± 110.44 min, p = 0.5040), docking time (p = 0.5642), and console time (p = 0.8571). Blood loss and the rate of intraoperative adverse events were also similar (p = 0.2475). For patients undergoing ureterostomy, the median operative times for the DV and KD groups were similar (220.00 [195.00–290.00] vs. 220.00 [215.00–290.00] min, p = 0.6796), as were the docking times (p = 0.7549) and console times (p = 0.3541). Blood loss and intraoperative adverse events were not significantly different between the groups (p = 0.7523).
Discussion
In recent years, minimally invasive techniques, such as laparoscopy, have been widely applied in surgery. As the concept of minimally invasive surgery has rapidly evolved, surgeons are no longer satisfied with the benefits of traditional laparoscopic techniques alone, as these procedures place higher demands on technical skills. Surgeons often need to maintain uncomfortable positions for long periods during laparoscopic surgery, causing significant discomfort. As a result, there has been a collective effort among surgeons to identify methods that provide greater benefits to both patients and surgeons. Robotic surgical platforms have addressed this issue, overcoming many of the limitations of traditional laparoscopy. Magnified 3D visualization helps surgeons to see the surgical field more clearly, and the four freely movable arms enable more complex surgical procedures while reducing the challenges of coordinating with surgical assistants. Previous studies have confirmed the feasibility, safety, and efficacy of robotic surgery32,33. In various urological tumor surgeries, robot-assisted laparoscopic systems retain the advantages of minimally invasive surgery while overcoming many of the shortcomings of traditional laparoscopy, providing outcomes comparable to those of open surgery. These systems allow surgeons to shift from “bedside surgery” to “console-based surgery,” improving ergonomics. However, immersive consoles also cause neck and shoulder discomfort for surgeons.
Multiple robotic surgical systems have been developed, both domestically and internationally. The Da Vinci surgical robot system is currently the most widely used robotic system worldwide34. Incomplete statistics show that there are approximately 3,000 Da Vinci systems globally35; however, in China, only approximately 100 units are available owing to high costs and significant surgical and maintenance expenses, which have hindered the widespread adoption of this technology36,37. Therefore, the development of new robotic surgery platforms and the advancement of innovative technologies are crucial38.
Building on the foundation of domestic medical robotics research, multidisciplinary collaboration has led to breakthroughs in key areas, such as surgical standardization, intelligent human–machine interaction, the integration of surgical information, and system versatility39,40. Advances in system architecture modeling and analysis, robotic mechanism optimization, 3D stereoscopic vision feedback for remote operations, virtual surgery simulation technology, and system interface technology have created a domestically developed laparoscopic robotic surgery system with proprietary intellectual property, the KangDuo Surgical Robot System41. The KangDuo SR1000 robot has been applied in clinical practice, yielding satisfactory results41,42,43.
In this study, we demonstrated that the overall efficacy of the KangDuo SR2000 system in radical cystectomy is not inferior to that of the Da Vinci system. The research results for the KangDuo SR2000 robotic system regarding efficacy and safety are encouraging. The effectiveness of the KangDuo SR2000 system was evaluated using one primary efficacy indicator and two secondary indicators. Both the KD and DV groups completed their surgeries as planned, with no conversion to laparoscopic or open surgery, thus achieving a 100% success rate. The time to first flatus and first bowel movement were similar between the groups, with both effectively preserving gastrointestinal function. Highly experienced surgeons performed surgeries, and there were no significant differences in the intraoperative sensation ratings. The KD group performed comparably for all other evaluated parameters to the DV group.
Furthermore, there were no significant differences between the KD and DV groups regarding the operative time, docking time, or console time. With improvements to the KangDuo SR2000 system, experienced surgeons could perform complex procedures, such as RC and ileal conduit surgery, which involve extensive suturing and anastomosis, as efficiently as with the Da Vinci system.
In our study, there were only 5 Clavien - Dindo Grade I postoperative complications, a result that is different from the generally high perioperative complication rates for radical cystectomy. After analysing the results, we believe that there may be several reasons for this. First, the rich experience of the surgical team played a key role. The surgeries involved in this study were all performed by senior surgeons with many years of experience in radical cystectomy in the Department of Urology of our hospital. Second, strict patient screening criteria were also an important factor. At the inclusion stage, we conducted a comprehensive and detailed assessment of patients, excluding those with serious underlying diseases and those in poor physical condition who could not tolerate the trauma of surgery.
Thirdly, the advanced KangDuo SR2000 and Da Vinci Xi robotic surgical systems provided a guarantee of safety, and the well-established perioperative management programme also played a positive role in reducing complications. However, despite the low complication rate in this study, this does not mean that the high-risk nature of radical cystectomy itself can be ignored. Future studies are still needed to further explore how to optimise surgical operations and perioperative management to reduce the complication rate in different healthcare settings and patient populations.
Additionally, we analyzed multiple preoperative and postoperative laboratory tests to investigate whether the two robotic systems affected patients’ internal environments differently. The laboratory results were similar in both groups. Overall, the short-term outcomes of this study are promising; however, further follow-up is necessary to assess the long-term results.
Moreover, we charted the learning curve for surgeons using the KangDuo system and found that as surgeons gained more experience with the system, the operative time decreased, indicating improved proficiency. Ileal conduit surgery, which typically takes longer than ureterostomy, somewhat affected the learning curve (Fig. 5).
The KangDuo SR1000 system has been widely applied in other surgical procedures, producing favorable outcomes43,44. The upgraded KangDuo SR2000 system is still in the clinical trial stage; however, the strengths of this study lie in its innovative design, prospective data collection, and rigorous follow-up, which provide strong evidence. Our study also fills a gap in the application of RARC in China by promoting the development of the KangDuo robotic system45. However, this study has some limitations. First, this was a comparison of short-term outcomes, and long-term follow-up data are required. Second, the sample size was relatively small, which may have affected the statistical significance of the results. Finally, this study was conducted at a single center; multicenter studies with larger sample sizes would be more convincing.
It is important to note that the technical tool of extracorporeal urinary diversion used in this study may limit the generalisability of the study results to some extent. Extracorporeal urinary bypass may not be routinely used in some specific medical centres due to equipment conditions, technical proficiency and other factors. Different healthcare providers may choose alternative methods of urine drainage when dealing with similar patients, which will lead to differences in therapeutic interventions for the patient population. For example, some primary care hospitals may prefer to use a simple double ureteral abdominal wall stoma, whereas larger speciality hospitals may use a more complex ileal access procedure or in situ cystectomy. Therefore, the results of this study need to be carefully considered in terms of the implications of such technological differences when generalising to areas with different levels of healthcare resources and habitual use of different urine drainage techniques. However, the choice of extracorporeal urinary diversion in this study was based on the specific needs of the study design, which aimed to compare the efficacy analysis of the KangDuo SR2000 with that of the da Vinci Robot in the same extracorporeal diversion procedure, and therefore subgroup analyses of the different extracorporeal diversion procedures were performed in this study. Despite potential issues with generalisability, we were able to provide unique insights and valuable data to the field through an in-depth study of patients with this specific technique, and expect more studies in the future to compare different urine drainage techniques to further clarify the scope of applicability and limitations of the results of this study. Two cases of in situ neobladdering, a complex form of urinary diversion, were also performed during the period of this study but were excluded, only ileal conduit and ureterostomy were included in this study. Recently, several studies have reported the use of robot-assisted in situ neobladdering, bringing new perspectives and possibilities to the field of RARC. For example, the literature46 describes the use of a novel robotic system for in situ neobladder surgery, which enables finer tissue suturing and reconstruction operations by optimising the dexterity of the robotic arm and the precision of its operation. Another paper47 focuses on the impact of a novel robotic system on surgical time and bleeding. The results of this study showed that RARC surgery time was significantly reduced and intraoperative haemorrhage was also reduced when operated by a surgical team skilled in the new robotic system. This may be attributed to the clearer three-dimensional field of view and more stable operational control provided by the new robotic system. In addition48, explores the feasibility of the novel robotic system in different medical institutions. It is pointed out in the paper that despite the potential advantages of the novel robotic system, there are still some obstacles to its widespread application in RARCs due to the uneven distribution of medical resources in different regions and differences in the threshold of technical access. Taken together, the novel robotic system shows potential advantages in the context of RARC, such as improved surgical precision and shorter surgical time, and can be applied to in situ neobladder as a mode of urinary diversion, but it also faces challenges in terms of cost, training, and dissemination.
It is also worth discussing that while both systems performed similarly on key metrics, there may be differences in certain details, such as surgeons’ experience with the systems and the steepness of the learning curves, which could affect real-world applications. Future studies should explore these aspects further. Another factor worth considering is the cost. Although this study did not directly compare the cost-effectiveness of the two systems, KangDuo 2.0, as a domestically produced device, may have an advantage in acquisition and maintenance costs49. This could be important for hospitals in regions with limited medical resources and may influence decision-making. Additionally, although this study focused on RC, future studies should compare these systems with other types of surgery to comprehensively assess their performance. Different types of surgeries with varying complexities may reveal different aspects of the system’s performance. Technological innovation is an ongoing process. This study reflects the current performance of the KangDuo 2.0 and Da Vinci Xi systems; however, both companies are continually upgrading their technologies. Future comparative studies are needed to keep up with the latest technological developments.Since the KangDuo SR2000 robot is still not on the market, its procurement, maintenance and operating costs, which are related to the privacy of KangDuo, have not been approved. Despite some shortcomings of the KangDuo SR2000, the SR2000 can still provide a new option for minimally invasive robotic surgery. Its development and manufacturing costs are lower than those of the Da Vinci Xi surgical robotic system, and although the price has not yet been determined, there is no doubt that the reduction in the development costs of the new robotic surgical robot and the price impact of the competitive effect will ultimately benefit more patients.
Conclusion
In conclusion, we report that experienced surgeons using the KangDuo SR2000 robotic system achieved results comparable to those obtained using the Da Vinci Xi robotic system for RARC. Therefore, the study provides strong supporting evidence for applying the KangDuo 2.0 system in complex urological surgeries. It showcases China’s innovation in the field of high-end medical equipment and offers valuable insights for clinical practice. As more clinical data are collected and technology advances, we expect domestic surgical robots to play an increasingly important role in the global market by providing patients with higher quality and more accessible medical services.
The trial registration number and date
This study, titled “Study on the Application of Laparoscopic Surgery Systems in Urological Surgeries,” with the trial registration number Chi CTR2400094197, was approved by the institutional review board and the trial registration date is 18/12/2024. All patients provided written informed consent. https://www.chictr.org.cn/searchproj.html.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Rozanec, J. J. & Secin, F. P. Epidemiology, etiology and prevention of bladder cancer. Arch. Esp. Urol. 7310, 872–878 (2020).
Alouini, S. Risk factors associated with urothelial bladder cancer. Int. J. Environ. Res. Public. Health 217 (2024).
Compérat, E. et al. Current best practice for bladder cancer: S narrative review of diagnostics and treatments. Lancet 400, 1712–1721 (2022).
Ahmadi, H., Duddalwar, V. & Daneshmand, S. Diagnosis and staging of bladder cancer. Hematol. Oncol. Clin. 353, 531–541 (2021).
Kirkali, Z. et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 666(Suppl 1), 4–34 (2005).
Bellmunt, J. Bladder cancer. Hematol. Oncol. Clin. 292, xiii–xiv (2015).
Dobruch, J. O. M. Bladder cancer: Current challenges and future directions. Medicina 57 (2021).
Klaassen, Z. et al. Treatment strategy for newly diagnosed T1 High-grade bladder urothelial carcinoma: New insights and updated recommendations. Eur. Urol. 745, 597–608 (2018).
DeGeorge, K. C., Holt, H. R. & Hodges, S. C. Bladder cancer: Diagnosis and treatment. Am. Fam. Physician 968, 507–514 (2017).
Sternberg, C. N. A critical review of the management of bladder cancer. Crit. Rev. Oncol. Hematol. 313, 193–207 (1999).
Brake, M., Loertzer, H., Horsch, R. & Keller, H. Recurrence and progression of stage T1, grade 3 transitional cell carcinoma of the bladder following intravesical immunotherapy with Bacillus Calmette-Guerin. J. Urol. 1636, 1697–1701 (2000).
Parekh, D. J. et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet 391, 2525–2536 (2018).
Pruthi, R. S. & Wallen, E. M. Robotic-assisted laparoscopic radical cystoprostatectomy. Eur. Urol. 532, 310–322 (2008).
Luchey, A. M., Agarwal, G. & Poch, M. A. Robotic-Assisted radical cystectomy. Cancer Control 223, 301–306 (2015).
Nix, J. et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. Eur. Urol. 572, 196–201 (2010).
Han, J. H. & Ku, J. H. Robot-assisted radical cystectomy: Where we are in 2023. Investig. Clin. Urol. 642, 107–117 (2023).
Hussein, A. A., Li, Q. & Guru, K. A. Robot-assisted radical cystectomy: Surgical technique, perioperative and oncologic outcomes. Curr. Opin. Urol. 321, 116–122 (2022).
Koie, T. et al. Utility of robot-assisted radical cystectomy with intracorporeal urinary diversion for muscle-invasive bladder cancer. Int. J. Urol. 263, 334–340 (2019).
Carrion, A. et al. Comparison of perioperative outcomes and complications of robot assisted radical cystectomy with extracorporeal vs intracorporeal urinary diversion. Actas Urol. Esp. Engl. Ed. 436, 277–283 (2019).
Khetrapal, P., Conroy, S., Kelly, J. D. & Catto, J. W. F. Comparing open-radical cystectomy and robot-assisted radical cystectomy: Current status and analysis of the evidence. Curr. Opin. Urol. 303, 400–406 (2020).
Khetrapal, P. et al. Robot-assisted radical cystectomy versus open radical cystectomy: A systematic review and Meta-analysis of perioperative, oncological, and quality of life outcomes using randomized controlled trials. Eur. Urol. 844, 393–405 (2023).
Rai, B. P. et al. Robot-assisted vs open radical cystectomy for bladder cancer in adults. BJU Int. 1256, 765–779 (2020).
Challacombe, B. J. et al. The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications. Eur. Urol. 604, 767–775 (2011).
Fairag, M. et al. Robotic revolution in surgery: Diverse applications across specialties and future prospects review Article. Cureus 161, e52148 (2024).
Zhang, Z. et al. Robot-assisted radical nephroureterectomy using the KangDuo surgical Robot-01 system versus the Da Vinci system: A multicenter prospective randomized controlled trial. Int. Braz. J. Urol. 506, 727–736 (2024).
Wen, Z. et al. KangDuo surgical robot versus Da Vinci robotic system in urologic surgery: A systematic review and meta-analysis. J. Robot. Surg. 191, 6 (2024).
Xiong, S. et al. Robotic urologic surgery using the KangDuo-Surgical Robot-01 system: A single-center prospective analysis. Chin. Med. J. 13624, 2960–2966 (2023).
Fan, S. et al. Robot-assisted pyeloplasty using a new robotic system, the KangDuo-Surgical Robot-01: A prospective, single-centre, single-arm clinical study. BJU Int. 1282, 162–165 (2021).
Xu, L. et al. Analysis of KangDuo-SR-1500 and KangDuo-SR-2000 robotic partial nephrectomy from an operative and ergonomic perspective: A prospective controlled study in Porcine models. J. Robot Surg. 181, 26 (2024).
Liu, Y. et al. Comparison of short-term outcomes of robotic-assisted radical colon cancer surgery using the Kangduo surgical robotic system and the Da Vinci Si robotic system: A prospective cohort study. Int. J. Surg. 1103, 1511–1518 (2024).
Fan, S. et al. Pyeloplasty with the Kangduo surgical robot vs the Da Vinci Si robotic system: preliminary results. J. Endourol. 3612, 1538–1544 (2022).
Alip, S. L., Kim, J., Rha, K. H. & Han, W. K. Future platforms of robotic surgery. Urol. Clin. 491, 23–38 (2022).
Koukourikis, P. & Rha, K. H. Robotic surgical systems in urology: What is currently available? Investig. Clin. Urol. 621, 14–22 (2021).
Solaini, L. et al. Robotic versus laparoscopic left colectomy: A systematic review and meta-analysis. Int. J. Colorectal Dis. 377, 1497–1507 (2022).
Chen, S. et al. The application of internal suspension technique in retroperitoneal robot-assisted laparoscopic partial nephrectomy with a new robotic system KangDuo surgical Robot-01: Initial experience. Asian J. Urol. 104, 482–487 (2023).
Li, X. et al. Robot-assisted partial nephrectomy with the newly developed KangDuo surgical robot versus the Da Vinci Si surgical system: A Double-center prospective randomized controlled noninferiority trial. Eur. Urol. Focus 91, 133–140 (2023).
Kaul, S. A. & Menon, M. Da Vinci assisted cystoprostatectomy and urinary diversion: A paradigm shift in surgical management of bladder cancer. Minerva Urol. Nefrol. 592, 149–157 (2007).
HemalAK et al. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol. Clin. 314, 719–729 (2004).
Braga, L. H., Pace, K., DeMaria, J. & Lorenzo, A. J. Systematic review and meta-analysis of robotic-assisted versus conventional laparoscopic pyeloplasty for patients with ureteropelvic junction obstruction: Effect on operative time, length of hospital stay, postoperative complications, and success rate. Eur. Urol. 565, 848–857 (2009).
Singh, I. & Hemal, A. K. Robot-assisted pyeloplasty: Review of the current literature, technique and outcome. Can. J. Urol. 172, 5099–5108 (2010).
Wang, J. et al. Partial nephrectomy through retroperitoneal approach with a new surgical robot system, KD-SR-01. Int. J. Med. Robot. 182, e2352 (2022).
Dai, X. et al. Comparison of KD-SR-01 robotic partial nephrectomy and 3D-laparoscopic partial nephrectomy from an operative and ergonomic perspective: A prospective randomized controlled study in Porcine models. Int. J. Med. Robot. 172, e2187 (2021).
Li, Z. et al. Robot-assisted modified bilateral dismembered V-shaped flap pyeloplasty for ureteropelvic junction obstruction in horseshoe kidney using KangDuo-Surgical-Robot-01 system. Int. Braz J. Urol. 493, 388–390 (2023).
Fan, S. et al. Robot-Assisted radical prostatectomy using the KangDuo surgical Robot-01 system: A prospective, Single-Center, Single-Arm clinical study. J. Urol. 2081, 119–127 (2022).
Xia, L., Wang, X., Xu, T. & Guzzo, T. J. Systematic review and Meta-Analysis of comparative studies reporting perioperative outcomes of Robot-Assisted partial nephrectomy versus open partial nephrectomy. J. Endourol. 319, 893–909 (2017).
Papalia, R. et al. ∆ delta neobladder: A novel stentless simplified totally intracorporeal robotic technique. Minerva Urol. Nephrol. 766, 773–781 (2024).
Rocco, B. et al. First case of robot-assisted radical cystectomy and intracorporeal neobladder reconstruction with the Hugo RAS system: Step-by-step surgical setup and technique. J. Robot. Surg. 175, 2247–2251 (2023).
Gaya, J. M. et al. Robot-assisted radical cystectomy and ileal conduit with Hugo(TM) RAS system: feasibility, setting and perioperative outcomes. Int. Braz. J. Urol. 496, 787–788 (2023).
Fan, S. et al. Totally intracorporeal robot-assisted bilateral ileal ureter replacement for the treatment of ureteral strictures using Kangduo surgical robot 2000 plus. Int. Braz. J. Urol. 506, 781–782 (2024).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Y.F., J.W., H.W., A.Z., Y.X. and W.X. The first draft of the manuscript was written by Y.F., all figures and tables were prepared by [Jing Wang] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Harbin Medical University (Date2024.08.13/No IITGC-3866-20231117).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in all Figures and Tables.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, Y., Wang, J., Zhou, A. et al. Short-term outcomes comparison of KangDuo 2.0 and Da Vinci Xi in radical cystectomy. Sci Rep 15, 11739 (2025). https://doi.org/10.1038/s41598-025-95382-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95382-3