Abstract
Although allogeneic hematopoietic cell transplantation (HCT) is an alternative treatment for relapsed or refractory (R/R) acute promyelocytic leukemia (APL), little is known regarding the utility of allogeneic HCT for R/R therapy-related APL (t-APL). We retrospectively analyzed data for 144 patients with APL (t-APL, n = 20 and de novo APL, n = 124) who received a first allogeneic HCT between 2008 and 2020. We found no significant differences in survival between the t-APL and de novo APL groups. The 3-year overall survival (OS) rates were 53.8% in the t-APL group and 52.4% in the de novo APL group. However, as previously reported, patients without complete remission (CR) at HCT had significantly worse OS than those with CR (P = 0.004). The 3-year OS rates were 61.1% in patients with CR and 36.5% in those without CR. These findings suggest that allogeneic HCT may be considered a viable treatment option for patients with t-APL and de novo APL, with an emphasis on achieving CR before transplantation to optimize outcomes. However, clinicians should be aware of the potential for worse outcomes in male patients and those with lower performance status, highlighting the need for personalized treatment approaches and careful patient selection.
Similar content being viewed by others
Introduction
With the introduction of combination therapy consisting of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), initial chemotherapy has become more effective for the treatment of acute promyelocytic leukemia (APL)1,2. In Japan, ATO became available for insurance coverage in December 2004. Combination therapy with ATRA and ATO is now the standard of care for low- to intermediate-risk APL. Despite the different origins of therapy-related APL (t-APL) and de novo APL, studies have shown that the prognoses of these two types of APL are similar when treated with a combination of ATRA and ATO3. Currently, treatment options for patients with relapsing or t-APL include ATO-based regimens, which have shown promising results with complete remission (CR) rates of 80–90% and 5-year overall survival (OS) rates of 50–70%. However, patients who fail to respond to ATO or experience multiple relapses may require allogeneic hematopoietic cell transplantation (HCT). The prognosis for these patients remains challenging, with 5-year OS rates ranging from 30 to 50% after allogeneic HCT4.
Given the rarity of t-APL, this study takes an exploratory approach to comparing outcomes between patients with t-APL and de novo APL undergoing allogeneic HCT. The study aims to provide valuable insights despite the inherent limitations of such a comparison and to offer essential insights into the prognosis and appropriate treatment strategies for these patient groups before insurance coverage for CPX-3515. CPX-351 is a liposomal formulation that combines two chemotherapy drugs, cytarabine and daunorubicin, in a fixed 5:1 molar ratio, and it has been shown to be effective for therapy-related leukemia.
Patients and methods
Study design and definitions
This retrospective study involved > 300 Japanese transplant centers in the Transplant Registry Unified Management Program database, which contains physician-reviewed data with informed consent and yearly follow-ups6,7. All methods in this study were performed in accordance with relevant guidelines and regulations. The study protocol was approved by the Data Management Committee of the Japanese Society for Hematopoietic Cell Transplantation and the Ethics Review Board of St. Mary’s Hospital (approval number: 24-1102). This observational study was conducted using data from the Transplant Registry Uniform Management Program database, and all participants provided informed consent.
In total, 144 patients aged > 15 years who received their first allogeneic HCT for t-APL (n = 20) and de novo APL (n = 124) were eligible for the study. All patients included in this study had APL with confirmed t(15;17) translocation and/or PML::RARA fusion. The presence of PML::RARA was verified in all cases using a combination of cytogenetic analysis, fluorescence in situ hybridization, and/or reverse transcription polymerase chain reaction. Any patients lacking confirmed PML::RARA fusion were excluded from the study to ensure a homogeneous patient population with classical molecular characteristics of APL. The selection criteria were APL according to the World Health Organization classification8, one allogeneic HCT between January 2008 and December 2020, no or one autologous HCT before the first allogeneic HCT, and availability of sufficient follow-up data including neutrophil engraftment and acute graft-versus-host disease (GVHD). We divided the patients into two groups based on a diagnosis of t-APL or de novo APL. Cytogenetic risk was diagnosed by multiple experts9. The preparative regimens were classified as myeloablative conditioning or reduced-intensity conditioning, based on the Center for International Blood and Marrow Transplant Research functional criteria10. Neutrophil engraftment was defined as the time required to reach an absolute neutrophil count of ≥ 500/μL for 3 consecutive days. Primary graft failure was defined as failure of neutrophil engraftment by day + 35. Incidences of grade II–IV acute GVHD and the presence or absence of chronic GVHD were defined as reported previously11,12. Patients were considered evaluable for chronic GVHD if they had engraftment. The percentages of patients who relapsed or were refractory to allogeneic HCT were determined (relapse). The survival and OS rates were calculated. GVHD-free, relapse-free survival (GRFS) was defined as being alive without grade III–IV acute GVHD, moderate to severe chronic GVHD requiring systemic immunosuppressive therapy, relapse, or death13. Chronic GVHD-free, relapse-free survival (CRFS) was defined as the absence of chronic GVHD requiring systemic immunosuppressive therapy, relapse, or death14. All data were censored at the date of the last reported follow-up. Analyzed outcomes included transplant-related mortality (TRM) (defined as death occurring while a patient is in continuous remission after HCT, encompassing all causes of non-leukemia death following the transplant procedure), disease-free survival (DFS), and OS.
Statistical analysis
The frequencies and descriptive statistics of patient-, disease-, and transplantation-related variables in the two groups were calculated. The distributions of categorical and continuous variables in the two groups were compared using Pearson’s chi-squared test or the Mann–Whitney U-test. The probabilities of neutrophil engraftment, acute and chronic GVHD, TRM, and relapse were calculated using cumulative incidence curves to accommodate competing risks. The 95% confidence intervals (CIs) for all probabilities and P-values for pairwise comparisons were derived from pointwise estimates and calculated using standard techniques. To accommodate competing risks, cumulative incidence curves for TRM and relapse were estimated, and their differences were compared using Gray’s test15. Competing risk events for TRM and relapse were relapse and death, respectively. The Fine and Gray model was used for each group to evaluate the effects of patient-, disease-, and transplantation-related variables on TRM16. Baseline patient variables for analysis included age at HCT (age of ≥ 55 years), sex, Karnofsky performance status (KPS of < 80)17 at HCT, disease status at HCT (CR vs. non-CR), prior autologous HCT, and HCT-specific comorbidity index (HCT-CI of > 2)18.
To evaluate survival, Kaplan–Meier curves generated for the two groups were compared using the log-rank test, and univariate and multivariate Cox regression analyses were performed. The Cox proportional-hazards model included patient- and transplantation-related variables. A two-sided P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html)19, a graphical user interface for R (The R Foundation for Statistical Computing; http://www.r-project.org) that extends the functionality of R Commander by adding statistical functions. Analyses were conducted using R version 4.3.1 and R Commander version 2.9-1.
Results
Patient characteristics
Table 1 shows the patient-related variables of the two groups of patients. Among the 20 patients with t-APL, the most frequent primary malignancy was breast cancer (n = 11), followed by gastric cancer (n = 4), cervical cancer (n = 1), germinal cell tumor (n = 1), peripheral T-cell lymphoma (n = 1), soft tissue sarcoma (n = 1), and tongue cancer (n = 1). Chemotherapy alone was the most frequent treatment for primary malignancies (n = 10), followed by chemotherapy plus radiotherapy (n = 6). The percentages of patients who were male (P = 0.001), had a higher KPS at HCT (P = 0.009), and had a lower HCT-CI at HCT were lower in the t-APL group than in the de novo APL group.
The data suggest a trend in which male patients with both t-APL and de novo APL have a higher frequency of additional chromosomal abnormalities than female patients; however, these differences did not reach statistical significance. Specifically, in the t-APL cohort, 40% (2 of 5) of male patients had additional chromosomal abnormalities compared with 20% (3 of 15) of female patients (P = 0.560). In the de novo APL cohort, 22% (18 of 81) of male patients exhibited these abnormalities compared with 9% (4 of 43) of female patients (P = 0.087). The lack of statistical significance suggests that these observed differences may be due to chance rather than a true biological or clinical difference between the sexes.
There were no statistically significant differences between the t-APL and de novo APL groups regarding either transplant-related characteristics (Table 2) or outcomes (Table 3).
The median follow-up period for patients diagnosed with t-APL was 114 months (range, 16–173 months). For patients with de novo APL, the median follow-up was 87 months (range, 3–167 months).
Overall, these findings comprehensively reflect the long-term outcomes associated with both t-APL and de novo APL.
Infectious complications
Two of 20 patients (10%) with t-APL were positive for hepatitis C virus and human immunodeficiency virus at the time of allogeneic HCT. However, these viral infections had no impact on the outcome of allogeneic HCT for these patients. The incidence of infections after allogeneic HCT was not significantly different between the t-APL and de novo APL groups (Supplementary Table 1).
TRM and relapse
There were no significant differences in the cumulative incidences of TRM between the t-APL and de novo APL groups (Supplementary Fig. 1A). The Fine and Gray model showed that male sex (hazard ratio [HR], 3.389) was significantly associated with TRM (Table 4).
There was no significant difference in the cumulative incidence of relapse between the t-APL and de novo APL groups (Supplementary Fig. 1B). The Fine and Gray model showed that non-CR at HCT (HR, 1.806) and prior autologous HCT (HR, 1.878) were significantly associated with relapse.
DFS, GRFS, CRFS, and OS
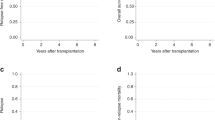
There were no significant differences in DFS, GRFS, CRFS, or OS between the t-APL and de novo APL groups (Supplementary Fig. 1C–E and Fig. 1). The 1- and 3-year OS rates were 80.0% and 53.8% (95% CI 55.1–92.0% and 29.9–72.8%) in the t-APL group and 65.1% and 52.4% (95% CI 56.0–72.8% and 43.1–60.8%) in the de novo APL group, respectively. Patients without CR at HCT had significantly worse OS than those with CR at HCT in the t-APL and de novo APL groups (P = 0.004) (Fig. 1). The 1- and 3-year OS rates were 71.1% and 61.1% (95% CI 60.7–79.1% and 50.3–70.2%) in patients with CR and 60.0% and 36.5% (95% CI 45.1–72.0% and 23.2–49.9%) in patients without CR, respectively. The 3-year OS rates in the t-APL and de novo APL group were 70.0% and 60.1% (95% CI 37.9–89.2% and 48.7–69.7%) in patients with CR and 37.5% and 36.5% (95% CI 10.0–65.9% and 21.8–51.3%) in those without CR, respectively.
Kaplan–Meier estimates of OS. Comparisons are shown between the t-APL and de novo APL groups and between patients with and without CR at HCT. There were no significant differences in OS between the t-APL and de novo APL groups, but patients with CR at HCT had significantly better OS than those without CR at HCT (P = 0.004). OS, overall survival; APL, acute promyelocytic leukemia; CR, complete remission; HCT, hematopoietic cell transplantation.
Multivariate Cox regression analysis identified male sex as an independent predictor of worse DFS (HR, 1.942), GRFS (HR, 1.693), CRFS (HR, 1.771), and OS (HR, 2.089) (Table 4). A KPS of < 80 at HCT was identified as an independent predictor of worse DFS (HR, 1.986) and OS (HR, 1.972). CR at HCT was an independent predictor of worse DFS (HR, 1.799), GRFS (HR, 1.583), and OS (HR, 1.753). Prior autologous HCT was identified as an independent predictor of worse DFS (HR, 1.622) and CRFS (HR, 1.753).
Discussion
The present study found no significant differences in DFS, GRFS, CRFS, or OS between the t-APL and de novo APL groups. However, as previously reported20, prior autologous HCT was identified as an independent predictor of worse relapses, DFS, and CRFS, and patients without CR at HCT had significantly worse relapses, DFS, GRFS, and OS than did those with CR at HCT.
The lack of statistically significant differences suggests that patients with t-APL, despite having therapy-related disease, did not fare worse in transplant outcomes compared with those with de novo APL. This information could be clinically relevant because it might indicate that transplantation is an equally viable treatment option for both groups of patients. However, it is essential to note that the absence of statistical significance does not necessarily mean the groups are identical. The study may have lacked sufficient statistical power to identify small differences that might exist. A large cohort study showed that although t-APL generally has a better prognosis than other therapy-related acute myeloid leukemias21, its outcomes may be slightly less favorable than those of de novo APL. However, with modern treatment approaches, particularly ATRA- and ATO-based regimens, the outcomes of patients with t-APL have substantially improved and may be approaching those of patients with de novo APL22. t-APL may lead to residual organ damage or decreased bone marrow function due to prior treatment, all of which might affect the prognosis. Some differences in the mutational landscape exist between t-APL and de novo APL, but these differences do not appear to be as clinically significant as once thought. The prognosis for both forms of the disease depends primarily on the specific mutations present, especially those influencing the treatment response, rather than whether the leukemia is therapy-related or de novo in origin. However, there is no significant difference in response to ATRA or ATO between t-APL and de novo APL4. Similar OS was observed between patients with t-APL and de novo APL despite the fact that patients with t-APL were more likely to have higher comorbidity indices and potentially harbor mutations associated with a poor prognosis. This difference between the patient groups might be attributed to several factors. First, the effectiveness of modern APL-specific therapies, such as ATRA and ATO, may mitigate some of the adverse prognostic factors associated with t-APL. Second, the graft-versus-leukemia effect of allogeneic HCT may overcome the potential resistance mechanisms in t-APL cells. Finally, improvements in the supportive care and management of transplant-related complications may reduce the impact of comorbidities on transplant outcomes. However, further research is needed to fully elucidate the biological and clinical factors contributing to these comparable outcomes.
Notably, the same treatment approach for de novo APL is recommended for t-APL, and a good prognosis can be expected with appropriate treatment. Whereas earlier studies reported relapse rates of up to 20% in patients with high-risk APL20, more recent data from the ATRA and ATO era show lower relapse rates. For example, the APL0406 trial reported a 2-year cumulative relapse incidence of only 1.9% in patients treated with ATRA plus ATO23. This marked reduction in relapse rates has prompted a reevaluation of the role of HCT in APL treatment. Although HCT was once considered the standard approach for patients in second remission, emerging evidence suggests that transplantation may be deferred in patients who achieve a second molecular CR with ATO-based salvage therapy23,24. Nevertheless, HCT remains a critical option for patients with persistent molecular disease or multiple relapses. A meta-analysis of autologous and allogeneic HCT for recurrent APL published recently found that TRM is a problem with allogeneic HCT and that autologous and allogeneic HCT have similar recurrence rates (24% vs. 23%)25. However, the benefits of HCT for t-APL have not been thoroughly studied, and its effectiveness remains unclear. In our study, TRM after allogeneic HCT for t-APL was similar to that for de novo APL, and TRM for t-APL with a high HCT-CI was not different from that for de novo APL. These findings suggest that although t-APL is a therapy-related leukemia—typically associated with a worse prognosis in other subtypes of acute myeloid leukemia—it responds similarly to treatment and demonstrates comparable outcomes to de novo APL, even in the setting of allogeneic HCT.
Our study showed that male sex was independently associated with worse TRM, DFS, GRFS, CRFS, and OS in patients with APL undergoing allogeneic HCT. This sex-based disparity in APL outcomes may be attributed to several factors:
-
Biological differences: Sex hormones influence immune function and hematopoiesis. Estrogen, for example, has been shown to protect hematopoietic stem cells and enhance immune responses26. However, there are no reports of estrogen being associated with treatment efficacy or complications in the context of allogeneic HCT.
-
Genetic factors: The National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic GVHD12 suggests that men may have a higher prevalence of additional chromosomal abnormalities, potentially contributing to more aggressive disease.
-
Pharmacokinetics: Differences in drug metabolism between sexes can impact treatment efficacy and toxicity27. For example, male patients may exhibit higher clearance rates for certain chemotherapeutic agents, potentially resulting in suboptimal drug exposure28. However, no data specifically link these agents to treatment efficacy or complications in the context of allogeneic HCT.
-
Transplant-related factors: Sex-mismatched donor–recipient pairs, particularly female donors to male recipients, have been associated with an increased risk of GVHD and mortality following allogeneic HCT. However, this risk generally ranges from approximately 20% to 25%29.
Further research is needed to elucidate the precise mechanisms underlying the observed sex-based disparity in APL outcomes and to develop targeted interventions to improve outcomes for male patients. Understanding these factors might lead to more personalized treatment approaches and potentially improve outcomes for male patients with APL.
Our study had several important limitations, most notably its retrospective, registry-based design and the small sample size of patients with t-APL. The limited number of patients with t-APL (n = 20) compared with de novo APL (n = 124) may have reduced our ability to detect significant differences between the groups and limited the generalizability of our findings. Additionally, the inherent differences in patient characteristics and treatment histories between patients with t-APL and de novo APL pose challenges for direct comparison. Despite these limitations, this exploratory analysis provides valuable insights into the potential efficacy of allogeneic HCT for both patient groups, especially given the rarity of t-APL. However, potential selection bias cannot be ruled out because of the lack of proper randomization, and we lacked data for HCT-ineligible patients. Moreover, the analysis did not include details on various conditioning regimens and post-transplant therapies, which may have influenced the outcomes. Our findings provide a comprehensive overview of the long-term outcomes associated with both t-APL and de novo APL within the study’s timeframe. However, the relatively short follow-up period restricted our ability to fully assess long-term TRM and complications. These limitations highlight the need for larger, prospective studies with longer follow-up periods to validate our findings and provide more robust evidence to guide clinical decision-making for the treatment of patients with t-APL and de novo APL.
In conclusion, our data suggest that the results of allogeneic HCT for patients with t-APL were comparable to those for patients with de novo APL. However, patients without CR require an additional range of treatment strategies and interventions aimed at optimizing outcomes before and after receiving allogeneic HCT containing CPX-351. This might be effective against therapy-related leukemia, although its effect on t-APL is unknown. An optimal therapy for relapsed or refractory APL has not been established, and further advances continue to be made.
Data availability
The data analyzed in this study are not publicly available because of ethical restrictions that exceed the scope of the recipients’ or donors’ consent for research use in the registry. Data may be available from the corresponding author upon reasonable request and with permission of the Japanese Society for Transplantation and Cell Therapy/Japanese Data Management Committee for Hematopoietic Cell Transplantation.
References
Lo-Coco, F. et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369, 111–121. https://doi.org/10.1056/NEJMoa1300874 (2013).
Burnett, A. K. et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol. 16, 1295–1305. https://doi.org/10.1016/S1470-2045(15)00193-X (2015).
Braun, T. et al. Evolving characteristics and outcome of secondary acute promyelocytic leukemia (APL): A prospective analysis by the French-Belgian-Swiss APL group. Cancer 121, 2393–2399. https://doi.org/10.1002/cncr.29389 (2015).
Lo-Coco, F., Hasan, S. K., Montesinos, P. & Sanz, M. A. Biology and management of therapy-related acute promyelocytic leukemia. Curr. Opin. Oncol. 25, 695–700. https://doi.org/10.1097/CCO.0000000000000013 (2013).
Lancet, J. E. et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 8, e481–e491. https://doi.org/10.1016/S2352-3026(21)00134-4 (2021).
Atsuta, Y. et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int. J. Hematol. 86, 269–274. https://doi.org/10.1532/IJH97.06239 (2007).
Atsuta, Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): Scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int. J. Hematol. 103, 3–10. https://doi.org/10.1007/s12185-015-1894-x (2016).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405. https://doi.org/10.1182/blood-2016-03-643544 (2016).
Yanada, M. et al. Effect of cytogenetic risk status on outcomes for patients with acute myeloid leukemia undergoing various types of allogeneic hematopoietic cell transplantation: An analysis of 7812 patients. Leuk. Lymphoma 28, 1–9. https://doi.org/10.1080/10428194.2017.1357173 (2017).
Giralt, S. et al. Reduced intensity conditioning regimen workshop: Defining the dose spectrum−Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 15, 367–369. https://doi.org/10.1016/j.bbmt.2008.12.497 (2009).
Przepiorka, D. et al. 1994 consensus conference on Acute GVHD grading. Bone Marrow Transplant. 15, 825–828 (1995).
Filipovich, A. H. et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 11, 945–956. https://doi.org/10.1016/j.bbmt.2005.09.004 (2005).
Holtan, S. G. et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125, 1333–1338. https://doi.org/10.1182/blood-2014-10-609032 (2015).
Ruggeri, A., Labopin, M., Ciceri, F., Mohty, M. & Nagler, A. Definition of GvHD-free, relapse-free survival for registry-based studies: An ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant 51, 610–611. https://doi.org/10.1038/bmt.2015.305 (2016).
Gray, R. J. A class of KK-sample tests for comparing the cumulative incidence of a competing risk. Ann. Statist. 16, 1141–1154 (1988).
Fine, J. & Gray, R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Karnofsky, D. A. & Burchenal, J. H. The clinical evaluation of chemotherapeutic agents in cancer. In Evaluation of Chemotherapeutic Agents (ed. MacLeod, C. M.) 191–205 (Columbia University Press, New York, 1949).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106, 2912–2919. https://doi.org/10.1182/blood-2005-05-2004 (2005).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Yanada, M. et al. Allogeneic hematopoietic cell transplantation for patients with relapsed acute promyelocytic leukemia. Transplant. Cell Ther. 28(847), e1-847.e8. https://doi.org/10.1016/j.jtct.2022.09.021 (2022).
Gross, S. et al. Therapy-related AML: Long-term outcome in a large cohort of AML-patients with intensive and non-intensive therapy. Blood Cancer J. 14, 160. https://doi.org/10.1038/s41408-024-01140-5 (2024).
Voso, M. T. et al. Acute promyelocytic leukemia: Long-term outcomes from the HARMONY project. Blood 145, 234–243. https://doi.org/10.1182/blood.2024026186 (2025).
Costa, A. et al. Response rates and transplantation impact in patients with relapsed acute promyelocytic leukemia. Cancers 16, 3214. https://doi.org/10.3390/cancers16183214 (2024).
Colita, A., Tanase, A. D., Tomuleasa, C. & Colita, A. Hematopoietic stem cell transplantation in acute promyelocytic leukemia in the era of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). Cancers 15, 4111. https://doi.org/10.3390/cancers15164111 (2023).
Mohty, R. et al. Efficacy of autologous and allogeneic hematopoietic cell transplantation in adults with acute promyelocytic leukemia: Results of a systematic review and meta-analysis. Transplant. Cell Ther. 30, 599.e1-599.e10. https://doi.org/10.1016/j.jtct.2024.03.024 (2024).
Nakada, D. et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558. https://doi.org/10.1038/nature12932 (2014).
Soldin, O. P. & Mattison, D. R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 43, 143–157. https://doi.org/10.2165/00003088-200948030-00001 (2009).
Galdas, P. M., Cheater, F. & Marshall, P. Men and health help-seeking behaviour: literature review. J. Adv. Nurs. 49, 616–623. https://doi.org/10.1111/j.1365-2648.2004.03331.x (2005).
Flowers, M. E. et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117, 3214–3219. https://doi.org/10.1182/blood-2010-08-302109 (2011).
Acknowledgements
We thank the patients and clinical staff for their participation in this study. We are very grateful to the Japanese Data Center for Hematopoietic Cell Transplantation for assisting with the data management and to St. Mary’s Hospital for their editorial support. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was supported by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) of the Japan Agency for Medical Research and Development (AMED).
Funding
The authors funded the article processing charges.
Author information
Authors and Affiliations
Contributions
SY contributed to the study design, data analysis, and manuscript preparation. MY, HA, TI, YA, JK, and TK on contributed to the manuscript preparation. HT, NU, KO, SO, ND, TO, ST, MS, YK, HN, TKaw, and OM provided the clinical data. TF, JK, and YA managed the clinical data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Compliance with ethical standards
The study was approved by the Data Management Committee of the Japanese Society for Hematopoietic Cell Transplantation and the Institutional Review Board of St. Mary’s Hospital.
Informed consent
The Transplant Registry Unified Management Program database contains physician-reviewed data. Observational studies based on the Transplant Registry Unified Management Program database are performed with informed consent.
Competing interests
MS owns stock in Celaid Therapeutics but declares no potential competing interests. SY, MY, HA, TF, Y Kanda, HT, NU, KO, SO, YO, ND, TO, ST, Y Kondo, HN, T Kawakita, MO, YA, and T Konuma declare no potential competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamasaki, S., Yanada, M., Araie, H. et al. Comparative analysis of allogeneic hematopoietic cell transplantation in patients with therapy-related and de novo acute promyelocytic leukemia A retrospective study. Sci Rep 15, 10967 (2025). https://doi.org/10.1038/s41598-025-95471-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95471-3