Abstract
The objective of this study is to evaluate the clinical presentations, treatment strategies, and prognostic factors for prostate diffuse large B-cell lymphoma (DLBCL), aiming to improve patient management and outcomes. We conducted a retrospective analysis of four prostate DLBCL cases treated at Beijing Chaoyang Hospital between 2014 and 2024, integrating these findings with data from the surveillance, epidemiology, and end results (SEER) dataset (2000–2021) to provide a broader context. All four patients underwent thorough diagnostic evaluations, and immunohistochemistry (IHC) confirmed their diagnoses. Kaplan-Meier survival curves and Cox regression analysis were applied to the SEER dataset to assess overall survival and treatment efficacy. P-values below 0.05 considered statistically significant. All four patients were diagnosed with prostate DLBCL via biopsy and confirmed by IHC, with extraprostatic involvement in three cases. Two patients achieved complete response, and one had partial response. In the SEER database, Kaplan-Meir analysis found that 59 patients had a 5-year survival rate of 59 months when it dropped in half, while multi-variable Cox regression highlighted age (HR 10.45, p < 0.001), surgery (HR 0.38, p = 0.0292), and radiation (HR 0.35, p = 0.0212) as the survival predictors. Although chemotherapy was administered in clinical practice, its impact was not statistically significant in our analysis. Prostate DLBCL is aggressive with diverse clinical presentations, making early detection and personalized treatment essential. Surgery and radiotherapy significantly improve patient outcomes, but the prognostic impact of chemotherapy, despite its widespread use, requires further validation through clinical trials.
Similar content being viewed by others
Introduction
Prostate lymphoma (PL) is extremely rare, accounting for only 0.09% of all prostate tumors and 0.1% of newly diagnosed lymphomas1,2. PL typically arises from stromal cells and is classified into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), with the latter predominantly represented by DLBCL. Other subtypes, such as Burkitt lymphoma and mantle cell lymphoma, are less common3,4,5. Most patients present with nonspecific lower urinary tract symptoms (LUTS), which can mimic benign prostatic hyperplasia (BPH), leading to diagnostic delays6,7. This diagnostic ambiguity frequently leads to delayed diagnosis and missed opportunities for early intervention. Prostate DLBCL is aggressive, progressing rapidly without timely intervention, often resulting in distant metastasis and poor outcomes. Early detection, accurate diagnosis, and individualized treatment are essential to improve survival rates in these patients. Given the absence of specific clinical guidelines, raising awareness of the disease among urologists and integrating early diagnostic techniques into clinical practice is crucial for better management.
This study began with a retrospective analysis of clinical data from four patients diagnosed with prostate DLBCL at Beijing Chaoyang Hospital, affiliated with Capital Medical University, between October 2014 and October 2024. To broaden the scope and validate our findings, we incorporated data from the SEER database, which allowed us to identify broader prognostic factors and trends. By integrating detailed case analyses from our clinical cohort with population-level insights from the SEER dataset, this research provides a comprehensive evaluation of clinical presentations, diagnostic approaches, treatment strategies, and prognostic indicators for prostate DLBCL. This dual approach strengthens the relevance of our findings, with the ultimate goal of improving both individualized patient management and overall outcomes.
Clinical information
We conducted a retrospective review of four patients diagnosed with prostate DLBCL at Beijing Chaoyang Hospital, Capital Medical University, between October 2014 and October 2024. Key clinical characteristics are summarized in (Table 1), where patient ages ranged from 59 to 82 years, and prostate-specific antigen (PSA) levels were normal in most cases, except one with an elevated value. Presenting symptoms varied, including hematuria and LUTS. Notably, one patient had a prior history of intra-abdominal lymphoma. Karnofsky scores and international prognostic inde (IPI) scores reflected varying prognoses, with Deauville scores available for one patient. Digital rectal examination results and prostate volumes also showed heterogeneity.
Methods
Clinical research method
All patients underwent comprehensive diagnostic evaluations, including transrectal ultrasound (TRUS), enhanced CT, and selective MRI. PET/CT was employed in Case 4 for pre- and post-treatment assessment. IHC analysis confirmed the diagnosis using markers like Cluster of Differentiation 20 (CD20), Ki-67, CD10, B-cell Lymphoma 6 (BCL-6), Multiple Myeloma Oncogene 1 (MUM1), and Paired Box Protein 5 (Pax-5). The specific antibodies used in this study were purchased from the following suppliers: CD20 and Pax-5: Zhongshan Golden Bridge Biotechnology Co., Ltd. (China); CD10: Roche Diagnostics (United States); Ki-67, BCL-6, MUM1: Maixin Biotech (China). Based on these findings, each patient received corresponding treatment, with follow-up evaluations monitoring their responses and survival outcomes. This study was approved by the Ethics Committee of Beijing Chaoyang Hospital and adhered to the Declaration of Helsinki (Ethics Approval No. 2024-science-582-1).
SEER database research method
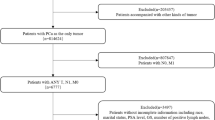
In addition to the clinical analysis, a survival analysis was conducted using the SEER dataset, which includes 59 cases of primary prostate DLBCL diagnosed between 2000 and 2021 across 17 cancer registries in the United States. Data, including patient demographics, treatment modalities, and survival outcomes, were extracted using SEER Stat version 8.3.4 from the National Cancer Institute (NCI)8. Kaplan-Meier survival curves and Cox regression were used to assess overall survival and evaluate the impact of factors such as age, SEER stage, Lugano stage, radiation, surgery, and chemotherapy. By integrating these population-based findings with our clinical case data, we achieved a more comprehensive understanding of individual patient outcomes in the context of broader trends.
Statistical analyses were performed using R version 4.2.3, with subgroup comparisons made using chi-square tests or Fisher’s exact test where applicable. P-values below 0.05 were considered statistically significant.
Results
Clinical findings
All four patients were diagnosed with prostate DLBCL via biopsy, confirmed through IHC analysis. Figures 1 and 2 illustrates the imaging and pathological findings, including changes in tumor size post-chemotherapy, MRI features, PET-CT results, and key immunohistochemical markers. Imaging varied across cases: Case 1 showed uneven glandular echoes and a soft tissue shadow near the right ureter on CT, while Case 2 presented with a pelvic soft tissue shadow with mild enhancement. Case 3 had a localized anterior prostate protrusion, and Case 4 exhibited multiple low echoes and external protrusions on TRUS, with irregular enhancement on MRI. Extraprostatic involvement was observed in three patients, affecting the seminal vesicles, bladder wall, and pelvic structures. Lymph node involvement was detected in Cases 1, 2, and 4, including the external iliac, inguinal, and retroperitoneal nodes, with additional involvement of diaphragmatic and abdominal aortic nodes in Case 4. Pathological analysis confirmed DLBCL in all cases, with CD20 positivity and Ki-67 proliferation indices between 80 and 90%. Cases 2 and 3 were classified as the Germinal Center B-cell (GCB) subtype, while Case 4 was non-GCB, with genetic mutations such as CD79B p.Y196H and MYD88 p.515R influencing treatment. Three patients received R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) chemotherapy, while Case 4 underwent Z-R-CHOP (Zanubrutinib, Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) due to genetic findings. Complete remission was achieved in Cases 3 and 4, with partial remission in Case 2. Case 1 refused further treatment and showed no response. Lymphoma-specific survival ranged from 6.7 to 47.6 months, with overall survival between 9.2 and 62.6 months. Detailed patient information can be found in the Supplementary Table.
Case 4: (A) Non-contrast CT scan, showing initial lesion. (B) Non-contrast CT scan, 3 months after starting chemotherapy, showing reduction in lesion size. (C) MRI T2-weighted image in coronal view. (D) Diffusion-weighted imaging (DWI) in coronal view. (E) MRI T2-weighted sagittal view. (F–H) PET-CT images displaying prostate involvement in axial (F), coronal (G), and sagittal (H) planes.
SEER database findings
A total of 59 patients were analyzed. Chi-square or Fisher’s exact tests assessed the relationships between diagnosis year, age, race, SEER Stage, Lugano Stage, radiation, chemotherapy, and time from diagnosis to treatment with survival at various intervals (one-year, two-year, three-year, and five-year). As shown in (Table 2), age was significantly associated with survival at one, two, three, and five years. SEER Stage correlated with two-year survival, and Lugano Stage with one-year survival. Radiation therapy showed a significant impact on two- and three-year survival (all p < 0.05). No significant associations were found for race, diagnosis year, chemotherapy, or time to treatment with survival outcomes (all p ≥ 0.05).
A comprehensive survival analysis, including an overall Kaplan-Meier survival curve, evaluated the impact of age, SEER stage, Lugano stage, and radiation therapy on overall survival (Fig. 3). The overall survival curve showed that survival dropped to 50% at approximately 59 months, highlighting the long-term survival challenges faced by the cohort.
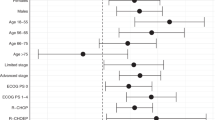
The multivariate Cox regression analysis (Fig. 4) identified several factors significantly associated with overall survival. Age was the strongest predictor, with patients aged 70 and older having a significantly higher hazard ratio (HR 10.45, 95% CI 3.39–32.27, p < 0.0001), indicating worse outcomes compared to younger patients. Both surgery and radiation therapy were protective, significantly enhancing survival (HR 0.38, p = 0.0292 and HR 0.35, p = 0.0212, respectively). Although chemotherapy, SEER stage, and Lugano stage did not reach statistical significance, trends suggested they may still influence survival outcomese.
Discussion
PL is extremely rare in clinical practice, with a low diagnostic rate1. It is generally classified into HL and NHL, with the latter being more aggressive, most commonly presenting as DLBCL9. Prostate DLBCL originates from mesenchymal tissues, with incidental granulomatous prostatitis possibly linked to its development and progression10,11. While urologists typically detect prostate lymphoma, most research focuses on hematological and pathological aspects. Our study provides novel clinical insights by integrating data from four cases with a large-scale SEER database analysis, offering a comprehensive evaluation of clinical presentation, therapeutic strategies, and outcomes in prostate DLBCL. This dual approach enhances the understanding of diagnostic and therapeutic challenges, bridging the gap between individualized case analysis and population-level trends.
Prostate DLBCL affects all age groups, but rapid, irregular prostate enlargement in younger patients (< 70 years) should raise suspicion for lymphoma. Some reports suggest that prostate DLBCL in younger patients may be linked to HIV-positive status and immunosuppression, leading to a poorer prognosis12,13. Notably, our SEER analysis confirmed a significant age-related survival difference, with younger patients (< 70 years) exhibiting superior survival outcomes compared to older patients (≥ 70 years) (HR = 10.45, p < 0.001). This trend was reflected in our cohort, where both younger patients achieved complete remission following aggressive treatment, emphasizing the prognostic advantage of early intervention. Prostate DLBCL symptoms are often nonspecific, including LUTS such as frequent urination, urgency, retention, and dysuria. Some patients also present with localized pain and systemic symptoms like fever, night sweats, and weight loss14. In some cases, the lymphoma is incidentally discovered during surgery for BPH and confirmed by pathology15. Digital rectal examination (DRE) typically reveals an enlarged, irregular, and firm prostate, though in cases with large pelvic masses, the boundaries can be difficult to assess16. PSA levels are usually within normal limits, though elevated levels may be observed due to concurrent prostatitis or epithelial hyperplasia, which necessitates close follow-up. Elevated lactate dehydrogenase (LDH) levels, which increase the IPI score, are associated with poorer outcomes17. The wide range of clinical presentations in our cases underscores the diagnostic challenges associated with prostate DLBCL, reinforcing the need to include it in differential diagnoses, even when PSA levels are normal.
Imaging plays a key role in the diagnosis and staging of prostate DLBCL but risks confusion with prostate cancer due to overlapping radiological features. Prostate size in DLBCL can vary significantly depending on the stage, with rapid growth and invasion into surrounding tissues complicating the assessment18. Although CT has limited value in evaluating the prostate, it is useful for assessing involvement extent, adjacent structures, and significant tumor reduction after treatment. For instance, enhanced CT often reveals irregular enlargement or pelvic masses with heterogeneous enhancement, suggesting a more aggressive disease course16. MRI frequently shows high diffusion-weighted imaging (DWI) signal intensity with reduced apparent diffusion coefficient (ADC), reflecting densely packed lymphoma cells that restrict water diffusion. This characteristic pattern, when observed alongside normal PSA levels, should raise suspicion for lymphoma, serving as an early radiological marker. PET/CT is valuable for assessing lymph node involvement and monitoring treatment response, with Deauville scoring guiding clinical decisions based on standardized uptake values (SUV)19.
The definitive diagnosis of prostate DLBCL relies on histopathology and immunohistochemistry, typically obtained through transrectal or transperineal biopsy. In some cases, DLBCL may be incidentally discovered during TURP or radical prostatectomy20,21. Pathology usually shows diffuse infiltration of large lymphoid cells with irregular nuclei. IHC confirms B-cell lineage, marked by CD20 and Pax-5, and high proliferation rates indicated by Ki-67, reaching up to 90%. Markers such as CD10, BCL-6, and MUM1 are used to further classify prostate DLBCL into GCB and non-GCB subtypes. CD10 and BCL-6 are typically expressed in GCB-type DLBCL, which is generally associated with a better prognosis, while MUM1 is often expressed in non-GCB-type, which is more commonly observed and typically associated with a poorer outcome. This highlights the importance of early intervention in prostate DLBCL, particularly in cases with the non-GCB subtype22. Pax-5 confirmed B-cell origin, while variable Myc Proto-Oncogene Protein (C-myc) expression highlighted tumorigenesis, suggesting a potential association with poor prognosis. Scattered CD3 and CD5 positivity, not indicative of T-cell origin, can also be seen in normal cells. PSA positivity, possibly related to epithelial hyperplasia, underscores the need for careful differentiation from prostate cancer.
Treating prostate DLBCL requires a multimodal approach, integrating surgery, chemotherapy, radiotherapy, immunotherapy, and targeted therapies. Surgery plays a crucial role in alleviating obstructive LUTS, particularly in localized or early-stage cases, improving symptoms through procedures like Transurethral Resection of the Prostate (TURP) or radical prostatectomy. However, in advanced stages with local invasion, surgery is not curative and may present risks such as prostate-rectal fistula. Nevertheless, our Cox regression analysis showed a significant survival benefit from surgery, particularly in patients without extensive extraprostatic involvement (HR = 0.38, p = 0.0292), supporting its inclusion in comprehensive treatment strategies. Chemotherapy is used in nearly all prostate lymphomas, though its efficacy requires further validation through clinical trials. While R-CHOP remains the standard regimen and R-CHP offers an alternative for high-risk patients, our multivariate analysis did not demonstrate a significant survival benefit (p = 0.5455). This result may reflect the influence of comorbidities, advanced age, and case variability, particularly in older patients whose health conditions may compromise treatment efficacy. The retrospective design and small sample size could also limit the statistical power of our findings. Future large-scale, prospective studies are essential to identify patient subgroups that may derive the most benefit from chemotherapy. Radiotherapy has shown improved survival in mid-term follow-ups (2–3 years) with significant benefits (HR 0.35, p = 0.0212). Standard radiotherapy regimens (30–45 Gy) delivered in fractionated cycles have consistently yielded long-term disease-free survival23,24,25,26. However, radiation may exacerbate LUTS and should be carefully considered, especially in advanced cases25. Immunotherapy and targeted therapies have significantly advanced, with anti-CD20 monoclonal antibodies like Rituximab forming the backbone of systemic treatment for prostate DLBCL. Emerging options, such as tafasitamab combined with lenalidomide, have shown promise in relapsed or refractory cases, offering alternatives for patients resistant to conventional treatments27. In genetically distinct subtypes harboring mutations such as CD79B or Myeloid Differentiation Primary Response 88 (MYD88) p.L265P, targeted therapies, including Zanubrutinib, have demonstrated favorable therapeutic efficacy, as observed in Case 4 of our cohort28. For patients who have failed multiple therapies, Chimeric Antigen Receptor (CAR) T-cell treatments, approved for relapsed DLBCL, offer a promising solution for high-risk cases29. Aggressive or refractory cases may benefit from intensive regimens like R-ESHAP, R-GCD, and RE-CHOP, further expanding treatment options12,30,31,32. It is crucial for urologists to be aware of these evolving therapies, but decisions should be made within a multidisciplinary team (MDT) that includes oncologists, urologists, and hematologists to ensure optimal management.
The overall survival curve showed that survival dropped to 50% at approximately 59 months, marking a critical finding not previously reported in prostate DLBCL studies. Prognostic factors in prostate DLBCL include age, disease stage, and treatment modalities. Our SEER-based analysis shows that older patients, particularly those over 70, have shorter overall survival, often due to comorbidities rather than lymphoma-specific causes. In contrast, younger patients under 60 tend to experience better long-term survival when appropriate treatment is administered. Advanced disease stages, such as distant SEER stages or Lugano stage IV, are associated with significantly worse survival outcomes, underscoring the importance of early detection. Multivariate Cox regression analysis demonstrated that surgery (HR = 0.38, p = 0.0292) and radiation therapy (HR = 0.35, p = 0.0212) significantly improve survival outcomes. These results highlight that early, aggressive intervention with multimodal strategies offers the best prognosis for prostate DLBCL patients.
While our study integrates a comprehensive institutional case analysis with nearly 30 years of SEER data, certain limitations must be acknowledged. The rarity of this disease leads to a small sample size, potentially reducing the statistical power of some analyses. Additionally, the retrospective nature of both our institutional cohort and SEER data may introduce biases, such as variability in treatment protocols and follow-up durations. Although treatments were personalized to reflect real-world clinical practice, this heterogeneity complicates direct comparisons across cases. We have conducted a thorough review of the relevant global literature, incorporating both our clinical data and SEER records. However, it is acknowledged that some sources may not have been included. To strengthen and build on our findings, further multicenter prospective studies are needed.
Conclusion
In conclusion, although prostate DLBCL is rare, early detection and individualized treatment can significantly improve outcomes. Clinicians should remain vigilant when encountering rapidly enlarging, irregular prostates, especially when PSA levels are normal or DWI shows high signal intensity with reduced ADC. A multidisciplinary approach, including surgery, radiation, and emerging targeted therapies, is essential for optimal management. Future prospective studies are needed to validate the role of chemotherapy and further refine treatment strategies.
Data availability
All data supporting the findings of this study are publicly available: https://seer.cancer.gov/seerstat/. Relevant patient information is provided in the supplementary files for reference.
Abbreviations
- DLBCL:
-
Diffuse large B-cell lymphoma
- SEER:
-
Surveillance, epidemiology, and end results
- IHC:
-
Immunohistochemistry
- PL:
-
Prostate lymphoma
- HL:
-
Hodgkin lymphoma
- NHL:
-
Non-hodgkin lymphoma
- LUTS:
-
Lower urinary tract symptoms
- BPH:
-
Benign prostatic hyperplasia
- PSA:
-
Prostate-specific antigen
- IPI:
-
International prognostic index
- TRUS:
-
Transrectal ultrasound
- CD20:
-
Cluster of differentiation 20
- LCA:
-
Leukocyte common antigen
- NCI:
-
National cancer institute
- GCB:
-
Germinal center B-cell
- R-CHOP:
-
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- Z-R-CHOP:
-
Zanubrutinib, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- DWI:
-
Diffusion-weighted imaging
- DRE:
-
Digital rectal examination
- LDH:
-
Lactate dehydrogenase
- ADC:
-
Apparent diffusion coefficient
- SUV:
-
Standardized uptake values
- BCL6:
-
B-cell lymphoma 6
- MUM1:
-
Multiple myeloma oncogene 1
- Pax-5:
-
Paired box protein 5
- C-myc:
-
Myc proto-oncogene protein
- TURP:
-
Transurethral resection of the prostate
- MYD88:
-
Myeloid differentiation primary response 88
- CAR:
-
Chimeric antigen receptor
- MDT:
-
Multidisciplinary team
References
Warrick, J. I., Owens, S. R. & Tomlins, S. A. Diffuse large B-cell lymphoma of the prostate. Arch. Pathol. Lab. Med. 138 (10), 1286–1289 (2014).
Sarris, A., Dimopoulos, M., Pugh, W. & Cabanillas, F. Primary lymphoma of the prostate: good outcome with doxorubicin-based combination chemotherapy. J. Urol. 153 (6), 1852–1854 (1995).
Brice, P., de Kerviler, E. & Friedberg, J. W. Classical hodgkin lymphoma. Lancet 398 (10310), 1518–1527 (2021).
Shankland, K. R., Armitage, J. O. & Hancock, B. W. Non-hodgkin lymphoma. Lancet 380 (9844), 848–857 (2012).
Bostwick, D. G., Iczkowski, K. A., Amin, M. B., Discigil, G. & Osborne, B. Malignant lymphoma involving the prostate: report of 62 cases. Cancer 83 (4), 732–738 (1998).
Chen, X., Tan, Z. & Duan, X. Primary diffuse large B-cell lymphoma of the prostate: A case report. Asian J. Surg. 46 (8), 3302–3303 (2023).
Ochirjav, E., Tsedenbal, B., Enkhjargal, M., Mendbayar, E. & Sharkhuu, E. Hematolymphoid tumor of prostate: diffuse large B cell lymphoma case report. Urol. Case Rep. 50, 102497 (2023).
SEER* stat software. National Cancer Institute Surveillance, Epidemiology, and End Results. Program(SEER). https://seer.cancer.gov/seerstat/ (2024).
Wang, J. et al. High frequency of bone marrow involvement in intravascular large B-cell lymphoma. Int. J. Surg. Pathol. 25 (2), 118–126 (2017).
Hassanzadeh, S., Mackrides, N., Rastegar, S. & Nejati, R. Diffuse large B-cell lymphoma with a background of extensive granulomatous inflammation: A potential pitfall for misdiagnosis. Cureus 13 (7), e16198 (2021).
Péricart, S. et al. Exclusive B-cell phenotype of primary prostatic lymphomas: a potential role of chronic prostatitis. Histopathology 76 (5), 767–773 (2020).
Ujiie, T. et al. Primary diffuse large B cell lymphoma of the prostate in a patient with HIV infection. IJU Case Rep. 6 (1), 30–32 (2023).
Gessese, T., Asrie, F. & Mulatie, Z. Human immunodeficiency virus related non-hodgkin’s lymphoma. Blood Lymphat Cancer 13, 13–24 (2023).
Chen, T. F., Lin, W. L., Liu, W. Y. & Gu, C. M. Prostate lymphoma with renal obstruction; reflections on diagnosis and treatment: two case reports. World J. Clin. Cases 11 (7), 1627–1633 (2023).
Harley, F., Dias, B., Dow, C. & Ooi, J. Primary non-metastatic extra-nodal diffuse large B-cell lymphoma of the prostate and seminal vesicle. Urol. Case Rep. 40, 101945 (2022).
Kakkar, A. et al. Primary diffuse large B-cell lymphoma of the prostate: A report of two cases with diagnostic considerations. J. Cancer Res. Ther. 11 (4), 977–979 (2015).
Ruppert, A. S. et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 135 (23), 2041–2048 (2020).
Wei, Y. et al. Analysis of three primary prostatic sarcoma cases and literature review. Prostate 84 (13), 1218–1223 (2024).
Enilorac, B. et al. Does PET reconstruction method affect Deauville score in lymphoma patients? J. Nucl. Med. 59 (7), 1049–1055 (2018).
He, H., Cheng, L., Weiss, L. M. & Chu, P. G. Clinical outcome of incidental pelvic node malignant B-cell lymphomas discovered at the time of radical prostatectomy. Leuk. Lymphoma 48 (10), 1976–1980 (2007).
Chu, P. G., Huang, Q. & Weiss, L. M. Incidental and concurrent malignant lymphomas discovered at the time of prostatectomy and prostate biopsy: a study of 29 cases. Am. J. Surg. Pathol. 29 (5), 693–699 (2005).
Hans, C. P. et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103 (1), 275–282 (2004).
Jiang, F. et al. 18F-prostate-specific membrane antigen and 18F-fluorodeoxyglucose PET/CT unmasked the characteristics of prostate lymphoma: A case report and literature review. Front. Med. (Lausanne) 9, 842093 (2022).
Ren, M. & Liu, Y. Primary diffuse large B-cell lymphoma of the prostate: a case report and review of the literature. J. Med. Case Rep. 15 (1), 546 (2021).
Wang, K., Wang, N., Sun, J., Fan, Y. & Chen, L. Primary prostate lymphoma: A case report and literature review. Int. J. Immunopathol. Pharmacol. 33, 2058738419863217 (2019).
Jafari, A., Mofid, B., Tabibi, A. & Kowsari, F. Primary prostate lymphoma managed with combined modality treatment: A case report. Urol. J. 16 (4), 412–414 (2019).
Cheson, B. D., Nowakowski, G. & Salles, G. Diffuse large B-cell lymphoma: new targets and novel therapies. Blood Cancer J. 11 (4), 68 (2021).
Schmitz, R. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl. J. Med. 378 (15), 1396–1407 (2018).
Trabolsi, A., Arumov, A. & Schatz, J. H. Bispecific antibodies and CAR-T cells: dueling immunotherapies for large B-cell lymphomas. Blood Cancer J. 14 (1), 27 (2024).
Kishimoto, N. et al. A case of primary malignant lymphoma of the prostate. Hinyokika Kiyo 57 (8), 445–449 (2011).
Duan, H., Deng, Z., Zhang, G. & Xie, T. Diffuse large B-cell lymphoma of the prostate: A case report. Asian J. Surg. 47 (1), 604–605 (2024).
Greve, P., Meyer-Wentrup, F. A. G., Peperzak, V. & Boes, M. Upcoming immunotherapeutic combinations for B-cell lymphoma. Immunother. Adv. 1 (1), ltab001 (2021).
Acknowledgements
We would like to express our sincere gratitude to the SEER database for providing the essential data for this research. We also thank Dr. Minghai Ma from Xi’an Jiaotong University and Dr. Zhiwen Luo from Peking Union Medical College for their constructive feedback and valuable insights on the development of this manuscript.
Funding
This work was supported by the Wu Jieping Foundation under the grant titled “The co-stimulatory molecule ICOS regulates the progression of Th17 cells in prostatic hyperplasia,” Beijing, China; Grant number: 320.6750.18013, and Capital Health Research and Development of Special Fund under the grant titled “multicenter randomized controlled clinical study of Qishao Tianxin prescription in the treatment of early urinary incontinence after radical prostatectomy for prostate cancer” Beijing, China; Grant Number 2020-2-2033.
Author information
Authors and Affiliations
Contributions
Yirui Wei and Weifeng He designed the research. Yirui Wei, Weifeng He and Dawei Xie collected and analyzed the data and drafted the manuscript. Dawei Xie and Jianwen Wang supervised the study. Pushen Yang and Hao Wang were involved in writing the manuscript. Yirui Wei and Jianwen Wang revised the manuscript and embellished the language. Jun Lu and Xiaolong Liang obtained the pathological images and interpreted the case results. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Review Committee of Beijing Chaoyang Hospital, Capital Medical University (Ethics Approval No. 2024-science-582-1). Informed consent was obtained from all patients for participation in the study.
Consent for publication
This study has been approved by all authors for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, Y., He, W., Xie, D. et al. Clinical and prognostic factors guiding diagnosis and treatment of prostate diffuse large B-cell lymphoma. Sci Rep 15, 12594 (2025). https://doi.org/10.1038/s41598-025-96315-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96315-w