Abstract
Patients with nephrotic syndrome, particularly those with primary membranous nephropathy (pMN), are at a heightened risk of thromboembolic events. The presence of phospholipase A2 receptor (PLA2R) antibody serves as an indicator of active primary membranous nephropathy. Identifying high-risk patients for thromboembolic events is crucial for facilitating effective communication between healthcare providers and patients, evaluating treatment outcomes, and assessing medical costs. This study aimed to develop a practical model for predicting the probability of thromboembolic events in patients with PLA2R-related primary membranous nephropathy. A total of 1384 patients diagnosed with PLA2R antibody-related primary membranous nephropathy were included in this study. The model group included 969 patients enrolled before August 2020, while the external validation group consisted of 415 patients enrolled later. Patients in the modeling group were divided into the thromboembolic and non-thromboembolic subgroups. Logistic regression analysis was performed, and a nomogram was established based on the results. The predictive performance of the nomogram was evaluated by the area under the receiver operating characteristic curve (AUC), calibration curves, and decision curve analysis (DCAs). The modeling group comprised 126 (13.0%) patients with thromboembolism, and significant differences were observed between the thromboembolism and non-thromboembolism subgroups. The risk factors included in the nomogram included age, Anti-PLA2R antibody, and 24-hour urine protein quantification. The AUC value of the nomogram was 0.741 (95% CI 0.695–0.788, P < 0.001). In addition, the calibration curves demonstrated acceptable agreement between the predicted outcomes by the nomogram and the actual values. DCA curves showed good positive net benefits in the predictive model. The external validation also confirmed the reliability of the prediction nomogram. This predictive nomogram including Anti-PLA2R antibody, age, and 24-hour urine protein quantification may facilitate the prediction of the thromboembolic risk in patients with PLA2R-related pMN.
Similar content being viewed by others
Introduction
PMN is recognized as an autoimmune disease and represents the glomerular disease with the highest incidence of thromboembolic events. The 2021 KDIGO guidelines underscore the significance of thromboembolic events in pMN, indicating the high venous thromboembolism (VTE) risk in patients with serum albumin levels of less than 20 g/L, and high arterial thromboembolism (ATE) risk in those with serum albumin levels of less than 30 g/L1,2. Studies conducted in China have indicated that 36% of pMN patients have a history of venous thromboembolic events3. Arterial thromboembolic events are more likely to occur within the first 6 months of pMN onset1. Furthermore, the heightened risk of thromboembolic events in pMN has been consistently affirmed since its discovery in the early 1980s. Most patients exhibit anti-M-type PLA2R antibody on podocytes during the course of the disease; these PLA2R antibody are closely correlated with membranous nephropathy activity and progression4,5,6. Furthermore, patients with elevated PLA2R antibody levels are exposed to a higher risk of multiple thromboembolic events, even in the absence of nephrotic syndrome7. In 2012, Sean J Barbour postulated a connection between PLA2R antibody and thromboembolic events8. Recent studies have demonstrated that PLA2R antibody serve as an independent risk factor for thromboembolic events7,8,9; however, further high-quality evidence is required due to limited research on this topic. Therefore, determining crucial risk factors for thromboembolic events in PLA2R-positive PMN patients holds significance. Thromboembolic diseases significantly impact treatment outcomes, hospitalization rates, disability, mortality, and prognosis. Hence, proposing a simple yet quantitative method to determine the risk of such diseases could provide new evidence for personalized treatment strategies.
Methods
Study design and patients
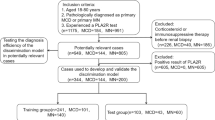
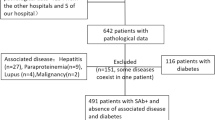
This retrospective study collected data on 1384 patients with PLA2R antibody-related pMN who received treatment at Zhengzhou University First Affiliated Hospital from January 2015 to July 2023. The prevalence of thromboembolic events and their risk factors were investigated at the time of diagnosis. All patients were newly diagnosed and had not been treated with drugs. Follow-up data were collected by review of the hospital’s electronic medical records.The external validation set was split from the whole data set in the initial study in chronological order and was used as a test set in this study to evaluate the generalizability of the constructed nomogram. Due to the relatively small amount of data (an order of magnitude of 10,000), a split ratio of 7:3 was recommended in the traditional machine learning procedure. Therefore, a total of 969 patients were assigned to the model group and 415 patients were included in the external validation group, with August 1, 2020, as the demarcation date. Inclusion criteria were as follows: (1) All patients were positive for PLA 2 R antibody by glomerular PLA 2 R immunohistochemical staining or serum PLA 2 R antibody detection, and were diagnosed with PLA2R antibody-related pMN; (2) All patients were over the age of 18; (3) Common causes of secondary MN were not identified, such as lupus, hepatitis, tumors, or drug-induced MN. The exclusion criteria included: (1) Concurrent glomerular diseases; (2) Lactating or pregnant women; (3) Lack of serum PLA2R antibody data at diagnosis; (4) Absence of lower limb and renal vein ultrasound, pulmonary angiography (CTPA), head magnetic resonance imaging (MRI), or head computed tomography (CT); (5) Exposure to classic risk factors for thromboembolism such as antiphospholipid syndrome, major surgery, and prolonged immobilization. The study flow chart is illustrated in Fig. 1.
The 2021 KDIGO recommendation specifies that when assessing PLA2R antibody using an enzyme-linked immunosorbent assay (ELISA), a cutoff value of 2 RU/mL should be applied to define an antibody-negative outcome1. In this study, the determination of PLA2R antibody-associated membranous nephropathy was based on the 2021 KDIGO guidelines10. All enrolled patients underwent renal and lower extremity vascular ultrasound examination at the initial diagnosis to determine whether renal vein, inferior vena cava, and deep vein thrombosis were present. Because of cost and the risk of contrast-induced nephropathy, head CT or MRI and CTPA were completed when patients presented with clinical signs and symptoms of cerebral infarction or PE. The study complies with the Declaration of Helsinki. The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. Due to the retrospective nature of the study, the ethics committee of the First Affiliated Hospital of Zhengzhou University waived the need of obtaining informed consent.
Clinical data collection
Comprehensive clinical data and laboratory parameters were collected from the patients, including gender, age, thromboembolic history, and indicators related to nephrotic syndrome and kidney function. A commercial ELISA kit from EUROIMMUN AG, Lübeck, Germany, was used to identify PLA2R antibody. The severity of proteinuria was assessed by 24-hour urine protein quantification.
Statistical analysis
Data were expressed as mean ± standard deviation, median, interquartile range, or percentages. The independent sample t-test was used to compare the thromboembolic and non-thromboembolic groups if the data followed a normal distribution. If the data did not conform to a normal distribution, the Mann–Whitney U test was used to compare the two groups. Categorical variables were compared using the χ2 test or Fisher exact probability method. Subsequently, thromboembolic formation predictions were estimated using logistic regression analysis. In addition, a nomogram was constructed based on the multivariate logistic regression analysis results, which included the age, PLA2R antibody levels, and 24-hour urine protein quantification. Moreover, the AUC of the above variables and nomogram was calculated, and calibration curves and DCAs were performed. Finally, the goodness-of-fit model was evaluated using the Hosmer–Lemeshow test. SPSS 24.0 software program and R software (version 3.6.3, http://www.R-project.org) were employed for statistical analysis, and two-sided p-values were calculated.
Results
Baseline characteristics
Between January 2015 and July 2023, a total of 1384 patients (557 females [40.25%]) met the inclusion criteria for this study. The model group comprised 969 patients enrolled before August 2020, while the external validation group included the remaining 415 patients (Table 1). The model group included 640 male patients (66.05%) and had an average age of 51 years (range: 42–60), a serum PLA2R antibody titer of 58.9 RU/mL (range:17.8-156.7), a 24-hour urine protein quantification of 4.86 g (range:2.71–7.77), a serum albumin level of 23 0.9 g/L(range:20 0.0–28 0.6), and a serum creatinine level of 72 µmol/L(range: 59–86).
Comparison between patients with thromboembolism and those without thromboembolism
In the model group, 126 patients (13.0%) experienced thromboembolic events, including 44 cases of deep vein thrombosis (DVT), 21 cases of renal and or inferior vena cava thrombosis (RVT), 14 cases of pulmonary embolism (PE), 1 case of splenic infarction, 32 cases of cerebral infarction, 4 cases of both PE and RVT, and 5 cases of both DVT and RVT. Four patients had both DVT and cerebral infarction, and one patient had extensive thrombus with DVT, RVT, and PE. The clinical characteristics are detailed in Table 2. No statistically significant differences in platelet count, total cholesterol level, low-density lipoprotein level, or APTT were found between patients with and without thromboembolic events. However, a notable disparity was observed in PLA2R antibody levels between patients with and without thromboembolic events, which is an important indicator of idiopathic membranous nephropathy. Patients who experienced thromboembolic events exhibited significantly higher PLA2R antibody titers [102.8 (43.7-363.3) vs. 54.4 (15.8-136.2) RU/mL; P < 0.001] compared to those without such events. In addition, the age of the thromboembolic group was significantly higher compared to the group without thromboembolic events [57 (49, 64) vs. 51 (42, 59) years, P < 0.001]. Creatinine levels were also notably elevated in the thromboembolic group [78 (65, 103) vs. 71 (59, 85) µmol/L, P < 0.001], along with increased 24-hour urine protein quantification [6.29 (3.53, 9.75) vs. 4.75 (2.58, 7.44) g, P = 0.001], d-dimer levels [0.60 (0.23, 2.05) vs. 0.29 (0.15, 0.66) g/ml, P < 0.001], fibrinogen levels [4.3 (3.7, 5.1) vs. 4.1 (3.5, 4.7) mg/dl, P = 0.006], and PT [9.9 (9.3, 10.6) vs. 9.7 (9.3, 10.2) mg/dl, P = 0.027]. Conversely, albumin [22.3 (17.7, 26.1) vs. 24.1 (20.2, 28.9) g/L, P < 0.001] and hemoglobin [125 (112, 137) vs. 132 (119, 144) g/L, P < 0.001] were significantly lower in the thromboembolic group (Table 2).
Effect of PLA2R antibody, patient age, and 24-hour urine protein quantification on thromboembolic risk prediction in Patients with PLA2R-related MN
The univariate logistic regression analysis and multivariable analysis identified four strong predictors of thromboembolic events of patients in the modeling group, including age (OR 1.03, 95% CI: 1.02, 1.05, P < 0.001), PLA2R antibody levels (PLA2R-Ab titers ≤ 36.9 RU/mL/PLA2R-Ab titers > 36.9 RU/mL) (OR 2.75, 95% CI: 1.67, 4.54, P < 0.001), 24-hour urine protein quantification (OR 1.05, 95% CI: 1.01, 1.10, P = 0.027), and d-dimer levels (OR 1.31, 95% CI: 1.17, 1.48, P < 0.001) (Tables 3 and 4). According to the best cut off value of roc curve, the cut off levels for PLA2R antibody titers (≤ 36.9 RU/mL and > 36.9 RU/mL) were determined. D-dimer is a fragment of degraded fibrin reflecting the activation of fibrinolysis and thrombosis, which was used as the marker of hypercoagulable state in patients with MN. As plasma D-dimer levels are affected by many factors, they were not included in the Nomogram.
Nomogram model establishment and validation
Based on the outcomes of the multivariable logistic model, a nomogram was constructed with 3 factors (Fig. 2). The discriminative ability of the nomogram was evaluated by the ROC curve and AUC value. The AUC value was 0.741 (95% CI 0.695–0.788) (Fig. 3). The calibration curves demonstrated good consistency between the predicted values and the real.
A novel nomogram predicting for the probability of thromboembolism events. To use the nomogram, the specific point for each variable of the patient lies on each variable axis. Draw a vertical line upward to determine the point at which each variable accepts. The sum of these points is located on the Total Points axis and draw a vertical line down to horizontal axis to determine the probability of immunological remission.Categorical variables were used for Anti-PLA2R antibody, with 1 referring to PLA2R-Ab titers ≤ 36.9 RU/mL and 2 referring to PLA2R-Ab titers > 36.9 RU/mL. To reduce skewness in the data, the 24-hour urine protein quantification was logarithmic.
outcomes (Fig. 4). The results of the Hosmer–Lemeshow test for the goodness-of-fit of the discriminative model showed a χ2 of 16.081 and a p-value of 0.041, indicating that the nomogram had a good fit. Finally, the DCA plot indicated positive net benefits in the predictive nomogram model among majority threshold probabilities (Fig. 5).
Calibration curves of the nomogram in the training and validation datasets. The y-axis indicates the observed cumulative incidence for i thromboembolism events, and the x-axis is the predicted probability of thromboembolism events based on the predictive model. The line adjacent to the ideal line represents the predictive accuracy.
The ROC, DCA, and calibration curves of the external validation datasets were analyzed to comprehensively validate and evaluate the prediction performance of the nomogram. The results showed that the nomogram displayed overall satisfactory and stable predictive performance, as evidenced by the AUC (95% CI) of 0.748 (0.687–0.809) in the validation datasets (Fig. 3). The calibration curve achieved approximately ideal agreement between observed outcomes and predictions in the external validation datasets, which further confirmed the prediction accuracy and robustness of the nomogram (Fig. 4). The results of the DCA curves suggested that the nomogram achieved a good clinical net benefit in the validation datasets (Fig. 5).
Discussion
The hypercoagulable state in pMN patients refers to any abnormality in the coagulation and anticoagulation process that increases the predisposition for thrombotic events in the body. The hypercoagulable state is an important component of the early diagnosis of thromboembolic diseases. However, the mechanism underlying the hypercoagulable state in NS remains incompletely understood. The risk of VTE is attributed to an increase in fibrinogen production and urinary loss of procoagulant and anticoagulant proteins, leading to a decrease in protein levels such as antithrombin III11. Furthermore, the risk of ATE is attributed to an increase in platelet activation and aggregation, which may be due to the loss of proteins that inhibit platelet formation or aggregation11,12.
In MN, pla2R antibody testing has been conducted in a rational manner for nearly 10 years. Nonetheless, this study is the first to establish a practical model to predict the probability of thromboembolic events in pla2R-related MN. According to the 2021 KDIGO guidelines, PLA2R antibody levels are an important criterion to assess the severity of pMN disease1. Serum PLA2R antibody levels are widely considered to reflect the overall state of the patient’s body in pMN13.
Our research revealed that patients with higher PLA2R antibody levels in pMN are more prone to thromboembolic complications. In vitro studies have shown that PLA2R can internalize and degrade secreted PLA2 (sPLA2) through the lysosomal pathway, clearing extracellular sPLA214. The M-type phospholipase A2 receptor 1 (PLA2R1) is the main antigen that generates PLA2R1-ab in patients with idiopathic MN4. sPLA2 can bind to M-type PLA2R and synthesize prostaglandin (PG) through trans-membrane signal transduction15. PG itself can inhibit platelet aggregation and vasodilation, thereby counteracting thrombosis. In vivo mouse experiments indicated that overexpression of sPLA2-V interferes with the physiological activation of endothelial protein C receptor, leading to impaired activation of protein C and exacerbating thrombosis16. Therefore, the PLA2R1 antibodies in pMN patients are hypothesized to bind to PLA2R and interfere with the normal physiological regulation of M-type PLA2R for sPLA2. This effectively reduces the production of prostaglandin through this pathway, preventing sPLA2 from being normally cleared, and reducing the protein C levels that inhibit thrombosis in the serum, resulting in a high incidence of thromboembolic events in pMN. On the other hand, a recent study by Htwe et al.17. reported that PLA2 can damage the pulmonary endothelium, and endothelial damage is a clear risk factor for thromboembolic events in the vasculature.
Hofstra JM et al. suggested that low albumin levels and proteinuria were risk factors for thromboembolism in nephrotic syndrome11, but in our study, albumin was not identified as a predictor of thromboembolic formation. A study conducted by Alexander Woywodt in 2022 showed that low albumin levels and proteinuria were risk factors for thromboembolism in anti-PLA2R-negative PMN patients. However, in anti-PLA2R-positive patients, low albumin levels were not predictive of thromboembolic events, Anti-PLA2R-positive patients may still suffer from thromboembolic events even if serum albumin levels are not low18. This is consistent with our study, but the mechanisms remain incompletely understood.
Age is an important risk factor for thromboembolic events, with a 7–8 times higher incidence of thromboembolic events in adults compared to children, and this has also been shown to be the case in MN19,20. Zhang et al.21 reported that > 60 years of age was an independent risk factor for VTE in NS patients (P < 0.05), which may be related to the increased activation of the coagulation system and decreased fibrinolysis with age, as well as decreased mobility and higher number of comorbidities22. Meanwhile, D-dimer levels represent the body’s coagulation state; a hypercoagulable state, accompanied by factors such as vascular endothelial injury, increases the risk of thromboembolic events23.
Collectively, these results suggest that the mechanism of thrombosis and embolism in PMNs may be more complex than previously thought. Our research findings may have implications for the risk assessment and risk calculation of thrombosis and embolism formation in PMN populations and provide evidence-based treatment recommendations for clinical decision-making. However, the results should be confirmed in larger cohorts and other ethnic groups.
Nevertheless, the limitations of the present study should be acknowledged. First, positive staining for PLA2R antigen on renal biopsy in anti-PLA2R antibody-negative patients may also reveal PLA2R-related MN, hindering the accurate identification of PLA2R-related MN. Second, despite the large cohort, the number of events was relatively small, which may limit the detection of risk factors. Finally, the true frequency of clinical events may be underestimated due to the passive and retrospective nature of the study, this article included newly diagnosed patients, most of the patients had not been treated with drugs, so the effect of drugs was not counted, which is also the defect of this article. We are following up, including the treatment of membranous nephropathy drugs, such as glucocorticoids, rituximab, cyclophosphamide, calcineurin inhibitors, thromboembolism drugs, such as aspirin, low molecular weight heparin, warfarin, rivaroxaban, and to observe the disappearance of thrombosis, because some patients have not been followed up, there is no complete results. The results of our study will be published when the statistics are complete.
Conclusion
A predictive nomogram including PLA2R antibody, age, proteinuria, and D-dimer levels was developed to predict the thromboembolic risk in patients with PLA2R-related MN. Primary membranous nephropathy patients should be closely monitored for these indicators and anticoagulation prophylaxis should be given to prevent thromboembolic diseases.
Data availability
Data is provided within the manuscript.
References
Kidney disease: Improving global outcomes glomerular diseases work group, KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1–S276. https://doi.org/10.1016/j.kint.2021.05.021 (2021).
Ruggenenti, P. et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 26, 2545–2558. https://doi.org/10.1681/asn.2014070640 (2015).
Hârza, M. et al. Histological diagnosis and risk of renal vein thrombosis, and other thrombotic complications in primitive nephrotic syndrome. Rom J. Morphol. Embryol. 54, 555–560 (2013).
Beck, L. H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl. J. Med. 361, 11–21. https://doi.org/10.1056/NEJMoa0810457 (2009).
Hofstra, J. M., Beck, L. H. Jr., Beck, D. M., Wetzels, J. F. & Salant, D. J. Anti-phospholipase A₂ receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 6, 1286–1291. https://doi.org/10.2215/cjn.07210810 (2011).
Kanigicherla, D. et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 83, 940–948. https://doi.org/10.1038/ki.2012.486 (2013).
Zhu, H. et al. Anti-PLA2R antibody measured by ELISA predicts the risk of vein thrombosis in patients with primary membranous nephropathy. Ren. Fail. 44, 594–600. https://doi.org/10.1080/0886022X.2022.2057861 (2022).
Barbour, S. J. et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 81, 190–195. https://doi.org/10.1038/ki.2011.312 (2012).
Jeyalan, V., Stauss, M., Liew Kang, S. & Ponnusamy, A. Pos-469a retrospective analysis of venous thromboembolism incidence amongst patients with membranous nephropathy: a single centre experience. Is anti- PLA2R an accessory or an accomplice? Kidney Int. Rep. 7, S209. https://doi.org/10.1016/j.ekir.2022.01.499 (2022).
Rovin, B. H. et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 100, 753–779. https://doi.org/10.1016/j.kint.2021.05.015 (2021).
Hofstra, J. M. & Wetzels, J. F. M. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Kidney Int. 89, 981–983. https://doi.org/10.1016/j.kint.2016.01.019 (2016).
Eneman, B., Levtchenko, E., van den Heuvel, B., Van Geet, C. & Freson, K. Platelet abnormalities in nephrotic syndrome. Pediatr. Nephrol. 31, 1267–1279. https://doi.org/10.1007/s00467-015-3173-8 (2016).
Hu, S. L. et al. Diagnostic value of phospholipase A2 receptor in idiopathic membranous nephropathy: a systematic review and meta-analysis. J. Nephrol. 27, 111–116. https://doi.org/10.1007/s40620-014-0042-7 (2014).
Rouault, M. et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry 46, 1647–1662. https://doi.org/10.1021/bi062119b (2007).
Murakami, M., Nakatani, Y., Atsumi, G. I., Inoue, K. & Kudo, I. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 37, 127–195. https://doi.org/10.1615/CritRevImmunol.v37.i2-6.20 (2017).
Tamayo, I. et al. Group V secretory phospholipase A2 impairs endothelial protein C receptor-dependent protein C activation and accelerates thrombosis in vivo. J. Thromb. Haemost. 12, 1921–1927. https://doi.org/10.1111/jth.12676 (2014).
Htwe, Y. M. et al. Group V phospholipase A(2) mediates endothelial dysfunction and acute lung injury caused by methicillin-resistant Staphylococcus aureus. Cells 10 https://doi.org/10.3390/cells10071731 (2021).
Wu, H. H. L. et al. Risk factors of venous thromboembolism in anti-PLA2R-positive and negative primary membranous nephropathy. Clin. Kidney J. 15, 1636–1638. https://doi.org/10.1093/ckj/sfac052 (2022).
Parker, K., Ragy, O., Hamilton, P., Thachil, J. & Kanigicherla, D. Thromboembolism in nephrotic syndrome: controversies and uncertainties. Res. Pract. Thromb. Haemost. 7, 102162. https://doi.org/10.1016/j.rpth.2023.102162 (2023).
Kerlin, B. A., Ayoob, R. & Smoyer, W. E. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin. J. Am. Soc. Nephrol. 7, 513–520. https://doi.org/10.2215/cjn.10131011 (2012).
Zhang, L. J. et al. Pulmonary embolism and renal vein thrombosis in patients with nephrotic syndrome: prospective evaluation of prevalence and risk factors with CT. Radiology 273, 897–906. https://doi.org/10.1148/radiol.14140121 (2014).
Nurmohamed, M. T., Büller, H. R. & ten Cate, J. W. Physiological changes due to age. Implications for the prevention and treatment of thrombosis in older patients. Drugs Aging. 5, 20–33. https://doi.org/10.2165/00002512-199405010-00003 (1994).
Furie, B. & Furie, B. C. Mechanisms of thrombus formation. N Engl. J. Med. 359, 938–949. https://doi.org/10.1056/NEJMra0801082 (2008).
Acknowledgements
Thanks to the support of Renal Pathology Laboratory, the First Affiliated Hospital of Zhengzhou University.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82370727).
Author information
Authors and Affiliations
Contributions
Z.Z., Y.G. and L.T. designed, performed, and analyzed the experiments. Z.Z. wrote and revised the manuscript. L.Y., Y.W. and Q.L. carried out the data collection, data analysis, and revised the manuscript. L.T. revised the manuscript and applied for funding support. L.W. and Y.Y. confrm the authenticity of the raw data. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhai, Z., Guo, Y., Yu, L. et al. Nomogram for thromboembolic events in primary membranous nephropathy associated with PLA2R antibody. Sci Rep 15, 12897 (2025). https://doi.org/10.1038/s41598-025-97203-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97203-z