Abstract
Given the controversial conclusion on the relationship between serum uric acid (SUA) levels and testosterone (T) levels in adult males, the purpose of this study is to explore the association between SUA levels and T levels in adult males. We conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2011 to 2016. The association was estimated using multiple linear regression model and results are presented as β with its 95% confidence intervals (95% CIs). This study enrolled 7791 males aged 18 years or older and found a negative correlation between serum uric acid levels and testosterone levels after controlling for confounding factors. Additionally, a non-linear association was observed, with an infection point of 4.4 mg/dL for serum uric acid levels. The effect sizes on the left and right sides of the inflection point were determined as 17.93 (− 3.61 to 39.48) and − 14.73 (− 18.51 to − 10.95), respectively. Elevated SUA levels were found to be linked to decreased T levels, even after controlling for confounders. Moreover, non-linear pattern in the relationship between SUA levels and T levels was also identified.
Similar content being viewed by others
Introduction
Serum uric acid (SUA) is the final product of purine nucleotide metabolism. It plays a role in scavenging oxygen free radicals, thus contributing to the restoration of normal body functioning. Moreover, SUA acts as a crucial antioxidant, aiding in the maintenance of stable blood pressure and combating oxidative stress1. In the United States, the prevalence of hyperuricemia was 21.2% and 21.6% in males and females, respectively2. A large number of studies have found that high serum uric acid levels have been linked to metabolic disorders including obesity, type 2 diabetes, cardiovascular disease, hepatic steatosis and insulin resistance3,4,5. Previous studies have confirmed that serum uric acid plays a role in the pathogenesis of metabolic syndrome, chronic kidney disease (CKD) and cardiovascular disease (CVD). This is achieved by disrupting biological processes such as oxidative stress, chronic inflammation, mitochondrial dysfunction, endothelial dysfunction, proliferation of vascular smooth muscle cells, and activation of the renin-angiotensin system3,6,7. A Mendelian randomization study conducted on a population of 10,000 individuals found that elevated SUA levels may increase the risk of metabolic syndrome. This increased risk is attributed to the elevation of blood pressure and triglyceride levels, as well as the reduction in HDL-C levels8. Another Mendelian randomization study, involving 3315 patients, revealed a causal association between SUA levels and adverse cardiovascular outcomes, including coronary artery disease, cardiovascular mortality and sudden cardiac death9.
Testosterone, secreted by Leydig cells in the testes, is essential for male reproductive organ maturation and the development of secondary sexual characteristics10. Testosterone deficiency has become an increasingly concerning and controversial issue worldwide, affecting approximately 7% of men at the age of 50 and exhibiting a higher prevalence with advancing age10. Research has shown that there is a common occurrence of low testosterone levels in individuals with obesity, metabolic syndrome, and insulin resistance11,12,13. Additionally, testosterone deficiency is associated with an increased risk of developing type 2 diabetes10,11. Studies have also indicated that long-term testosterone replacement therapy can significantly reduce body mass index (BMI), improve insulin resistance, and enhance blood glucose control14,15,16. Furthermore, testosterone therapy has been found to improve metabolic control, leading to decreased blood glucose values and lower mean glycated hemoglobin levels in men with type 2 diabetes and visceral obesity16.
Based on the association of serum uric acid levels and testosterone levels with metabolic syndrome, there have been studies investigating their relationship, but the results are controversial. Some studies show a negative correlation17,18, while others indicate a positive association between serum uric acid levels and testosterone levels19,20. Therefore, we hypothesize that the relationship between serum uric acid levels and testosterone levels is more complex than a simple linear association, and this relationship may be modulated by factors such as age, metabolic status, and lifestyle. While testosterone levels tend to stabilize in adult males and reach a peak at 18 years of age21,22, other factors may influence these levels thereafter. Thus, we conducted an analysis of the National Health and Nutrition Examination Survey (NHANES) dataset, including male adults aged 18 years and older, to explore the relationship between serum uric acid levels and serum testosterone levels in adult males.
Results
General characteristics
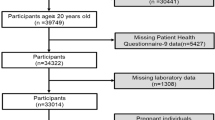
The weighted demographic characteristics are shown in Table 1. A total of 7791 eligible subjects were included in this study Fig. 1. The mean age was 47.62 ± 18.51 years, the mean serum testosterone level was 417.32 ± 189.56 ng/dL, and the mean SUA level was 6.02 ± 1.30 mg/dL. SUA levels were categorized based on SUA quartiles: Q1 (0.40 < SUA ≤ 5.00 mg/dL), Q2 (5.10 < SUA ≤ 5.80 mg/dL), Q3 (5.90 < SUA ≤ 6.70 mg/dL), and Q4 (6.80 < SUA ≤ 11.70 mg/dL). Significant differences were noted in the four groups in terms of age, race, BMI, waist circumference, PIR, smoking status, drinking status, ALT, AST, hsCRP, BUN, SCr, cholesterol, triglyceride, eGFR (estimated glomerular filtration rate), prevalence of hypertension, diabetes, hyperlipidemia, ASCVD, gout and CKD (all P < 0.05). In addition, we found that with increasing SUA quartiles, the serum testosterone levels gradually decreased (P < 0.01).
Associations between serum uric acid and testosterone
Table 2 reveals the association between serum uric acid levels and testosterone levels. In the crude model, testosterone levels is negatively correlated with serum uric acid levels (β = − 30.22, 95% CI − 33.97 to − 26.46, P < 0.0001). After adjusting for age, race and BMI in model I, the relationship between serum uric acid levels and testosterone levels was still robust (β = − 15.56, 95% CI − 19.73 to − 11.39, P < 0.0001). In model II, we further adjusted for ALT, AST, hypertension, diabetes, hyperlipidemia, ASCVD, CKD, smoking status and drinking status, their association persisted (β = − 15.66, 95% CI − 19.76 to − 11.55, P < 0.0001). This similar trend was seen when we treated SUA levels as a quartile in the sensitivity analysis (P for trend < 0.0001).
Non-linear relationship between uric acid and testosterone
In the present work, we looked at the possibility of a non-linear connection between SUA levels and testosterone levels by utilizing smooth curve fits (Fig. 2), we observed a non-linear connection between SUA levels and testosterone levels (after adjusting age, race, BMI, ALT, AST, hypertension, diabetes, hyperlipidemia, ASCVD, CKD, smoking status and drinking status). Using a two-piecewise linear regression model, we were able to identify that the inflection point was located at 4.4 mg/dL (Table 3). On the right side of the inflection point, a negative correlation between serum uric acid levels and testosterone levels was observed (β = − 14.73, 95% CI − 18.51 to − 10.95, P < 0.0001). However, on the left side of this inflection point, no statistically significant association was detected (β = 17.93, 95% CI − 3.61 to 39.48, P = 0.1028).
Adjusted association of serum uric acid levels with testosterone levels in adult males. A non-linear relationship between serum uric acid levels and testosterone levels was found. Red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Adjusted: age, race, BMI, ALT, AST, hypertension, diabetes, hyperlipidemia, ASCVD, CKD, smoking status and drinking status. BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASCVD, atherosclerotic cardiovascular disease; CKD, Chronic kidney disease.
Subgroup analysis
The results of the stratified analyses examining the relationship between serum uric acid levels and testosterone levels are shown in Fig. 3. Significant interaction was observed for age, drinking status, CKD, diabetes, hypertension, hyperlipidemia and gout (all P for interaction < 0.05), but not for BMI, ASCVD, or smoking status (all P for interaction > 0.05). Among these strata, participants with higher serum uric acid levels showed a significant decrease in testosterone levels, particularly among those who were younger in age, current alcohol drinkers, and without CKD, diabetes, hypertension, hyperlipidemia and gout.
Effect size of serum uric acid levels on testosterone levels in subgroup analysis. Each stratification adjusted for all the factors (age, race, BMI, ALT, AST, hypertension, diabetes, hyperlipidemia, gout, ASCVD, CKD, smoking status and drinking status) except the stratification factor itself. BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease.
Discussion
A nonlinear association of serum uric acid levels and testosterone levels was presented in adult males in the present study. The inflection point was identified at 4.4 mg/dL. On the left side of the inflection point, the association did not reach statistical significance. However, a negative correlation between SUA levels and testosterone levels was observed on the right side of the inflection point.
A series of studies have reported a linear association between SUA levels and testosterone levels18,20,23,24,25. However, the conclusions remain controversial, and most of these studies did not consider nonlinearity or perform subgroup analysis. A prospective study conducted by Yahyaoui et al.23 involving 47 female-to-male transsexuals over a 2-year period revealed a significant increase in serum uric acid (SUA) levels and a decrease in the fraction excretion of uric acid (FEUA) following cross-sex hormone treatment, indicating a positive relationship between testosterone levels and SUA levels. Conversely, the study by Marinello and Rosen20,24, which found normal testicular endocrine function in gout patients, with no significant difference in serum testosterone levels between male patients with gout, asymptomatic hyperuricemia, and controls. Additionally, Borbélyová's research on the effects of gonadectomy and long-term hypogonadism in male middle-aged rats showed no significant impact on serum uric acid levels25. In contrast, studies by Han and Wang et al. found that serum uric acid levels might be negatively associated with serum testosterone levels in adult males18,26. Furthermore, Lu et al. reported a negative correlation between total testosterone levels and SUA levels in diabetic patients27. Possible explanations for the differences include: (I) relatively small sample sizes; (II) variations in inclusion criteria; (III) differences existed in research methods, statistical analysis techniques, and adjusted covariates. In this study, we discovered a nonlinear relationship between SUA levels and testosterone levels with an inflection point of 4.4 mg/dL. The results indicate that the negative linear association between SUA levels and testosterone levels is only observed in participants with a SUA level of 4.4 mg/dL or higher. In individuals with relatively lower SUA levels, this linear relationship cannot be detected. In the present study, we found significant interactions with age, drinking status, CKD, diabetes, hypertension, hyperlipidemia, and gout. Among younger individuals, the negative correlation between SUA and testosterone levels was stronger. This may be attributed to several factors. First, younger individuals typically have a higher metabolic rate, which affects the synthesis and metabolism of testosterone28. Additionally, the endocrine system of younger individuals is often more sensitive and active, and higher SUA levels may exert a more pronounced influence on the endocrine system29.
The mechanisms underlying the relationship between serum uric acid levels and testosterone levels are currently not well understood, but several possibilities have been proposed. Firstly, the deposition of uric acid crystals in testicular tissue can lead to oxidative damage and directly affect testosterone secretion30. Second, elevated SUA levels can induce metabolic syndrome through mitochondrial oxidative stress and inhibition of AMP-activated protein kinase31,32. Hyperuricemia can also cause insulin resistance and decreased insulin secretion by endothelial dysfunction, and reduced nitric oxide bioavailability33. Metabolic syndrome and insulin resistance have been shown to be associated with increased conversion of testosterone to estradiol and decreased production of testosterone by Leydig cells34. Third, testosterone levels can reciprocally influence uric acid levels, as low testosterone levels may lead to insulin resistance, reducing uric acid clearance and potentially elevating serum uric acid levels35,36,37. Decreased testosterone levels may reduce protein synthesis and increase endogenous purine levels, resulting in elevated uric acid levels30.
However, there are some limitations to our research. First, we were unable to draw a causal relationship between SUA levels and T levels because of the cross-sectional nature of our study. Second, the dataset used in this study was obtained from a nationwide survey in the United States, so it is uncertain whether the findings can be generalized to populations of other racial backgrounds. Third, testosterone level was measured only once, whereas the diagnosis of low testosterone typically requires at least two recorded values taken on different days. It is important to note that serum testosterone levels are not static but rather dynamic, which may introduce measurement bias in our findings. Fourth, some covariates in our study relied on self-reported data, which introduces potential recall bias. Fifth, cross-sectional studies may fail to fully capture the intricate relationships between variables and are susceptible to selection bias and confounding factors. This could potentially influence our results. Sixth, due to limited data availability, we focused solely on analyzing the relationship between SUA and testosterone without further investigating the effects of SUA on free testosterone or bioactive testosterone. Finally, due to limited research, we have not explored the relationship between serum uric acid levels and hypogonadism. Further investigations are necessary to fully elucidate the complex interplay between serum uric acid and testosterone levels in adult males and to understand the implications of these associations on overall health.
In summary, our study is the first to demonstrate a non-linear correlation between serum uric acid (SUA) levels and testosterone levels in adult males. We found that participants with higher SUA levels exhibited significantly lower testosterone levels, particularly among those with younger age, current alcohol consumption, and the absence of CKD, diabetes, hypertension, and hyperlipidemia.
Methods
Data source and study population
The data for this study were obtained from three consecutive cycles (2011–2012, 2013–2014, and 2015–2016) of NHANES, a national cross-sectional survey designed to assess the health and nutritional status of the United States. NHANES has been approved by National Center for Health Statistics (NCHS) Ethics Review Board, is a major project of the NCHS within the Centers for Disease Control and Prevention (CDC). All research methods of the NHANES were conducted in accordance with the Declaration of Helsinki. The NHANES database is publicly accessible. The data we extracted to validate the study were used to describe the demographic characteristics. An illustration of the participant selection process is depicted in Fig. 1. The study design and data used in this study are available at https://www.cdc.gov/nhcs/nhanes/.
Main variables
Serum uric acid concentration was measured by the timed endpoint method, as detailed in the NHANES Laboratory Procedures Manual38. The hydrogen peroxide reacts with 4-aminoantipyrine (4-AAP) and 3,5-dichloro-2-hydroxybenzene sulfonate (DCHBS) under the catalysis of peroxidase to form colored products. The system monitored absorbance changes at 520 nm, which directly indicated SUA levels. Values were reported in mg/dL, but can be converted to μmol/L by multiplying by 59.48. Serum testosterone levels were quantified using the isotope dilution liquid chromatography tandem mass spectrometry (ID-LC–MS/MS) method following the National Institute for Standards and Technology’s (NIST) reference guidelines. The detection limits for the assays were established as 0.36 mg/dL for serum testosterone and 0.5 mg/dL for serum uric acid. Concentrations below these limits were handled by replacing them with the respective limit of detection divided by the square root of two, ensuring accurate low-level measurements18.
Covariates
Covariates were selected according to previous studies. Age, race, BMI, ALT, AST, hypertension, diabetes, hyperlipidemia, ASCVD, CKD, smoking status and drinking status were included as adjustment variables in the adjusted models. Based on the duration of their smoking history, the participants were categorized into three distinct groups: non-smokers, former smokers, and current smokers. Participants who reported consuming alcohol in amounts exceeding 0 g/week were classified as drinkers. As for the drinking status groups: never (had < 12 drinks in lifetime); former (did not drink last year but drink ≥ 12 drinks in lifetime); mild (≥ 2 drinks/day for males); moderate (≥ 3 drinks/day for males), current heavy alcohol use (≥ 4 drinks/day for males)39,40. The eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation. 41.
Statistical analysis
The statistical analysis process was conducted in four steps. First, we divided the study population into four groups based on serum uric acid quartiles. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as percentages. Second, following the recommendation of the Strengthening the reporting of observational studies in epidemiology (STROBE) statement42, linear regression models were applied to estimate the independent correlation between serum uric acid and testosterone before or after the adjustment of confounders. Third, the generalized additive models (GAM) were used to find the non-linear correlations between serum uric acid and testosterone, and the piecewise linear regression model was used to find the threshold impact of serum uric acid on testosterone. Fourth, subgroup analyses were conducted with stratified linear regression models, and changes and interactions between subgroups were identified through likelihood ratio tests. Data analysis was performed using R (The R Foundation; http://www.r-project.org; version 4.2.0) and EmpowerStats (www.empowerstats.net, X&Y solutions, Inc. Boston, Massachusetts). A two-sided P value of less than 0.05 was considered as statistical significance.
Data availability
The datasets generated and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- BMI:
-
Body mass index
- PIR:
-
Poverty-to-income ratio
- ASCVD:
-
Atherosclerotic cardiovascular disease
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CKD:
-
Chronic kidney disease
- CI:
-
Confidence interval
References
Wang, Q., Wen, X. & Kong, J. Recent progress on uric acid detection: A review. Crit. Rev. Anal. Chem. 50, 359–375. https://doi.org/10.1080/10408347.2019.1637711 (2020).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthr. Rheum 63, 3136–3141. https://doi.org/10.1002/art.30520 (2011).
Wan, X. et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 64, 925–932. https://doi.org/10.1016/j.jhep.2015.11.022 (2016).
Krishnan, E., Pandya, B. J., Chung, L., Hariri, A. & Dabbous, O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: A 15-year follow-up study. Am. J. Epidemiol. 176, 108–116. https://doi.org/10.1093/aje/kws002 (2012).
Fenech, G., Rajzbaum, G., Mazighi, M. & Blacher, J. Serum uric acid and cardiovascular risk: State of the art and perspectives. Joint Bone Spine 81, 392–397. https://doi.org/10.1016/j.jbspin.2014.01.008 (2014).
Zhu, Y. et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 447, 707–714. https://doi.org/10.1016/j.bbrc.2014.04.080 (2014).
Yanai, H., Adachi, H., Hakoshima, M. & Katsuyama, H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22179221 (2021).
Biradar, M. I., Chiang, K. M., Yang, H. C., Huang, Y. T. & Pan, W. H. The causal role of elevated uric acid and waist circumference on the risk of metabolic syndrome components. Int. J. Obes. 2005(44), 865–874. https://doi.org/10.1038/s41366-019-0487-9 (2020).
Kleber, M. E. et al. Uric acid and cardiovascular events: A Mendelian randomization study. J. Am. Soc. Nephrol. 26, 2831–2838. https://doi.org/10.1681/asn.2014070660 (2015).
Grossmann, M. et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 93, 1834–1840. https://doi.org/10.1210/jc.2007-2177 (2008).
Traish, A. M., Feeley, R. J. & Guay, A. Mechanisms of obesity and related pathologies: Androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J. 276, 5755–5767. https://doi.org/10.1111/j.1742-4658.2009.07305.x (2009).
Fui, M. N., Dupuis, P. & Grossmann, M. Lowered testosterone in male obesity: Mechanisms, morbidity and management. Asian J. Androl. 16, 223–231. https://doi.org/10.4103/1008-682x.122365 (2014).
Muraleedharan, V. & Jones, T. H. Testosterone and mortality. Clin. Endocrinol. 81, 477–487. https://doi.org/10.1111/cen.12503 (2014).
Kelly, D. M. et al. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine 54, 504–515. https://doi.org/10.1007/s12020-016-1019-1 (2016).
McInnes, K. J. et al. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes 61, 1072–1081. https://doi.org/10.2337/db11-1136 (2012).
Boyanov, M. A., Boneva, Z. & Christov, V. G. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male Off. J. Int. Soc. Study Aging Male 6, 1–7 (2003).
Zhang, Q., Chen, W., Yun, C. & Wang, J. The application value of serum 25(OH)D3, uric acid, triglyceride, and homeostasis model assessment of insulin resistance in male patients with hyperuricemia combined with hypogonadism. BMC Endocr. Disord. 21, 102. https://doi.org/10.1186/s12902-021-00765-y (2021).
Han, Y. et al. Exploration of the association between serum uric acid and testosterone in adult males: NHANES 2011–2016. Trans. Androl. Urol. 10, 272–282. https://doi.org/10.21037/tau-20-1114 (2021).
Kurahashi, H. et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr. J. 60, 1321–1327. https://doi.org/10.1507/endocrj.ej13-0203 (2013).
Marinello, E., Riario-Sforza, G. & Marcolongo, R. Plasma follicle-stimulating hormone, luteinizing hormone, and sex hormones in patients with gout. Arthritis Rheum. 28, 127–131. https://doi.org/10.1002/art.1780280203 (1985).
Nguyen Hoai, B., Do Truong, T., Nguyen Cao, T., Pham Minh, Q. & NguyenBuiDuy, V. Age-specific testosterone thresholds: Reconsidering diagnostic criteria for hypogonadism across generations of Vietnamese men. Aging Male Off. J. Int. Soc. Study Aging Male 27, 2422379. https://doi.org/10.1080/13685538.2024.2422379 (2024).
Correction: a validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PloS one 10, e0117674, https://doi.org/10.1371/journal.pone.0117674 (2015).
Yahyaoui, R. et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J. Clin. Endocrinol. Metab. 93, 2230–2233. https://doi.org/10.1210/jc.2007-2467 (2008).
Rosen, R., Tomer, Y., Carel, R. & Weinberger, A. Serum 17-beta-estradiol and testosterone levels in asymptomatic hyperuricaemic men. Clin. Rheumatol. 13, 219–223. https://doi.org/10.1007/bf02249015 (1994).
Borbélyová, V. et al. Does long-term androgen deficiency lead to metabolic syndrome in middle-aged rats?. Exp. Gerontol. 98, 38–46. https://doi.org/10.1016/j.exger.2017.08.016 (2017).
Wang, Y. & Charchar, F. J. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci. Rep. 11, 17323. https://doi.org/10.1038/s41598-021-96959-4 (2021).
Wan, H. et al. The associations between gonadal hormones and serum uric acid levels in men and postmenopausal women with diabetes. Front. Endocrinol. 11, 55. https://doi.org/10.3389/fendo.2020.00055 (2020).
Kelly, D. M. & Jones, T. H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 217, R25-45. https://doi.org/10.1530/joe-12-0455 (2013).
Hackney, A. C. Endurance exercise training and reproductive endocrine dysfunction in men: Alterations in the hypothalamic-pituitary-testicular axis. Curr. Pharm. Des. 7, 261–273. https://doi.org/10.2174/1381612013398103 (2001).
Cao, W. et al. Association between sex hormone and blood uric acid in male patients with type 2 diabetes. Int. J. Endocrinol. 2017, 4375253. https://doi.org/10.1155/2017/4375253 (2017).
King, C. et al. Uric acid as a cause of the metabolic syndrome. Contrib. Nephrol. 192, 88–102. https://doi.org/10.1159/000484283 (2018).
Sharaf El Din, U. A. A., Salem, M. M. & Abdulazim, D. O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 8, 537–548. https://doi.org/10.1016/j.jare.2016.11.004 (2017).
Li, C., Hsieh, M. C. & Chang, S. J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 25, 210–216. https://doi.org/10.1097/BOR.0b013e32835d951e (2013).
Corona, G. et al. Testosterone and metabolic syndrome: a meta-analysis study. J. Sex. Med. 8, 272–283. https://doi.org/10.1111/j.1743-6109.2010.01991.x (2011).
Dimopoulou, C., Goulis, D. G., Corona, G. & Maggi, M. The complex association between metabolic syndrome and male hypogonadism. Metab. Clin. Exp. 86, 61–68. https://doi.org/10.1016/j.metabol.2018.03.024 (2018).
Perez-Ruiz, F. et al. Renal clearance of uric acid is linked to insulin resistance and lower excretion of sodium in gout patients. Rheumatol. Int. 35, 1519–1524. https://doi.org/10.1007/s00296-015-3242-0 (2015).
Facchini, F., Chen, Y. D., Hollenbeck, C. B. & Reaven, G. M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266, 3008–3011 (1991).
Li, G. Y., Qian, X. D., Ma, C. M. & Yin, F. Z. The dose-response relationship between sex hormones and hyperuricemia in different gender: NHANES 2013–2016. Front. Endocrinol. 13, 1035114. https://doi.org/10.3389/fendo.2022.1035114 (2022).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6, 399–410. https://doi.org/10.1002/hep4.1803 (2022).
Hicks, C. W., Wang, D., Matsushita, K., Windham, B. G. & Selvin, E. Peripheral neuropathy and all-cause and cardiovascular mortality in U. S. adults: A prospective cohort study. Ann. Int. Med. 174, 167–174. https://doi.org/10.7326/m20-1340 (2021).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Acknowledgements
Not applicable.
Funding
This project was supported by National Natural Science Foundation of China [No. 82201879 to Jingfei Chen]; Hunan Provincial Natural Science Foundation of China [No. 2022JJ40675 to Jingfei Chen]; Scientific Research Project of Hunan Provincial Health Commission [No. B202305037231 to Jingfei Chen]; Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University (to Jingfei Chen); China Postdoctoral Science Foundation (to Jingfei Chen).
Author information
Authors and Affiliations
Contributions
All the authors made a significant contribution to this work. J.C (Jingfei Chen) designed the study. W.C (Wenxiu Chen) and W.S (Wanjuan Song) wrote the manuscript. H.Z (Hanzhi Zhang), W.S (Wanjuan Song) and X.S (Xin Su) collected, analyzed, and interpreted the data. J.C (Jingfei Chen) and J.C (Jianlin Chen) critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, W., Song, W., Zhang, H. et al. Potential links between serum uric acid levels and testosterone levels in adult males: a cross-sectional study. Sci Rep 15, 16924 (2025). https://doi.org/10.1038/s41598-025-97352-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97352-1