Abstract
Volitional eyes closing (EC) can promote the interoceptive thoughts that vary considerably among individuals. Although this behavior is known to recruit a large-scale brain network as its neural underpinning, individual variability in such network recruitment remains unexplored. Here, we compared the intersubject variability in functional connectivity (IVFC) between the EC and eyes opening conditions. It was found that the IVFC significantly increased during the EC condition, which could be replicated in different public datasets and multi-resolution parcellations. Moreover, the EC-enhanced IVFC focused on selective subsets of FCs, with predominant impact on the default-mode, dorsal attention, and visual networks. Finally, a positive relationship was discovered between EC-enhanced IVFC and deep involvement in the task-unrelated thoughts that was measured with an additional dataset. Collectively, these results suggested that enhanced individual difference characterize the functional network of volitional EC, with widespread impact on cognitive systems and potential connection to task-unrelated thoughts.
Similar content being viewed by others
Introduction
People tend to close their eyes when engaged in difficult cognitive tasks, with improved memory1, imagination2, and creativity3. This behavior, known as volitional eyes closing (EC), has attracted many researchers to investigate its neural correlates. Previous investigations on such issue demonstrated that volitional EC is underpinned by a large-scale brain network4,5. However, the network underpinning of EC was only examined by the group-averaged approach, where individual difference of brain function was discounted as mere “noise”. Notably, recent studies on intrinsic functional connectivity (FC) have highlighted that the individual difference exhibit a trait-like nature (unimpeded by day-to-day variation)6, and characterize the person-specific features of brain organization (acting as an identifying fingerprint)7. Moreover, individual differences in FC have been shown to reflect neural processes that are unique to each person8, and to predict behavioral performance with high accuracy9. As a result, interpreting the individual difference in context has emerged as a burgeoning frontier of cognitive neuroscience10, offering valuable insights into the neural correlates of volitional EC.
In this study, we hypothesize that the EC would enhance the intersubject variability of functional connectivity (IVFC), compared with the eyes opening (EO) condition. IVFC is not static but can adapt to the environmental demands11,12. A typical example of such demands is the manipulation of mental state (such as resting, watching a movie, and performing a sensorimotor task)13. Accordingly, volitional EC, which shifts mental state from the exteroceptive to interoceptive mode14, has great potential to impact the IVFC. Notably, interoceptive mental state can promote the internal thoughts that are unrelated to current task15. Behavioral studies on the task-unrelated thoughts (TUTs), such as mind wandering, have demonstrated the rich diversity of their contents among individuals16,17, possibly due to their implicit direction towards personal concerns18 and autobiographical memory19. Given the close connection between mental content and its underlying neural activity20, it is reasonable to anticipate that EC could greatly enhance the IVFC. Indeed, neuroimaging studies on functional network also support this hypothesis. Substantial IVFC has been found to associate with integrated information processing, characterized by abundant long-range FCs11. Consistently, researches on the large-scale network of volitional EC revealed a distinctive feature of integration, ranging from the increased communication efficiency between ROIs4 to the promoted cognitive interplay between modules5. Further analysis on the network’s temporal properties even unveiled that a hyper-connected state with abundant long-range connectivity, dominated the brain dynamics of the EC21. Despite the theoretical analyses mentioned above, empirical research on the EC-enhanced IVFC has not been conducted.
Here, we systematically investigated the adaptation of IVFC in response to volitional EC. Specifically, we first established the EC-enhanced IVFC in different public datasets and multi-resolution parcellations (from 100 to 1000 ROIs)22. Next, we identified the “critical FCs” for EC-enhanced IVFC and assessed their impact on distinct functional systems (based on their spatial distribution). Finally, we associated the EC-enhanced IVFC with deep involvement in TUTs, using an additional dataset on mind wandering. Our results suggested that enhanced individual difference characterize the functional network of volitional EC, with widespread impact on cognitive systems and potential connection to task-unrelated thoughts.
Materials and methods
Participants
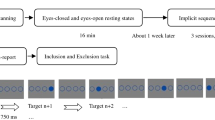
Openly available datasets on EC/EO (https://www.nitrc.org/projects/eceo_rsfmri_9) and mind wandering (https://osf.io/43dp5).
were used in this study. For the EC/EO dataset, nine groups of subjects were originally contained23,24 but only five of them (group 1, 4, 6, 7, and 8) were currently chosen to analyze (Figure S1). The other four groups were excluded due to the following reasons: group 2–3 and 5 were collected from the same cohort as group 1 (over different visit), and 4 (with different TR), respectively; group 9 were scanned with different parameters from the others. Moreover, the five selected groups were further divided into two cohorts, based on the sites of MRI scanning: group 6 and 8 were scanned in Beijing Normal University (Beijing cohort); group 1,4, and 7 in Hangzhou Normal University (Hangzhou cohort). To achieve robust conclusions, the current study focused on the Beijing cohort and replicated the main results in the Hangzhou cohort as validation. Lastly, seven and five subjects in Beijing and Hangzhou cohorts were excluded due to poor image quality, yielding 134 subjects as the final sample for analyses (N = 56 and 78 for Beijing and Hangzhou cohorts, respectively).
Detailed description of EC/EO dataset could be found in23,24. Briefly, subjects were scanned in two consecutive sessions of EC/EO condition, with the order of sessions being counter-balanced to ensure the fair distribution. Each session lasts for eight minutes, in which subjects were instructed to keep their eyes naturally closed or open, but not to focus on any particular thoughts. After each session, the experimenter briefly conversed with the participant to confirm that he/she did not fall asleep.
For the mind wandering dataset, 30 subjects were scanned while performing a fast-paced sustained attention to response task (two subjects were excluded due to ending the experiment prematurely), in which the technique of probe-caught experience sampling was used to assess the frequency of TUTs25. Participants were instructed to respond to every digit (randomly chosen from 1 to 9) with a button press using their right index finger unless the target stimulus appeared (digit 3). The train of stimuli was occasionally interrupted by a thought probe to track ongoing fluctuations in attentional focus, which was formulated as “Where was your attention during the previous trials?”. To respond to the probe, participants had to use left and right response buttons to move an arrow above a 4-point sidebar ranging from 1 (off-task) to 4 (on-task). In the current study, participants’ “off-task” responses were interpreted as their TUTs frequency, which was subsequently analyzed (see Sect. 2.6).
Image acquisition and preprocessing
The scanning parameters of EC/EO datasets could be found in23,24. Briefly, imaging data were acquired on the 3T-MRI clinical scanner with an eight-channel head coil. The resting-state scans were collected through a standard gradient-echo echo planar imaging sequence, which lasted for eight minutes and included 240 volumes. Another high-resolution anatomical scan was obtained for each participant using a 3D T1-weighted magnetization-prepared rapid gradient echo (MP- RAGE). The datasets were processed with FreeSurfer (v7.3.2; https://surfer.nmr.mgh.harvard.edu), ANTs (v2.3.5; http://stnava.github.io/ANTs/), and AFNI (v.23.2.08; https://afni.nimh.nih.gov), which was openly available at https://github.com/lufee10/myPrep. Details for the processing were described as follow:
-
(1)
FreeSurfer’s recon-all was run on the anatomical volume to estimate the tissue maps for each subject, from which lateral ventricle and white matter mask were created and converted into NIFTI format (@SUMA_MakeSpec_FS) for use in AFNI26.
-
(2)
ANTs’ commands were run on the anatomical volume of each subject to 1 > correct for intensity non-uniformity (N4BiasFieldCorrection),
-
2 > denoise image (DenoiseImage),
-
3> strip the skull (antsBrainExtraction.sh), and
-
4> non-linearly warp to MNI template (antsRegistration).
-
-
(3)
AFNI (afni_proc.py) was run on the functional images of each subject to 1 > discard the first five images and remove “spikes”,
-
2> correct for slice time differences and head motion artifacts,
-
3> normalize to MNI template with a resampling size (3x3x3 mm3),
-
4> smooth by 6 mm full-width half maximum Gaussian kernel,
-
5> filter with a band pass (0.01 - 0.1 HZ), and
-
6> regress out several nuisance variables, including the six rigid body motion parameters with their derivatives and linear polynomial baseline terms, and physiological noise from white matter and CSF voxels
Notably, the fast ANATICOR27 was implemented to provide white matter regressor, and the first three principal components of the ventricles was computed as the cerebrospinal fluid regressor28. Finally, time points were censored from the regression model whenever the per-time point motion (Euclidean norm of the motion derivatives) exceeded 0.2 mm or when at least 5% of the brain voxels were seen as outliers from the trend.
The scanning parameters and preprocessing of mind wandering dataset have been described in25. Briefly, participants were scanned on the 3T Philips Achieva MRI system with a 32-channel head coil. The functional images were acquired with a fast-echo (FE) echo-planar imaging (EPI) sequences (FOV = 200 × 104 × 200 mm, TR = 2250 ms, TE = 29.94 ms, flip-angle = 800, voxel size = 2.5 mm isotropic), and the anatomical images were collected with a turbo fileld-echo (TFE) sequence (FOV = 240 × 220 × 188 mm, TR = 8192 ms, TE = 3760 ms, voxel size = 1 mm isotropic).
Then the images were processed with FSL (v6.0; https://fsl.fmrib.ox.ac.uk/fsl/docs/#/) and custom Python scripts (v.2.7.15) using the Nipype framework (v1.1.8;29). Specifically, the anatomical images were skull-striped and segmented into gray matter, white matter, and cerebrospinal fluid. The functional images were (1) smoothed with a 6 mm full-width half-maximum Gaussian kernel; (2) motion and slice-time corrected; and (3) high-pass filtered at 1/44 Hz. Then the general linear model was constructed to regress out (1) several nuisance variables, including the six rigid body motion parameters, average white matter, and cerebrospinal fluid; and (2) task regressors that were prepared by convolving stimulus, thought probe, and response onsets with a standard hemodynamic response function to model task-dependent BOLD signal. The preprocessed images (in native space) were downloaded from https://osf.io/43dp5, which were aligned to the MNI space before analyses in the current study (with FSL’s flirt).
EC-effect on the IVFC (with the EC/EO dataset)
The IVFC was estimated and analyzed by taking the steps as follow:
-
(1)
Constructing the FC matrix of subject: for each subject, the time series of Schaefer’s 100 ROIs22 were extracted. Then the correlations between time series were computed and Fisher z-transformed, yielding a 100 × 100 FC matrix for each subject.
-
(2)
Generating the distance matrix of group: for each group, subjects’ FC matrix were vectorized by extracting the upper triangle 4,950 elements. Then the correlation distances between FC vectors were calculated, which generated a N x N distance matrix for each group (N refers to the subject number).
-
(3)
Computing the IVFC and its profile: for each distance matrix that excluded the diagonal elements, the i-th row-average was computed as the IVFC of subject i. Additionally, the entire i-th row was considered as the IVFC profile of subject i.
-
(4)
Statistical testing: the IVFC was compared between EC and EO conditions by the paired t-test. This analysis aimed to reveal the group effect of EC in the functional variability across individuals. Additionally, the IVFC profile was compared between conditions for each subject, by the paired t-test. Notably, the mean value of IVFC profile is taken as the IVFC. Therefore, such comparison could test for a subject, whether he/she underwent a significant EC-induced alteration in IVFC30. We expected that most subjects would exhibit a significant EO-to-EC promotion of IVFC profile, which strongly supports the group finding of EC-enhanced IVFC.
“Critical FCs” for the EC-enhanced IVFC (with the EC/EO dataset)
A two-steps procedure developed by our previous work30 was implemented to identify the FCs that were “critical” for the EC-enhanced IVFC:
-
(1)
Measuring the “FC-wise loading” for the IVFC: The first step involved assessing the contribution of each FC to the IVFC. As mentioned in Sect. 2.3, the IVFC of any subject i equals to the averaged correlation distance between subject i and the others within group, which was calculated as.
where \(\:{X}_{i},\:{X}_{j}\) refers to the FC vector for subject i and j, respectively; n refers to the subject number.
Additionally, the correlation between two vectors (\(\:{X}_{i}\) and \(\:{X}_{j}\)) was computed as the sum of “element-wise product,” after the vectors are Z-score normalized31, which was calculated as.
where m, \(\:{X}_{i,k}\) ,\(\:Var\left({X}_{i}\:\right)\), and \(\:{\stackrel{-}{X}}_{i}\) refer to the length, k-th element, variance, and mean of \(\:{X}_{i}\), respectively; \(\:{Z}_{i,k}\) refers to the k-th element of the Z-score normalized \(\:{X}_{i}\)Therefore, the FC-wise loading for the IVFC of subject i was defined as one minus averaged “element-wise product” between subject i and the others, where any k-th “loading” was calculated as.
-
(2)
Identifying the “critical FCs” for the EC-enhanced IVFC: The second step entailed the FC-wise comparison on the “loading” of IVFC between the two conditions. Any connectivity with significant EO-to-EC promotion in \(\:Loading\left(k\right)\) was identified as the “critical FC” for the EC-enhanced IVFC (p < 0.0001).
Local impact of the EC-enhanced IVFC (with the EC/EO dataset)
This study explored the functional systems being predominantly impacted by the training-enhanced IVFC, based on the spatial distribution of “critical FCs”. Specifically, the proportion of “critical FCs”, calculated as \(\frac{{number~of\prime \prime critical~FCs\prime \prime }}{{number~of~all~FCs}}\), was first computed for each functional network based on Yeo’s atlas32. Then the permutation-based tests were conducted for each proportional value to identify the networks with substantial “critical FCs” as those being predominantly impacted. The permutation tests held the null hypothesis that any FC has an equal probability to become the “critical FCs”. Accordingly, it would not consider the observed proportion as significant, if it is comparable to the case where “critical FCs” were randomly selected from all FCs. The procedures of permutation testing were as follows:
-
(1)
Randomly selecting 617 FCs (i.e., the number of “critical FCs” identified in Sect. 3.2) as the shuffled “critical FCs”;
-
(2)
Computing the proportion of shuffled “critical FCs” as the “shuffled proportion”;
-
(3)
Iterating the above steps for 10, 000 times to generate the empirical distribution of test statistics;
-
(4)
Calculating the p value as the ratio of times when the “shuffled proportion” is greater than the “observed proportion,” with 5% being set as the threshold of statistical significance.
As a validation, brain regions with substantial “critical FCs” were identified by the ROI-wise permutation tests, following procedures similar to those described above. Briefly, a total of 617 FCs were randomly selected (10, 000 times) as the shuffled “critical FCs”, based on which the “shuffled regional proportion” was calculated. Then the p value was computed as the ratio of times when the “shuffled regional proportion” is greater than the “observed regional proportion”. We expected that most ROIs with substantial “critical FCs” (p < 0.05) would be located in the functional systems being predominantly impacted by the training-enhanced IVFC.
Relation to deep involvement in TUTs (with the EC/EO and Mind wandering dataset)
Participants in the mind wandering dataset were initially categorized into either the frequent TUTs subgroup (N = 14) if their TUTs frequency was above the median, or the occasional TUTs subgroup (N = 14) if it was below. This categorization produced a significant difference in the TUTs frequency between subgroups (t (26) = 4.768, p = 6.21e-5, Cohen d = 1.802). Subsequently, the IVFC for each subject was estimated within their respective subgroup (see Sect. 2.3). As a preliminary analysis, this metric was compared between the subgroups. We expected that subjects with frequent TUTs would exhibit higher IVFC than the others, indicating a potential association between the TUTs and IVFC.
To extend such relationship into the present context, we next assessed each subject’s propensity for frequent TUTs, in both EC and EO conditions. This metric was defined as the normalized functional similarity of subject i in EC/EO condition to the cohort of frequent TUTs, computed as
where \(\:{X}_{i},\:{X}_{j},\:{X}_{k}\) refers to the FC vector for subject i, j and k, respectively; m and n refers to the subject number within frequent and occasion TUTs subgroup, respectively.
Paired t-test between conditions was conducted on the above metric to demonstrated that deep involvement in TUTs characterized the EC condition. Moreover, moderation effect of EC condition was examined towards the linear correlation between the IVFC and the propensity for frequent TUTs. We expected that the EC condition could promotion such linear correlation, which suggested that the elevated propensity for frequent TUTs may serve as a potential cause of the enhanced IVFC.
Results
Volitional EC significantly enhanced the IVFC
First, we sought to analyze the EC-effect on the individual difference of FCs, at both group and individual levels. At group level, paired t-test on the IVFC revealed the significant increase from the EO to EC condition (Figure 1A, t (55) = 7.147, p = 2.164e-9, Cohen d = 1.026). Notably, such EC-enhanced IVFC could be replicated under multi-resolution parcellations, with ROIs number ranging from 100 to 1000 (Figure S2). At individual level, paired t-test on the IVFC-profile unveiled a consistent result with the group analysis. It found that most subjects (67.857%) exhibited significant EO-to-EC promotion of IVFC-profile, while only a small minority (3.571%) showed the opposite trend (Figure 1B). As a validation, the EC-effect on the individual difference of FCs was replicated in a different dataset (Hangzhou cohort), at both group (Fig. 1C, t (55) = 4.747, p = 9.001e-6, Cohen d = 0.497) and individual levels (Fig. 1D). Therefore, our data provided convincing evidences for the EC-enhanced functional variability across individuals.
Volitional EC significantly enhanced the IVFC. (A) Comparison of IVFC at the group level. (Left panel) The EC condition exhibited significantly higher IVFC than the EO condition. (Right panel) Most subjects increased their IVFC in the EC condition. Asterisk (***) indicates p < 0.001. Error bars indicate standard error of the mean.(B) Comparison of IVFC profile at the individual level. Most subjects (67.857%) exhibited significant EO-to-EC promotion of IVFC-profile, while only a small minority (3.571%) showed the opposite trend. Note that the values of EO-to-EC promotion have been sorted among participants for improved visualization. (C) Replication of (A) in a different cohort. Asterisk (***) indicates p < 0.001. Error bars indicate standard error of the mean. (D) Replication of (B) in a different cohort.
The EC-enhanced IVFC was concentrated in the default-mode, dorsal attention, and visual networks
Second, we attempted to identify those FCs that were “critical” for the EC-enhanced IVFC. Connectivity-wise tests on the “loading” for IVFC discovered a total of 617 FCs (12.4% FCs within the whole brain network) as the “critical FCs”. These FCs linked all nodes of the whole network (Figure 2A), showing a large-scale cortical basis for the EC. Notably, simply removing these “critical FCs” from network precluded the significant EC-promotion of IVFC (t (55) = 0.610, p = 0.545, Cohen d = 0.089, Fig. 2B). Moreover, removing these “critical FCs” would also preclude the EC-enhanced IVFC in a different dataset (Hangzhou cohort) that was unrelated to identifying the “critical FCs” (t (77) = 1.581, p = 0.118, Cohen d = 0.172, Fig. 2C). These observations demonstrated that the “critical FCs”, despite their small proportion, were essential for the EC-enhanced IVFC.
The EC-enhanced IVFC was concentrated in selective subsets of FCs. (A) The spatial distribution of “critical FCs”. Brain regions were color-coded according to Schaefer’s atlas (seven networks). The figure was generated by pyCircos V0.3.0 (https://github.com/ponnhide/pyCircos). (B) Comparison of IVFC after removing the “critical FCs” from network. Simply removing these “critical FCs” from network precluded the significant EC-promotion of IVFC. Error bars indicate standard error of the mean.(C) Replication of (B) in a different cohort. Error bars indicate standard error of the mean. Error bars indicate standard error of the mean
Next, we aimed to evaluate the local impact of EC-enhanced IVFC on distinct functional systems. To this end, we analyzed the spatial distributions of the “critical FCs” across distinct networks. Permutation-based tests indicated that the DMN (p = 0.008), DAN (p = 0.015) and Vis (p = 0.019) networks exhibited significantly high proportion of “critical FCs” (Fig. 3A). To validate this finding, we also identified the ROIs with substantial “critical FCs”, by the permutation-based tests described in Sect. 2.5. Consistently, 89.6% ROIs (26 in 29 ROIs) with significantly high proportion of “critical FCs” were discovered to be affiliated with the three networks (41.3%, 17.2% and 31.1% for DMN, DAN and Vis networks, respectively) (Fig. 3B). Therefore, our results showed the heterogenous influence of EC-enhanced IVFC on distinct functional systems, with the DMN, DAN and VIS networks being predominantly impacted.
The EC-enhanced IVFC predominantly impacted the default-mode, dorsal attention, and visual networks.(A) The proportion of “critical FCs” across distinct networks. (Left panel) Asterisk (*) indicates the networks with considerable “critical FCs” (permutation testing, p < 0.05). Dashed line denotes the averaged proportion for all networks (12.4%). (Right panel) The observed proportions (red dashed line) for the Vis, DAN, and DMN were significantly higher than the shuffled proportion (black dashed line), respectively (permutation testing, p < 0.05). (B) The proportion of “critical FCs” across distinct ROIs. (Left panel) The regional proportion of “critical FCs” was heterogenous across distinct ROIs. (Right panel) Most ROIs with considerable “critical FCs” (permutation testing, p < 0.05) were affiliated with the DMN (41.3%), DAN (17.2%), and Vis (31.1%). The figure was generated by BrainSpace V0.1.4 (https://brainspace.readthedocs.io/en/latest/index.html).
The EC-enhanced IVFC is potentially linked to deep involvement in the task-unrelated thoughts
Finally, we intended to explore the relationship between the EC-enhanced IVFC and deep involvement in TUTs. This exploration was aided by another dataset where subjects performed a sustained attention task, with both their TUTs frequency and neurocognitive function being measured. A preliminary analysis on this dataset showed that subjects with frequent TUTs possessed higher IVFC than those with occasional TUTs (t (26) = 3.425, p = 0.002, Cohen d = 1.294), which implied that a potential connection may exist between the TUTs and IVFC.
To extend such relationship into the present context, we next assessed each subject’s propensity for frequent TUTs (see Sect. 2.6), in both EC and EO conditions. Paired t-test on this metric discovered a significant increase from the EO to EC condition (t (55) = 2.662, p = 0.010, Cohen d = 0.386), which suggested that deep involvement in TUTs characterize the EC condition. Furthermore, such metric was found to positively correlate with IVFC in the EC (Fig. 4A, r = 0.479, p = 0.002), but not in the EO condition (r = 0.175, p = 0.196).As a validation, we replicated the result in the Hangzhou cohort. It found consistent increase of propensity for frequent TUTs, from the EO to EC condition (t (77) = 3.013, p = 0.003, Cohen d = 0.375). Additionally, such metric positively correlated with IVFC in the EC (Fig. 4B, r = 0.541, p = 3.09e-7), but not in the EO condition (r = 0.034, p = 0.772).
The EC-enhanced IVFC is potentially linked to deep involvement in the task-unrelated thoughts. (A) Subject’s propensity for frequent TUTs positively correlated with IVFC in the EC, but not in the EO state. Note that values have been z-normalized for improved visualization. (B) Replication of (A) in a different cohort.
Then an additional two-step hierarchical regression was conducted with the IVFC as dependent variable. In step 1, group (dummy coded: 0 = EO, 1 = EC) and the propensity for frequent TUTs were entered as the independent variables in the model. In step 2, the interaction term between the above two independent variables was entered in the model. As expected, in step 1, the two independent variables accounted for a significant amount of variance in the dependent variable (R2 = 0.305, F [2, 109] = 23.972, p < 0.001). Moreover, comparing step 2 with step 1, there was a significant increase in the multiple R2 ( Δ R2 = 0.029, F_changed (1, 108) = 4.608, p = 0.034), suggesting the moderation effect of eyes condition toward the correlation between the IVFC and propensity for frequent TUTs. No outlier (Cook’s distance > 1) was found for all the regression models above, suggesting that these significant findings were not statistical artifact. The results implied that the deep involvement in TUTs (measured as the EC-elevated propensity for frequent TUTs) may serve as a potential cause for the enhanced IVFC during the EC condition.
Discussion
This study explored the adaptation of IVFC in response to volitional EC. Our main findings are as follows: (1) the IVFC significantly increased during the EC condition; (2) the EC-enhanced IVFC focused on selective subsets of FCs, which predominantly impacted the default-mode, dorsal attention, and visual networks; and (3) deep involvement in the task-unrelated thoughts positively associated to the EC-enhanced IVFC. Jointly, our findings suggested that volitional EC could enhanced the individual difference of brain function, with widespread impact on cognitive systems and potential link to task-unrelated thoughts.
Enhanced individual difference of functional network during volitional EC
Understanding the average typical brain and its individual difference constitute two complementary goals of cognitive neuroscience33. However, previous studies on neural correlate of EC mainly adopted the group-averaged approach which discounted the individual difference as mere “noise”. Notably, the averaged correlate of EC identified in previous studies exhibited a distinctive feature of functional integration4,5, which suggested that it should encompass substantial individual difference11. This hypothesis aligns with the diverse mental contents of interoceptive state34,35, but requires empirical examination.
The present study tested such hypothesis by comparing the IVFC between EC and EO conditions, in different public datasets. We found that the EC displayed significantly higher IVFC than the EO condition in these datasets (Fig. 1A and B). Moreover, this finding could be replicated under the multi-resolution parcellations, with ROIs number ranging from 100 to 1,000 (Figure S2). Elevated IVFC has been consistently observed in brain regions with complex cognitive processes. For example, association areas responsible for advanced cognitions (such as language) showed higher IVFC than the unimodal ones36. Similarly, nonprimary auditory cortices involved in processing complex sound (such as music) exhibited higher IVFC than the primary ones12. Therefore, the EC-enhanced IVFC found here may reflect the increased capacity of brain network to address complex issue. This finding has implications for the understanding of EC-improved behaviors, such as creativity, which rely on the intricate interplay of multiple cognitive processes37.
We also observed that the majority of subjects exhibited significant EO-to-EC promotion of IVFC-profile (Fig. 1B and D). Such observation added weight to the finding of EC-enhanced IVFC, by showing that it is not only a central tendency of group, but also a prevalent phenomenon among individuals. On the one hand, it suggested that the EC may serve as an optimal condition to elicit rich patterns of brain function. Previous studies that aimed to evoke variable brain activity (such as naturalistic viewing paradigms) have proved fruitful for studying meaningful idiosyncrasies (Yeshurun et al., 2021)38, particularly in subclinical populations13. One recent study on mental disorders even indicated the disrupted intersubject variability as a prominent feature of functional organization among the schizophrenia39. Thus, we speculated that the substantial IVFC of EC may become a valuable source for the early diagnosis of abnormal brain functions in the future. On the other hand, it implied that the averaged neural correlate of EC may be readily divided into multiple subtypes, rather than adhering to a stereotyped general pattern. We encourage future studies to examine such implication and investigated the potential causes (such as specific cognitive traits or personality factors) for the emergence of distinct neural subtypes.
Key players in the newfound plasticity: default-mode, dorsal attention, and visual network.
The EC-enhanced IVFC profoundly affected default-mode, dorsal attention, and visual networks, given substantial “critical FCs” connected to them (Figs. 2A and 3A). DMN is recognized as an intrinsic system specialized for internally oriented thoughts40. Functional disruption of the network would cause many psychiatric diseases with impaired regulation of spontaneous internal mentation, such as attention-deficit hyperactivity41 and major depression disorders42. Moreover, DMN is particularly sensitive to external influences towards the IVFC38. Research on cognitive training showed such network as being preferentially impacted in its functional variability30. Thus, our findings are compatible with the previous studies of DMN. Given the positive correlation between IVFC and functional integration11, our finding could reflect the promoted integrative capacity of DMN that has been observed in the averaged correlate of EC4,5.
DAN is conventionally associated with the control of stimulus-driven attention43,44. Consistently, the EC condition, which presumably decrease the exogenous attention towards environmental stimulus, has been found to reduce the activation of DAN-related areas14,45. Notably, recent studies on DAN emphasized that their intrinsic FCs are highly variable FCs across subjects46, with significant heterogeneity particularly observed in the connections to DMN47,48. Moreover, the functional variability of DAN-DMN interactions would adapt to various cognitive contexts, which may facilitate the dynamical transitions between distinct mental states49. Therefore, our finding of abound “critical FCs” of DAN (with substantial DAN-DMN interactions), both complements previous studies on EC and agrees with the recent theory of DAN. We speculated this finding may reflect the improved capacity of individuals to dynamically reorient their ongoing mind back to the introspective mental state50.
Visual network is the primary functional community that receives, integrates, and processes visual information51. This network has been repeatedly observed to increase its activity because the EC involves considerable visual imagination of various sensory experiences14. Our work supported the essential role of visual network in underpinning the EC-related neural processes, from the novel perspective of individual difference. Large individual difference characterized the function of visual network52,53, which revealed the distinct cognitive strategies adopted by individuals to sustain similar tasks of visual processing8. Therefore, one may speculate that EC could boost the strategic variability in visual imagination, which would favor the rich diversity of interoceptive thoughts. We encouraged future research with relevant behavior tests to confirm the hypothesis.
A potential connection between EC-enhanced IVFC and deep involvement in TUTs
An intriguing question is what causes the elevated IVFC during EC. Previous studies suggested that heterogenous TUTs predominate the EC condition54,55. Moreover, our preliminary analysis revealed that subjects with frequent TUTs exhibited the strengthened IVFC. Therefore, it is worthwhile to explore the active role of TUTs in the EC-enhanced IVFC.
This study measured each subject’s propensity for frequent TUTs in EC and EO condition, respectively. We found that this metric exhibited a significant increase from the EO to EC condition. Furthermore, the metric significantly correlated with IVFC during the EC, which is, however, not the case during EO condition (Fig. 4). Therefore, our results suggested that the prevalent TUTs during EC condition may serve as a potential cause for the enhanced IVFC. These results are consistent with the proposal that the “freely moving thoughts” is a possible source of FC variability34,56. Indeed, the TUTs are rarely constrained by a fixed topic but largely related to personal concerns17,35,57, which gives plenty of room for the person-specific neural processes58,59. Moreover, the heterogenous construct of TUTs was underpinned by the integration of multi-sensory information60. Thus, we speculated that the EC, with reduced constraint from external input and promoted multi-sensory activity, offers good opportunity for the occurrence of TUTs to enhance the individual difference of brain function. We encouraged future research to pay more attention to the active role of TUTs during the EC condition.
Conclusion
This study used distinct datasets to demonstrate that the EC could enhance the individual difference of functional network. Moreover, such enhancement was discovered to focus on selective subsets of FCs, with predominant connections to the default-mode, dorsal attention, and visual networks. Finally, a potential link was observed between the EC-enhanced functional variability and deep involvement in TUTs. Collectively, our results suggested that enhanced individual difference characterize the functional network of volitional EC. This work has implications for a better understanding of the neural mechanisms that underlies the introspective mental state.
Data availability
Both the EC/EO (https://www.nitrc.org/projects/eceo_rsfmri_9) and mind wandering (https://osf.io/43dp5) datasets are openly available. The scripts for preprocessing EC/EO dataset could be accessed at https://github.com/lufee10/myPrep.
References
Perfect, T. J., Andrade, J. & Eagan, I. Eye closure reduces the cross-modal memory impairment caused by auditory distraction. J. Exp. Psychol. Learn. Mem. Cogn. 37, 1008 (2011).
Wang, B., Ginns, P. & Mockler, N. Sequencing tracing with imagination. Educ. Psychol. Rev. 34, 421–449 (2022).
Ritter, S. M., Abbing, J. & Van Schie, H. T. Eye-closure enhances creative performance on divergent and convergent creativity tasks. Front. Psychol. 9, 1315 (2018).
Xu, P. et al. Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage 90, 246–255 (2014).
Zhang, Y. et al. Modular brain network in volitional eyes closing: enhanced integration with a marked impact on hubs. Cereb. Cortex 34, (2024).
Seitzman, B. A. et al. Trait-like variants in human functional brain networks. Proceedings of the National Academy of Sciences 116, 22851–22861 (2019).
Michon, K. J., Khammash, D., Simmonite, M., Hamlin, A. M. & Polk, T. A. Person-Specific and precision neuroimaging: current methods and future directions. Neuroimage 119589 (2022).
Seghier, M. L. & Price, C. J. Interpreting and utilising intersubject variability in brain function. Trends Cogn. Sci. 22, 517–530 (2018).
Shen, X. et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 12, 506–518 (2017).
Dubois, J. & Adolphs, R. Building a science of individual differences from fMRI. Trends Cogn. Sci. 20, 425–443 (2016).
Mueller, S. et al. Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595 (2013).
Ren, J. et al. Individual variability in functional organization of the human and monkey auditory cortex. Cereb. Cortex. 31, 2450–2465 (2021).
Finn, E. S. et al. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage 160, 140–151 (2017).
Marx, E. et al. Eye closure in darkness animates sensory systems. Neuroimage 19, 924–934 (2003).
Terasawa, Y., Fukushima, H. & Umeda, S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Hum. Brain Mapp. 34, 598–612 (2013).
Seli, P. et al. Mind-wandering as a natural kind: A family-resemblances view. Trends Cogn. Sci. 22, 479–490 (2018).
Fox, K. C. R., Nijeboer, S., Solomonova, E., Domhoff, G. W. & Christoff, K. Dreaming as Mind wandering: evidence from functional neuroimaging and first-person content reports. Front. Hum. Neurosci. 7, 412 (2013).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N Y Acad. Sci. 1316, 29–52 (2014).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience vol. 20 593–608 Preprint at (2019). https://doi.org/10.1038/s41583-019-0212-7
Haynes, J. D. & Rees, G. Decoding mental States from brain activity in humans. Nat. Rev. Neurosci. 7, 523–534 (2006).
Weng, Y. et al. Open eyes and closed eyes elicit different Temporal properties of brain functional networks. Neuroimage 222, 117230 (2020).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 28, 3095–3114 (2018).
Zhang, X. & Zang, Z. Evaluate the efficacy and reliability of functional gradients in within-subject designs. Hum. Brain Mapp. 44, 2336–2344 (2023).
Yue, J. et al. Higher reliability and validity of Wavelet-ALFF of resting-state fMRI: from multicenter database and application to rTMS modulation. Hum. Brain Mapp. 44, 1105–1117 (2023).
Groot, J. M. et al. Probing the neural signature of Mind wandering with simultaneous fMRI-EEG and pupillometry. Neuroimage 224, 117412 (2021).
Saad, Z. S. & Reynolds, R. C. Suma Neuroimage 62, 768–773 (2012).
Jo, H. J., Saad, Z. S., Simmons, W. K., Milbury, L. A. & Cox, R. W. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52, 571–582 (2010).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 (2007).
Gorgolewski, K. et al. and Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform 5, (2011).
Zhang, Y. et al. Enhanced intersubject similarity in functional connectivity by long-term abacus training. Cereb. Cortex. 33, 8633–8644 (2023).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015).
Yeo, B. T. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. (2011).
Lebreton, M., Bavard, S., Daunizeau, J. & Palminteri, S. Assessing inter-individual differences with task-related functional neuroimaging. Nat. Hum. Behav. 3, 897–905 (2019).
Kam, J. W. Y. et al. Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. Proceedings of the National Academy of Sciences 118, e2011796118 (2021).
Wang, H. T. et al. Dimensions of experience: exploring the heterogeneity of the wandering Mind. Psychol. Sci. 29, 56–71 (2018).
Cui, Z. et al. Individual variation in functional topography of association networks in youth. Neuron 106, 340–353e8 (2020).
Zhuang, K. et al. Connectome-based evidence for creative thinking as an emergent property of ordinary cognitive operations. Neuroimage 227, 117632 (2021).
Yeshurun, Y., Nguyen, M. & Hasson, U. The default mode network: where the idiosyncratic self Meets the shared social world. Nat. Rev. Neurosci. 22, 181–192 (2021).
Sun, X. et al. Disrupted intersubject variability architecture in functional connectomes in schizophrenia. Schizophr Bull. 47, 837–848 (2021).
Andrews-Hanna, J. R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270 (2012).
Norman, L. J., Sudre, G., Price, J., Shastri, G. G. & Shaw, P. Evidence from big data for the default-mode hypothesis of ADHD: a mega-analysis of multiple large samples. Neuropsychopharmacology 48, 281–289 (2023).
Rashidi-Ranjbar, N. et al. Association of functional connectivity of the executive control network or default mode network with cognitive impairment in older adults with remitted major depressive disorder or mild cognitive impairment. Neuropsychopharmacology 48, 468–477 (2023).
Sprague, T. C. & Serences, J. T. Attention modulates Spatial priority maps in the human occipital, parietal and frontal cortices. Nat. Neurosci. 16, 1879–1887 (2013).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Riedl, V. et al. Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. J. Neurosci. 34, 6260–6266 (2014).
Dixon, M. L. et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences 115, E1598–E1607 (2018).
Dixon, M. L. et al. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage 147, 632–649 (2017).
Anderson, J. S., Ferguson, M. A. & Lopez-Larson, M. Yurgelun-Todd, D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 1, 147–157 (2011).
Dixon, M. L., Fox, K. C. R. & Christoff, K. A framework for Understanding the relationship between externally and internally directed cognition. Neuropsychologia 62, 321–330 (2014).
Corbetta, M., Patel, G. & Shulman, G. L. The reorienting system of the human brain: from environment to theory of Mind. Neuron 58, 306–324 (2008).
Grill-Spector, K. & Malach, R. The human visual cortex. Annu. Rev. Neurosci. 27, 649–677 (2004).
Takemura, H. Investigating human visual cortex variability. Nat. Rev. Neurosci. 24, 270 (2023).
Hasnain, M. K., Fox, P. T. & Woldorff, M. G. Intersubject variability of functional areas in the human visual cortex. Hum. Brain Mapp. 6, 301–315 (1998).
Faber, M., Krasich, K., Bixler, R. E., Brockmole, J. R. & D’Mello, S. K. The eye–mind wandering link: identifying gaze indices of Mind wandering across tasks. J. Exp. Psychol. Hum. Percept. Perform. 46, 1201 (2020).
Smilek, D., Carriere, J. S. A. & Cheyne, J. A. Out of Mind, out of sight: eye blinking as indicator and embodiment of Mind wandering. Psychol. Sci. 21, 786–789 (2010).
Elton, A. & Gao, W. Task-related modulation of functional connectivity variability and its behavioral correlations. Hum. Brain Mapp. 36, 3260–3272 (2015).
Andrews-Hanna, J. R. et al. A Penny for your thoughts: dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Front. Psychol. 4, 900 (2013).
van der Heiden, L., Scherpiet, S., Konicar, L., Birbaumer, N. & Veit, R. Inter-individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: an fMRI study. Neuroimage 65, 387–394 (2013).
Ochsner, K. N. & Feldman Barrett, L. A multiprocess perspective on the neuroscience of emotion. Emotion: Curr. Issues Future Dir. 38–81 (2001).
Hung, S. M. & Hsieh, P. J. Mind wandering in sensory cortices. Neuroimage: Rep. 2, 100073 (2022).
Acknowledgements
We thank the editors and reviewers for their suggestions to improve the manuscript.
Funding
This work was supported by the Health and Medical Science and Technology Plan Project in Ningbo City (No. 2024Y19).
Author information
Authors and Affiliations
Contributions
Yi Zhang and Xin Shi designed the study; Yi Zhang, Guiyang Lv and Panpan Han analyzed the data; Yi Zhang, Guiyang Lv, Nianqiang Peng and Longhui Li wrote the first draft of the paper; Yi Zhang, Xin Shi, Guiyang Lv, Panpan Han, Nianqiang Peng, Longhui Li, Yue Liu and Jun Gu edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Lv, G., Han, P. et al. Enhanced individual difference of functional brain network induced by volitional eyes closing. Sci Rep 15, 13037 (2025). https://doi.org/10.1038/s41598-025-97621-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97621-z