Abstract
Thrombocytopenia is one of the side effects of VPA. This study aimed to evaluate the incidence and risk factors of thrombocytopenia after intravenous VPA treatment in children with neurosurgical operations. Pediatric patients undergoing neurosurgical operations treated with intravenous VPA were enrolled in this retrospective study. According to platelet count after intravenous injection of VPA, the pediatric patients were divided into the thrombocytopenia group and the non-thrombocytopenia group. Binary logistic regression analysis was used to explore the risk factors for thrombocytopenia. A total of 252 children with neurosurgical operations were included in this study, and the incidence of thrombocytopenia was 12.3% (31/252). Univariate analysis showed that baseline platelet count, duration of VPA therapy, and blood loss were associated with the occurrence of thrombocytopenia after intravenous administration of VPA. Binary logistic regression revealed that baseline platelet count (OR 0.995, 95% CI 0.991–0.999) and blood loss (OR 0.995, 95% CI 0.991–0.999) were independent risk factors for thrombocytopenia after intravenous VPA in children undergoing neurosurgical operations. Our data show that thrombocytopenia is common in neurosurgical operations children treated with intravenous VPA, and that baseline platelet count is an independent risk factor for thrombocytopenia. Regular monitoring of baseline platelet count is important for whether to short-term prophylactic use intravenous VPA in children undergoing neurosurgical operations.
Similar content being viewed by others
Introduction
The incidence of perioperative seizures during neurosurgical operations is 0.5–50%1,2,3, and seizures often lead to serious complications including the increased cerebral metabolic rate of oxygen (CMRO2), increased intracranial pressure, delayed emergence from anesthesia, and disturbance of the surgical field, especially in pediatric patients1. Previous study4 has found that early and late post-neurosurgical status epilepticus were associated with elevated inpatient mortality. Therefore, to limit or prevent secondary neurological damage, seizures must be treated immediately. Currently, whether antiepileptic drugs should be used routinely for the perioperative period of neurosurgical operations remains controversial. However, actual patterns of postoperative seizure prophylaxis highly depend on the experience of surgeons. It is reported that between 63 and 81% of US neurosurgeons prescribe anti-epilepsy drugs (AEDs) postoperatively to seizure patients with brain tumors5.
Valproic acid (VPA) is a widely used AED for prophylaxis during the perioperative period to prevent seizure in pediatric patients undergoing neurosurgical operations6,7. However, perioperative intravenous VPA can trigger thrombocytopenia, which can lead to an increased risk of bleeding in surgical patients8,9. Researchers found that thrombocytopenia occurs rather frequently, in nearly 40% of patients with intravenous VPA therapy during the neurological intensive care unit (NCU) stay10. Therefore, it is necessary to investigate the relationship between VPA and thrombocytopenia in surgical patients.
Although thrombocytopenia has been reported in some studies, the exact relationship between thrombocytopenia and intravenous VPA in adult patients is controversial, and data are lacking in pediatric patients9,11,12. The purpose of this study was to determine the incidence and risk factors for thrombocytopenia after prophylactically intravenous VPA in pediatric patients undergoing neurosurgical operations.
Method
Study design
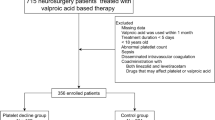
Pediatric patients who underwent neurosurgical operations and were treated with intravenous VPA in the ward between September 2017 and April 2020 at Wuhan Children’s Hospital were enrolled in the present study. Patients were included in our research if they had surgery for (1) intractable epilepsy; (2) Intracranial space-occupying; (3) lesions; (4) Hydrocephalus; (5) traumatic brain injury (TBI) (6) Intracranial hemorrhage (ICH) and subarachnoid hemorrhage (SAH); (7) other neurosurgical procedures. We excluded patients who (1) had thrombocytopenia (platelet count < 150 × 109/L) at the time of admission; (2) were diagnosed with any underlying diseases; (3) were lacking complete data; (4) were diagnosed or interventions considered strongly associated with thrombocytopenia, such as abnormal immune function or use of immunosuppressive agents; (5) Patients who may have developed thrombocytopenia prior to their operation (refers to individuals with pre-existing low platelet counts (due to chronic conditions such as immune thrombocytopenia, liver disease, chemotherapy, or autoimmune disorders) unrelated to the surgical intervention itself) were also excluded. The protocol was conducted under the Declaration of Helsinki and its later amendments or comparable ethical standards and was approved by the Ethics Committee of Wuhan Children’s Hospital of Huazhong University of Science and Technology in China (Serial number: 2015015), and due to the retrospective nature of the study, the need for informed consent was waived by the Ethics Committee of Wuhan Children’s Hospital of Huazhong University of Science and Technology.
The demographic data, laboratory results, concomitant medications, blood loss, and intravenous VPA dosage were extracted from the electronic medical records. Furthermore, the data were processed to discover the total daily dose of VPA, duration of VPA therapy, and VPA dose adjusted by body weight.
A platelet count below 150 × 109/L was defined as thrombocytopenia. Patients typically started VPA on 0–3 days prior to the operation. Due to the short average lifespan of platelets (8–9 days) and the focal point for the study being the development of thrombocytopenia associated with intravenous VPA therapy, the platelet counts from the next day after the first dose to within 3 days after the last dose of intravenous VPA were recorded. If multiple platelet count measurements were obtained during this period, the nadir was used in our study.
Statistical analysis
Data analyses were conducted using Statistical Package for Social Sciences 22.0 software (SPSS Inc., Chicago, IL). According to the distribution of continuous data, we expressed continuous data as mean and SD or median and interquartile range, and independent samples t-test or Mann–Whitney U tests were used for continuous data. The categorical data were expressed as proportions and analyzed by the Chi-square test or Fisher’s exact test.
The binary logistic regression model was used to identify the risk factors of thrombocytopenia with adjustment for potential variables and were expressed with odds ratios (ORs) and 95% confidence intervals (CIs). The variables include platelet count at admission, duration of VPA therapy, VPA use of more than 3 days, concomitant drugs that may cause thrombocytopenia (include ceftazidime, linezolid, vancomycin, ceftriaxone, ganciclovir, levetiracetam, diazepam, phenobarbital, lamotrigine, clonazepam, carbamazepine, oxcarbazepine, nitrazepam, midazolam, topiramate and risperidone), blood transfusion, and blood loss. A P value < 0.05 was considered statistically significant. The Bonferroni correction method was used for post hoc tests.
Results
Patients’ characteristics
A total of 252 pediatric patients with intravenous VPA were included, and their characteristics were summarized in Table 1. Finally, 12.3% (31/252) of pediatric patients experienced thrombocytopenia after intravenous VPA and 87.7% (221/252) did not. The most common reason for intravenous VPA therapy was seizure prevention after intracranial space-occupying lesions. Univariate analysis of the potential risk factors associated with thrombocytopenia showed that baseline platelet count, duration of VPA therapy, and blood loss were significantly associated with the development of thrombocytopenia. It is noteworthy that there was a trend for pediatric patients to develop thrombocytopenia when VPA was used beyond 3 days, although the difference was not significant. No statistical difference was found between pediatric patients with thrombocytopenia and those without thrombocytopenia regarding their gender, age, body weight, hospital stay, total daily dose of VPA, VPA dose adjusted by body weight, previous exposure to VPA, blood transfusion, use of ventilator, infection, and cause of therapy.
Risk factors for thrombocytopenia
The risk factors significantly influenced the development of thrombocytopenia through univariate analysis were analyzed using binary logistic regression analysis. In addition, given the tendency to develop thrombocytopenia in children, the concomitant drugs and VPA use for more than 3 days and blood transfusion were also analyzed as variables. As shown in Table 2, baseline platelet count (OR 0.995, 95% CI [0.991–0.999]) and blood loss (OR 0.995, 95% CI [0.991–0.999]) were found as independent risk factors for the development of thrombocytopenia. Nevertheless, the difference was no longer significant after Bonferroni correction (Pcorrected = 0.008).
Discussion
It is well known that VPA is closely associated with the development of thrombocytopenia. In this study, we estimated the potential risk factors of thrombocytopenia after intravenous VPA therapy in pediatric patients undergoing neurosurgical operations. We identify that lower baseline platelet count, duration of VPA therapy, and blood loss have significant associations with thrombocytopenia in pediatric patients. Meanwhile, in binary logistic regression analysis, we found that baseline platelet count and blood loss were independent risk factors for thrombocytopenia in pediatrics undergoing neurosurgical operations. To the best of our knowledge, this study is the first to evaluate the incidence and risk factors for thrombocytopenia after VPA injection in pediatric patients undergoing neurosurgical operations.
Thrombocytopenia is one of the most studied side effects of VPA, which had an incidence range from 3 to 40% in previous study13. Dong found that thrombocytopenia occurs in nearly 40% of patients with intravenous VPA therapy during the neurological intensive care unit stay10. Similarly, an RCT study with a sample size of 306 pediatric patients showed 64 (21%) children suffering thrombocytopenia after taking VPA14. The rate of thrombocytopenia previously reported in the study was higher than in our research. In our study, we found the incidence of thrombocytopenia is 12.3% in pediatric patients with intravenous VPA therapy. This discrepancy might be partly attributed to age and gender which some studies suggested thrombocytopenia is more frequent in women and elderly patients11,15,16,17,18. Moreover, the latest retrospective cohort study with 124 pediatric patients reported a 2.4% prevalence of thrombocytopenia after being treated with VPA, which is similar to our results19.
Previous studies have explored the association between VPA and thrombocytopenia, however, studies in patients unveiled different observations. A prospective study consisting of 24 pediatric patients put forward that VPA significantly decreased platelet count20, alternatively, research enrolled 87 pediatric patients reported that concurrent VPA showed a high incidence of thrombocytopenia compared to those without VPA21. On the contrary, a study in Italy implies that no statistically significant differences in platelet count between patients with VPA and those without VPA22. Some parallel results were obtained, and a study in the USA suggested that VPA therapy in pediatric patients demonstrates comparable incidence regarding thrombocytopenia across a range of therapeutic serum concentrations of 50–120 ug/mL19. In accordance with these results, our research failed to find a significant difference in daily VPA dose between pediatric patients with and without thrombocytopenia. A reason might be that our study only analyzed platelet change within 3 days after the last dose of intravenous VPA, whereas the study reported it takes 1 month to decrease platelet20.
Many studies noted that VPA level is strongly associated with the development of thrombocytopenia in pediatric patients13. Because of the study design, data of VPA plasma concentration were not monitored in our research. However, previous study supposed that a longer duration of VPA might be associated with higher blood levels, which results in the incidence of thrombocytopenia increasing10. Meanwhile, a study enrolled 20 patients with chronic administration of VPA reported 3 patients exceeding the upper limit of the therapeutic range22. Furthermore, a prospective systematic study including 24 newly diagnosed epileptic children treated with VPA suggested that VPA caused decreased platelet count during 1 month of therapy20. In our study, univariate analysis showed a significant association between the duration of VPA and thrombocytopenia in pediatrics undergoing neurosurgical operations. Unfortunately, our study did not identify the duration of VPA as an independent risk factor for thrombocytopenia in pediatrics undergoing neurosurgical operations. This could be attributed to differences in the population structures between our study and others.
Apart from VPA, several risk factors of thrombocytopenia in children were identified in our study, including baseline platelet count, duration of VPA therapy, VPA use of more than 3 days, concomitant medications, blood transfusion, and blood loss. Baseline platelet count has a significant influence on the development of thrombocytopenia is not novel. Our study indicates that baseline platelet counts were independent risk factors for the development of thrombocytopenia, which is in accordance with previous findings19,23.
In the current study, our analysis of deposited data from pediatric patients undergoing neurosurgical operations shows that blood loss was significantly higher in the non-thrombocytopenia group as compared to the thrombocytopenia group. It appears that thrombocytopenia is not caused by bleeding, which may be explained by several reasons: (1) Platelets returned to normal levels 48–72 h after bleeding, while our study recorded the platelet count within 3 days after the last dose of intravenous VPA; (2) Bleeding leads to an increase in thrombopoietin (TPO) levels, which stimulates the production of platelets; (3) A retrospective analysis did not find any donors with clinical thrombocytopenia among 939 regular donors who underwent 11,464 procedures24. In addition, platelet counts were calculated by estimating the number of platelets per microliter from a blood cell smear, which remained unaffected by blood loss25. Furthermore, the researchers enrolled 33 regular repeat platelet apheresis donors in a study and followed up to 12 months for their hematological, biochemical, and immunological parameters showed no significant difference from the baseline which was following our results26.
Previous studies suggested that VPA was an influential factor associated with an increased risk of coagulation dysfunction9,27. However, our results indicated that patients treated with short-term prophylactic VPA developed thrombocytopenia more related to the baseline platelet count other than VPA dosage and duration of VPA therapy. It is suggested that neurosurgeons should not be overly concerned about the occurrence of thrombocytopenia when using VPA to prevent epilepsy in the short term after surgery, especially for patients with normal basal platelet levels.
It is worth noting that the associations between the baseline platelet count or blood loss and thrombocytopenia could not pass the strict Bonferroni’s correction. However, Bonferroni’s correction is very strict and conservative, our study could provide an incentive for multicenter and larger population studies to validate the findings. Therefore, unadjusted P values were used.
In future studies, we plan to focus more on the medical burden associated with short-term usage of VPA. We will further collect and analyze the data, on whether adverse reactions caused by short-term VPA usage need drug intervention, or platelet transfusion, or cause bleeding and other related complications and prolong the hospital stay and other medical costs. This will provide more guidance and clinical evidence for neurosurgeons to use VPA after surgery.
Limitation
It should be noted that VPA plasma concentrations were not collected in pediatric patients due to the retrospective character of this study. Therefore, the influences of VPA plasma concentration on thrombocytopenia were neglected in this study, which may weaken the accuracy of the results. Future studies will focus on multicenter and large cohort studies to clarify the effect of VPA plasma concentration on thrombocytopenia in pediatric patients undergoing neurosurgical operations. Infection and severity of illness are often associated with thrombocytopenia28,29,30 and may be confounders of thrombocytopenia, however, in-depth analyses were not conducted which represents another limitation. Despite these limitations, our study provides a reference for using VPA in children’s surgery.
Conclusion
Our research suggested that baseline platelet count and duration of VPA therapy have significant associations with the development of thrombocytopenia, and baseline platelet count was an independent risk factor of thrombocytopenia in pediatric patients undergoing neurosurgical operations. Surgeons may have reduced concerns about thrombocytopenia when using intravenous VPA as a short-term prophylactic measure in pediatric patients undergoing neurosurgical procedures. Additional studies involving larger groups of participants are necessary to confirm the findings.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kutteruf, R. et al. Incidence and risk factors for intraoperative seizures during elective craniotomy. J. Neurosurg. Anesthesiol. 31, 234–240. https://doi.org/10.1097/ANA.0000000000000506 (2019).
Wong, J. M. et al. Patterns in neurosurgical adverse events: Open cerebrovascular neurosurgery. Neurosurg. Focus 33, E15. https://doi.org/10.3171/2012.7.FOCUS12181 (2012).
Howe, J., Lu, X., Thompson, Z., Peterson, G. W. & Losey, T. E. Intraoperative seizures during craniotomy under general anesthesia. Seizure 38, 23–25. https://doi.org/10.1016/j.seizure.2016.03.010 (2016).
Jin, M. C. et al. Status epilepticus after intracranial neurosurgery: Incidence and risk stratification by perioperative clinical features. J. Neurosurg. 135, 1752–1764. https://doi.org/10.3171/2020.10.Jns202895 (2021).
Youngerman, B. E. et al. Patterns of seizure prophylaxis after oncologic neurosurgery. J. Neurooncol. 146, 171–180. https://doi.org/10.1007/s11060-019-03362-1 (2019).
Cai, Q. et al. Preoperative antiepileptic drug prophylaxis for early postoperative seizures in supratentorial meningioma: A single-center experience. J. Neurooncol. 158, 59–67. https://doi.org/10.1007/s11060-022-04009-4 (2022).
Sayegh, E. T., Fakurnejad, S., Oh, T., Bloch, O. & Parsa, A. T. Anticonvulsant prophylaxis for brain tumor surgery: Determining the current best available evidence. J. Neurosurg. 121, 1139–1147. https://doi.org/10.3171/2014.7.JNS132829 (2014).
Gerstner, T. et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia 47, 1136–1143. https://doi.org/10.1111/j.1528-1167.2006.00587.x (2006).
Arruda, V. R. et al. Assessment of need for hemostatic evaluation in patients taking valproic acid: A retrospective cross-sectional study. PLoS ONE 17, e0264351. https://doi.org/10.1371/journal.pone.0264351 (2022).
Kim, D. W., Kim, W., Lee, C. H. & Chun, Y. I. Thrombocytopenia during intravenous valproic acid therapy in the neurological intensive care unit. J. Clin. Pharm. Ther. 45, 1014–1020. https://doi.org/10.1111/jcpt.13125 (2020).
Nasreddine, W. et al. Predicting the occurrence of thrombocytopenia from free valproate levels: A prospective study. Seizure 94, 33–38. https://doi.org/10.1016/j.seizure.2021.11.018 (2022).
Kurahashi, H., Takami, A., Murotani, K., Numoto, S. & Okumura, A. Decreased platelet count in children with epilepsy treated with valproate and its relationship to the immature platelet fraction. Int. J. Hematol. 107, 105–111. https://doi.org/10.1007/s12185-017-2323-0 (2018).
Kumar, R., Vidaurre, J. & Gedela, S. Valproic acid-induced coagulopathy. Pediatr. Neurol. 98, 25–30. https://doi.org/10.1016/j.pediatrneurol.2019.04.019 (2019).
Delgado, M. R., Riela, A. R., Mills, J., Browne, R. & Roach, E. S. Thrombocytopenia secondary to high valproate levels in children with epilepsy. J. Child Neurol. 9, 311–314. https://doi.org/10.1177/088307389400900318 (1994).
Trannel, T. J., Ahmed, I. & Goebert, D. Occurrence of thrombocytopenia in psychiatric patients taking valproate. Am. J. Psychiatry 158, 128–130. https://doi.org/10.1176/appi.ajp.158.1.128 (2001).
Conley, E. L. et al. Prevalence and risk of thrombocytopenia with valproic acid: Experience at a psychiatric teaching hospital. Pharmacotherapy 21, 1325–1330. https://doi.org/10.1592/phco.21.17.1325.34418 (2001).
Nasreddine, W. & Beydoun, A. Valproate-induced thrombocytopenia: A prospective monotherapy study. Epilepsia 49, 438–445. https://doi.org/10.1111/j.1528-1167.2007.01429.x (2008).
Buoli, M., Serati, M., Botturi, A. & Altamura, A. C. The risk of thrombocytopenia during valproic acid therapy: A critical summary of available clinical data. Drugs R&D 18, 1–5. https://doi.org/10.1007/s40268-017-0224-6 (2017).
Young, M. R., Bisaccia, E. K., Romantseva, L. & Hovey, S. W. Valproic acid serum concentration and incidence of toxicity in pediatric patients. J. Child Neurol. 37, 461–470. https://doi.org/10.1177/08830738221083480 (2022).
Kose, G. et al. Valproate-associated coagulopathies in children during short-term treatment. J. Child Neurol. 24, 1493–1498. https://doi.org/10.1177/0883073808331084 (2009).
McNamara, N. A. et al. Thrombocytopenia in pediatric patients on concurrent cannabidiol and valproic acid. Epilepsia 61, e85–e89. https://doi.org/10.1111/epi.16596 (2020).
Zighetti, M. L. et al. Effects of chronic administration of valproic acid to epileptic patients on coagulation tests and primary hemostasis. Epilepsia 56, e49-52. https://doi.org/10.1111/epi.12952 (2015).
Tseng, Y. J. et al. Safety range of free valproic acid serum concentration in adult patients. PLoS ONE 15, e0238201. https://doi.org/10.1371/journal.pone.0238201 (2020).
Lazarus, E. F., Browning, J., Norman, J., Oblitas, J. & Leitman, S. F. Sustained decreases in platelet count associated with multiple, regular plateletpheresis donations. Transfusion 41, 756–761. https://doi.org/10.1046/j.1537-2995.2001.41060756.x (2002).
Guo, P. et al. Evaluation of artificial intelligence-assisted morphological analysis for platelet count estimation. Int. J. Lab. Hematol. 46, 1012–1020. https://doi.org/10.1111/ijlh.14345 (2024).
Hans, R. et al. Serial analysis of hematological, biochemical, and immunological parameters alterations in regular healthy voluntary donors during plateletpheresis donation. Asian J. Transfus. Sci. 17, 157–163. https://doi.org/10.4103/ajts.ajts_119_22 (2023).
Zhu, R. et al. The prevalence and risk factors of coagulopathy in pediatric patients undergoing surgery for epilepsy. J. Neurosurg. Pediatr. 32, 1–8. https://doi.org/10.3171/2023.6.Peds23196 (2023).
Zhang, R. et al. Relationship between thrombocytopenia and prognosis in children with septic shock: A retrospective cohort study. Platelets 35, 2363242. https://doi.org/10.1080/09537104.2024.2363242 (2024).
Schupp, T. et al. Diagnostic and prognostic role of platelets in patients with sepsis and septic shock. Platelets 34, 2131753. https://doi.org/10.1080/09537104.2022.2131753 (2022).
dos Santos Medeiros, S. M. et al. Predictive biomarkers of mortality in patients with severe COVID-19 hospitalized in intensive care unit. Front. Immunol. 15, 1416715. https://doi.org/10.3389/fimmu.2024.1416715 (2024).
Author information
Authors and Affiliations
Contributions
Zhaosong Du and Ying Li participated in the research design. Zhaosong Du and Maochang Liu performed the data analysis and drafted the manuscript. Jun Wang and Gang Nie collected the data in the electronic medical record.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, Z., Wang, J., Nie, G. et al. Risk factors for thrombocytopenia associated with intravenous valproic acid therapy in pediatric patients undergoing neurosurgical operations. Sci Rep 15, 13675 (2025). https://doi.org/10.1038/s41598-025-98870-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98870-8