Abstract
Ground-nesting birds on islands are particularly vulnerable to the introduction of terrestrial carnivores because the former often lack defensive behaviors, displaying high levels of naivety under absence of co-evolutionary history. Relatively few studies have addressed such potentially adaptive responses. In this study, we investigated whether two bird species, upland geese (Chloephaga picta) and flightless steamer ducks (Tachyeres pteneres) have modified their nesting strategies as a response to the novel predatory pressure imposed by the invasive American mink (Neogale vison) on Navarino Island, southernmost Chile, since its arrival in 2001. We used discriminant analysis and pairwise Wilcoxon tests to compare two data sets (n = 140 nests in total) regarding the macro- and microhabitat where nests were placed, separated by a time span of 15 years. We found that upland geese currently nest in less dense habitat (macrohabitat), hide their nests in shorter shrubs with lower top and side cover (microhabitat), and breed later in the season. In contrast, flightless steamer ducks retain almost the same nesting habitat characteristics. We discuss our findings in the context of ecological and evolutionary restrictions to adaptation.
Similar content being viewed by others
Introduction
Invasive terrestrial carnivores are of great conservation concern because they can modify an entire ecosystem through predation1. They are the leading cause of extinction or endangerment of birds, mammals, and reptiles, especially on islands2. The frequent lack of competitors or predators in insular environments often result in invasive terrestrial carnivores rapidly achieving high population densities1,3. On the other hand, native prey species on islands often lack co-evolutionary history with terrestrial predators4, which may preclude the rise of defensive traits against them1,5,6. The resulting naivety seems to be the most likely explanation for the extreme impacts on native prey by introduced predators on islands, compared to neighboring continental settings7.
Ground-nesting birds are generally more exposed to terrestrial predators than species nesting on trees or cliffs, and particularly vulnerable during the incubation and fledgling periods when the adults constitute easy prey due to their reduced mobility8. Thus, the presence of a novel predator can lead to breeding failure9,10 and mortality among adult birds during incubation11. Consequently, predator avoidance or defensive behaviors during incubation may evolve as reproductive strategies12,13,14. A common predator avoidance strategy involves multiple breeding attempts in several locations within and between seasons, given that predators might return to nesting sites they have discovered13. Other strategies include egg crypsis, an innate predator response where birds lay eggs that resemble the colors and textures of their environment unspecific to the predator appearence15, and nest concealment16, which varies depending on whether predators are raptors preying from above, or terrestrial predators scanning the ground17. Nesting birds responding to avian predators that use visual cues to find prey while flying tend to place their nests inside dense vegetation18. However, this strategy may be less effective for hiding from terrestrial predators that often use olfactory cues to find their prey because dense vegetation may obstruct escaping routes19,20. Also, heavily concealed nests may reduce the chance of detecting an approaching terrestrial predator, resulting in an increased risk of predation while the bird is sitting on the nest16. Therefore, concealing the nest in less dense vegetation may be a good compromise for responding to aerial and terrestrial predators simultaneously (e.g., Gómez-Silva et al.21).
Regardless of the concealment strategy, an antipredator response to novel predation pressures requires time to evolve16. Often, prey species fail to adapt to sudden increases of novel predators and become locally extirpated2. The outstanding learning abilities of many birds may facilitate behavioral adaptation22, particularly for species that have a co-evolutionary history with predator species similar to those introduced, i.e., their native relatives23. While some evidence suggests this (e.g., birds better adapting their nests to the return of foxes Vulpes lagopus to Iceland than to the introduction of American mink Neogale vison24), more research is needed to understand how birds are coping with the expansion of novel predators across ecosystems.
The naivety of prey to an invasive predator can be measured on an ordinal scale from 1 to 4, with: Level-1 meaning no recognition at all; Level-2, recognition of danger but adopting a wrong response; Level-3, adopting a correct response but with an ineffective outcome; and Level-4, over-responding to predation, i.e., lethal effects are reduced but at high cost from sublethal effects5,6. Studies on islands with American mink (hereafter mink) as introduced predators have shown that birds may quickly identify them as predators, but with different outcomes. For example, the Icelandic common eider (Somateria mollissima borealis) moved its nesting habitats to islands farther from the shore and less accessible to mink, avoiding predation to some extent24, or switched to larger islands to avoid mink encounters in the intertidal zones, which is relatively ineffective, i.e., Level-3 naivety24.
Navarino Island, located in the Cape Horn Biosphere Reserve (CHBR), southernmost Chile (55°S, 67°W), provides an ideal scenario to study the behavioral adaptation of ground-nesting birds to novel predatory pressures. This island lacks native terrestrial predators25, and although two species of mustelids have been recorded in the CHBR—southern river otter (Lontra provocax) and marine otter (L. felina)26—there are no records on Navarino Island25, and they do not regularly prey on birds26. Mink was first recorded on Navarino Island in 200127, and since then, it has become a successful generalist predator28,29,30,31. Schüttler et al.28 found that ground-nesting upland geese (Chloephaga picta) and flightless steamer ducks (Tachyeres pteneres) were particularly vulnerable to mink nest predation (18.2% and 52.6%, respectively) and concluded that higher levels of mink predation were associated with greater nest concealment, nests built along rocky coastlines, and earlier breeders.
Our study aims to investigate whether upland geese and flightless steamer ducks remain naive or have modified their nesting strategy after two decades of mink presence on Navarino island. We compared macro- and microhabitat breeding characteristics using nest data collected by Schüttler et al.28 in 2005–2007 and by Gómez-Silva et al.21 in 2021–2022, hereafter labeled + 5y and + 20y after mink invasion. We predicted that these two bird species should currently recognize mink as a nest predator after two decades of co-existence and should show an adaptive response, because there are four mink relatives in the distribution range of both bird species23, including lesser grison (Galicitis cuja), Patagonian weasel (Lyncodon patagonicus), southern river otter, and marine otter32. Specifically, we predicted that after 15 years: (1) The area covered by vegetation around a nest will show lower values of plant density, to improve ground predator detection16; (2) shrub height and the percentage of lateral and top cover of a nest will decrease, to provide a better view of the arrival of terrestrial predators16; (3) nests will be built preferentially on sandy versus rocky coasts, to avoid higher levels of mink presence28; and (4) nests will be built towards the end of the breeding season, when breeding success is expected to be higher28.
Methodology
Study area
We worked along the northern accessible coastline of Navarino Island, located in the Cape Horn Biosphere Reserve, Chile (55°S, 67°W) (Fig. 1). Common ecosystems present are Southern beech forests (Nothofagus spp.), Magellanic tundra (Sphagnum spp.), shrublands and grasslands (particularly along the coastlines), as well as high-Andean plant communities35. The only native predators are avian36,37: Southern crested caracara (Caracara plancus), Chilean skua (Catharacta chilensis), Kelp gull (Larus dominicanus), and Chimango caracara (Milvago chimango) are common in coastal habitats. All terrestrial predators are introduced mammals: Free-ranging domestic cats (Felis silvestris catus), domestic dogs (Canis lupus familiaris), and mink38. Among these predators, the mink is of greatest concern28,30,31 and should therefore be prone to trigger adaptive responses in prey. Although free-ranging dogs definitely prey on birds (10.2% of 59 prey occurrences in dog feces39), their predation rates on nests are less compared to mink (i.e., 18.2% preyed nests of upland geese by mink vs 2.3% by dogs, and 52.6% preyed nests of flightless steamer ducks by mink vs 10.5% by dogs28). Regarding cats, camera-trap data indicates that they are far less abundant than mink (Schüttler et al., unpublished data).
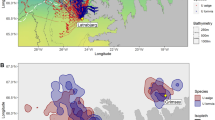
Location of nests of ground-nesting upland geese (CPI = Chloephaga picta) and flightless steamer ducks (TPT = Tachyeres pteneres) in the breeding seasons of 2005–2007 (+ 5 years after mink invasion) and 2021–2022 (+ 20 years after mink invasion), on Navarino Island, southernmost Chile. Limits of Patagonia following Jaksic and Martínez33. Blue symbols depict nests found five years after mink invasion (n = 100), orange symbols are those found 20 years after (n = 40); circles depict nests of upland geese, triangles those of flightless steamer ducks. Map created using QGIS34.
Target species
We focused our study on ground-nesting upland geese and flightless steamer ducks, from which we had previously collected nest data21,28. Upland geese occur as residents from Patagonia and Tierra del Fuego to the Malvinas (Falkland) Islands. During winter, they migrate to warmer zones, as far north as central Argentina. For breeding, they return to wet grasslands and coastal habitats where they nest relatively close to wetlands, using reeds and shrubs to conceal their nests40,41. Flightless steamer ducks have a more limited distribution, occurring strictly on the coast of Patagonia and Tierra del Fuego to the Cape Horn archipelago. They nest near rocky coasts or on islets in dense shrubland40,42.
Nest search
Our study compares nest records obtained during the breeding season (November-January) of 2005–2006 and 2006–2007 (+ 5y after mink invasion)28, with those of 2021–2022 (+ 20y after mink invasion)21. During the + 5y breeding season, Schüttler et al.28 aimed at quantifying mink predation on ground-nesting waterbirds and searched for nests by walking along seven (2005–2006) and nine (2006–2007) transects of 4 km each along the northern coastline of Navarino Island (covering 28 km in 2005–2006 and 36 km in 2006–2007), repeating visits to known nests after approximately eight days (mean = 8.2 d, SD = 1.9). During the + 20y season, Gómez-Silva et al.21 aimed for an extrapolation of breeding habitats of vulnerable waterbirds accounting for imperfect nest detection and walked along 91 transects of 500 m each, covering a total of 45.5 km along the northern coast, involving one repetition of the entire transect. Additionally, in the + 20y season, ten randomly selected islets (mean = 3 ± 2.9 ha, range 0.5–8.5 ha) adjacent to the coastline were included, yielding 11 transects (length covered on islets = 5.5 km). Despite the difference in the survey designs, both studies were based on randomized transects in overlapping coastal zones. Further details on methods can be found in21,28.
During the nest search—in both the + 5y and the + 20y breeding seasons—observers recorded indicators of nesting, such as territorial behavior (e.g., the presence of a guarding male in the case of upland geese), eggshells, or the presence of chicks as cues for the presence of nests. After finding a nest, observers recorded associated macro- and microhabitat variables following Schüttler et al.28 (Table 1). The macrohabitat assessment included measures of the dominant habitat in an area of 10 × 10 m around the nest (i.e., nest distance to the shore, habitat type, shore type, slope of the coast) and Julian date. The microhabitat assessment included measures of nest ___location directly (i.e., height of shrubs where the nest was built, side and top nest concealment). During both assessments, observers took care that breeding was not interrupted for long, working as fast as possible, and leaving the area quickly to enable female birds to return to their nests without delay21,28.
Statistical analyses
We first compared nests of upland geese on islets versus those on the main island of Navarino (+ 20y season) and found that the only significantly different variable was distance to the coastline (mean ± SD; 10.9 ± 8.05 m vs 23.5 ± 14.5 m; W = 45, p value = 0.03, n = 30). Therefore, we excluded distance from the analysis (used in Schüttler et al.28) and thus were able to pool the islets/main island data sets. For nests of flightless steamer ducks, we could not compare the habitat variables between islets and the main island because most nests were located on islets (nine vs one on Navarino island), so we pooled them. To check which macro- and microhabitat variables best separated the + 5y and + 20y nesting features of upland geese (n = 107 nests) and flightless steamer ducks (n = 33 nests; classes = scenarios), we performed a linear discriminant analysis (DA), which is a statistical method that uses eigenvalues to predict various groups in a dataset based on linear combinations of the variables. Following Schüttler et al.28, we used the “ade4” package for the R environment43. We used the dudi.mix function, which performs a multivariate analysis with mixed quantitative variables and factors. We performed a DA based on the results of a principal component analysis (PCA) to preclude information redundancy. Monte-Carlo permutation tests assayed the validation of the DA. The PCA of the ade4 package standardizes variables to zero mean and one unit variance. In parallel, we also performed univariate statistical analysis between the + 5y and + 20y variables (Table 1) for both species. Toward this, we checked for normal distribution via Shapiro tests and then performed Wilcoxon rank sum tests (also known as Mann Whitney U test) for all comparisons. The statistical analysis was conducted in R version 4.2.243; p values < 0.05 were considered significant.
Results
In the + 5y scenario, 100 nests were found in total, corresponding to 1.18 nests/km (33 nests, 28 km in 2005–2006) and 1.22 nests/km (44/36 in 2006–2007) of upland geese and 0.39 nests/km (11/28 in 2005–2006) and 0.33 nests/km (12/36 in 2006–2007) of flightless steamer ducks. In the + 20y scenario (2021–2022), a total of 40 nests were found, corresponding to 0.66 nests/km (30/45.5) of upland geese and 0.22 nests/km (10/45.5) of flightless steamer ducks.
A single discriminant analysis was performed for both scenarios and both species (CPI + 5y, CPI + 20y, TPT + 5y, TPT + 20y) based on the results of the PCA (five variables, n = 140). In the PCA, two variables revealed an Eigenvalue of > 1; the first principal component explained 36% of the variance, and the second 19%. For the first component loadings, height (0.52), side cover (0.52), and top cover (0.51) yielded loadings > |0.5|. Variable loadings of the second component > |0.5| were Julian date (0.64) and rocky shores (0.86). We excluded the variable slope due to low loadings (CS1 = 0.10) and the variable height because it was redundant with top- and side-cover variables. For the DA, three discriminant functions were generated: the Eigenvalue of the first axis was 0.58, the second was 0.15, and the third was 0.03. The discrimination of these three axes was significant using the Monte-Carlo permutation test based on 1000 permutations (p < 0.001). The centroids were well separated between the two species as they had distinct nesting habits28. Variables also discriminated between the two scenarios, being more marked for upland geese than for flightless steamer ducks (Fig. 2). The first axis was determined by shore type (± 0.55), side cover (0.67), and top cover (0.93); the second axis was determined by Julian date (-0.71) and side cover (0.55) (arrows in Fig. 2).
Results of the linear discriminant analysis of five macro- and microhabitat variables for nesting upland geese (CPI, nests depicted as circles) and flightless steamer ducks (TPT, as triangles), in two scenarios (+ 5y after mink invasion in blue and + 20y in orange, n = 140 nests) on Navarino Island, southernmost Chile. The arrows represent the relationship between canonical scores and variables; length and direction represent the variable’s discriminatory power. Variance %: F1 = 76%, F2 = 20%. The plot shows a separation between upland geese and flightless steamer ducks and also between temporal scenarios.
The Wilcoxon rank sum tests showed that for upland geese, macro- and microhabitat variables showed significant differences between side cover (2.35 ± 1.05 in the + 5y scenario vs 1.63 ± 0.86 in the + 20y scenario; W = 681, p value = 0.003), height (24.2 ± 13 cm vs 16.8 ± 15.6 cm; W = 734, p = 0.011), Julian date (19.4 ± 12.4 days vs 28.3 ± 16.3 days SD; W = 1399, p = 0.02), and type of habitat (1.75 ± 0.69 vs 1.46 ± 0.51; W = 765, p = 0.008). For flightless steamer ducks, we did not detect any significant differences between pairwise variables in either scenario; only slight trends for side cover (3.57 ± 0.79 vs 3.3 ± 0.68; W = 84, p = 0.168), slope (1.61 ± 0.72 vs 2.2 ± 1.03; W = 153, p = 0.112), and Julian date (30.4 ± 12.3 days vs 36.7 ± 9.57; W = 152, p = 0.152). Thus, between 2005 and 2021, upland geese significantly modified their nesting habitat to less dense shrubs with lower height and side cover and shifted their breeding to a later time in the breeding season (Fig. 3). In contrast, nests of flightless steamer ducks were similar between the + 5y and + 20y scenarios, with a slight modification to lower side cover, steeper coastline, and later nesting.
Violin charts for four significantly different macro- and microhabitat variables of nesting upland geese on Navarino Island, southernmost Chile, considering two temporal scenarios of mink presence. Blue dots represent nests found in the + 5y scenario and orange dots those found in the + 20y scenario. Gray dots provide mean values, the boxplot shows median and quartiles. *p values < 0.05; **p values < 0.01. Habitat ranges from 0 (= bare soil) to 4 (= forest), side cover varies from 1 to 4 indicating increase in nest concealment.
Discussion
Our study addressed whether ground-nesting waterbirds adapted their nesting strategies to an invasive predator after approximately two decades of co-existence. Indeed, for one of the two waterbirds—the upland geese—, we found significant differences in their breeding habits in the + 5y versus + 20y scenarios of mink presence, but no significant differences for flightless steamer ducks.
Tests of our four predictions
Regarding our prediction that nests would be built in habitats with lower vegetation density, our results indicate that upland geese, but not flightless steamer ducks, significantly modified their nesting habitat towards sparser patches. One could argue that the change in habitat with lower vegetation density could be due to a change in the habitat itself, with less dense vegetated areas being available for nesting nowadays. If this was the case, however, we should have observed this effect on both bird species; but flightless steamer ducks continue selecting habitats with dense vegetation (1.83 ± 0.71 in the + 5y scenario vs 2.0 ± 0.0 in the + 20y scenario; W = 125, p value = 0.58). Similarly, our prediction that vegetation concealment around the nest would be reduced was supported by data from upland geese, which changed their nesting habitat towards shrubs with lower height and less side and top cover in the immediate surroundings of the nest. For flightless steamer ducks, we detected only a modest trend to lower side cover. Apparently, upland geese adapted their nesting behavior by shifting from more protection from avian predators (higher level of nest concealment) to more protection from terrestrial predators (lower level of nest concealment), thus being able to increase their vigilance to the arrival of the new predator16. This echoes Schüttler et al.’s28 conclusion that nests of upland geese experienced lower levels of mink predation than nests of flightless steamer ducks, which have higher levels of nest concealment (10% for 23 nests vs 44% for 79 nests, respectively; see also Liljesthröm et al.44).
Further, we predicted an adaptation for nesting on sandy coasts versus rocky outcroppings, because nests on sandy coasts are more successful28 and because mink prefer heterogeneous coastal habitats45 (although they prefer flat versus rocky-steep shores in the presence of otters46). Neither bird species showed a clear preference for breeding in sandy coasts after 20 years of mink invasion; flightless steamer ducks showed evidence of a slight trend towards selecting steeper coasts. Although mink may prefer rocky outcrops, they are nonetheless habitat generalists31,46,47. Additionally, coastlines with heterogenous habitats may provide more feeding opportunities. This is particularly relevant for flightless steamer ducks, which feed mainly on marine invertebrates42,48 and might depend on such food resources during the breeding season, a period with exceptionally high energy requirements.
Finally, following Schüttler et al.28 who found later breeders to be more successful, we predicted that birds will nest more actively towards the end of the breeding season. Indeed, both bird species shifted their nesting dates towards the end of the breeding season: upland geese significantly so (i.e., 9 days later) and flightless steamer ducks as a trend (i.e., 6 days later). Climate change could also explain later nesting49. Although climate change is usually linked to earlier breeding dates49, in some species it is also linked with later laying (e.g., Ficedula hypoleuca in Laaksonen et al.50). In this regard, only long-term monitoring may help disentangle the different drivers of breeding time change51.
Differences in predatory responses between the two bird species
Our results suggest that upland geese are showing an adaptive response to mink predation while flightless steamer ducks are not. In the case of flightless steamer ducks, none of the macro- and microhabitat variables were significantly different between the + 5y and + 20y scenarios and the discriminant analysis only indicated a slight trend towards less side cover of nests and later breeding. The lack of expected adaptive response in flightless steamer ducks may owe to several reasons: (1) ecological limitations; (2) choice of a different adaptive strategy; or (3) evolutionary reasons.
With regard to ecological limitations, we speculate that flightless steamer ducks cannot respond with a macrohabitat shift to the arrival of mink because of their ecological specialization (the aforementioned dependence on marine invertebrates42). It is also possible that lowering nest concealment to achieve better visibility of terrestrial predators may generate changes in the microclimate of the nest13, potentially harmful for egg/nestling survival or the incubation ability of adults52. Therefore, flightless steamer ducks may have acquired another strategy to avoid mink predation, such as selecting predator-free habitats for breeding16. Indeed, nine of the ten nests in the + 20y scenario were found on islets21. Flightless steamer ducks have previously been described to use islets to avoid terrestrial predators in Argentinian Tierra del Fuego44,53.
Another possible explanation for the different responses between flightless steamer ducks and upland geese could be explained by evolution. The former do not migrate: they spend the entire year along a limited area of shoreline, which is defended as their territory42. Even though they are endemic to southern Chile and Patagonia and have co-evolved with terrestrial canids (e.g., foxes Lycalopex culpaeus, L. griseus) and mustelids (e.g., southern river and marine otters)26, they may lack behavioral adaptation because most islands in the Cape Horn Biosphere Reserve were predator-free before the arrival of mink, cats, and dogs38. In contrast, upland geese migrate every winter to northerly regions41, and even if they do not breed there, they likely recognize potential predators more than flightless steamer ducks, which lack that migratory experience.
Conclusion
Our study provides evidence that both species have recognized invasive mink as nest predators but with different adaptive responses. Upland geese responded with lower nest concealment at macro- and microhabitat scales, whereas flightless steamer ducks probably shifted their nests to supposedly predator-free islets. We speculate that upland geese have responded as expected but possibly with an ineffective outcome (level-3 naivety) considering that breeding success rates in both scenarios were similar (33% or 6/18 nests in Gómez-Silva et al.21 and 37% or 39/79 in Schüttler et al.28). Flightless steamer ducks, instead, may still lack a correct adaptation (level-2 naivety)5,6. Nevertheless, a higher sample size would have been desirable to achieve more reliable results for flightless steamer ducks. Despite the evidence for anti-predatory adaptation, it is not clear whether these adaptive responses will translate into higher nesting success in the short term (see Anton et al.23 who suggest that around 200 generations may be required to erode naivety). Although the survey design was different between the + 5y and + 20y breeding seasons—which could affect nest density comparison—, we think that a decreasing trend in local nest abundance has occurred (i.e., 1.18–1.22 nests/km in 2005–2007 vs 0.66 nests/km in 2021–2022 in upland geese and 0.33–0.39 nests/km vs 0.22 nests/km in flightless steamer ducks). Thus, controlling mink populations in important breeding habitats21,54 should be a conservation priority irrespective of the capacities of birds to develop adaptive responses.
Data availability
The data that supports this study are available in the Supplementary Material.
References
David, P. et al. Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol. Res. 56, 1–60 (2017).
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G. & Dickman, C. R. Invasive predators and global biodiversity loss. Proc. U. S. Natl. Acad. Sci. 113(40), 11261–11265 (2016).
MacArthur, R. H., Diamond, J. M. & Karr, J. R. Density compensation in island faunas. Ecology 53(2), 330–342 (1972).
Kier, G. et al. A global assessment of endemism and species richness across island and mainland regions. Proc. U. S. Natl. Acad. Sci. 106(23), 9322–9327 (2009).
Banks, P. B. & Dickman, C. R. Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol. Evol. 22(5), 229–230 (2007).
Carthey, A. J. & Banks, P. B. Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev. 89(4), 932–949 (2014).
Cox, J. G. & Lima, S. L. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21(12), 674–680 (2006).
Bartoszewicz, M. & Zalewski, A. American mink, Mustela vison diet and predation on waterfowl in the Słońsk Reserve, western Poland. Folia Zool. 52(3), 225–238 (2003).
Gómez-Serrano, M. Á. & López-López, P. Nest site selection by Kentish plover suggests a trade-off between nest-crypsis and predator detection strategies. PLoS ONE 9(9), e107121 (2014).
Smith, P. A., Bart, J., Lanctot, R. B., McCaffery, B. J. & Brown, S. Probability of detection of nests and implications for survey design. Condor 111(3), 414–423 (2009).
Keitt, B. S., Tershy, B. R. & Croll, D. A. Nocturnal behavior reduces predation pressure on Black-vented Shearwaters Puffinus opisthomelas. Mar. Ornithol. 32, 173–178 (2004).
Fontaine, J. J. & Martin, T. E. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol. Lett. 9(4), 428–434 (2006).
Lima, S. L. Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 84(3), 485–513 (2009).
Siegel-Causey, D. & Kharitonov, S. The evolution of coloniality. Curr. Ornithol. 7, 285–330 (1990).
Langerhans, R. Evolutionary Consequences of Predation: Avoidance, Escape, Reproduction, and Diversification (Springer, 2007).
Götmark, F., Blomqvist, D., Johansson, O. C. & Bergkvist, J. Nest site selection: A trade-off between concealment and view of the surroundings?. J. Avian Biol. 26(4), 305–312 (1995).
Vazquez, M. S. & Amico, G. C. Nest predation in Patagonian wetlands: Predator assemblage and microhabitat characteristics. Emu Austral Ornithol. 123, 24–34 (2023).
Clark, R. G. & Nudds, T. D. Habitat patch size and duck nesting success: The crucial experiments have not been performed. Wildl. Soc. Bull. 19(4), 534–543 (1991).
Guyn, K. L. & Clark, R. G. Cover characteristics and success of natural and artificial duck nests. J. Field Ornithol. 68(1), 33–41 (1997).
Hughes, N. K., Price, C. J. & Banks, P. B. Predators are attracted to the olfactory signals of prey. PLoS ONE 5(9), e13114 (2010).
Gómez-Silva, V., Crego, R. D., Jaksic, F. M., Flores-Brenner, G. & Schüttler, E. Understanding ground-nesting habitat selection by waterbirds to prioritize invasive predator control on islands. Basic Appl. Ecol. 78, 14–22 (2024).
Ruland, F. & Jeschke, J. M. How biological invasions affect animal behaviour: A global, cross-taxonomic analysis. J. Anim. Ecol. 89(11), 2531–2541 (2020).
Anton, A., Geraldi, N. R., Ricciardi, A. & Dick, J. T. Global determinants of prey naiveté to exotic predators. Proc. R. Soc. B 287(1928), 20192978 (2020).
Jónsson, J. E., Rickowski, F. S., Ruland, F., Ásgeirsson, Á. & Jeschke, J. M. Long-term data reveal contrasting impacts of native versus invasive nest predators in Iceland. Ecol. Lett. 26(12), 2066–2076 (2023).
Anderson, C. B. et al. Exotic vertebrate fauna in the remote and pristine sub-Antarctic Cape Horn Archipelago, Chile. Biodivers. Conserv. 15, 3295–3313 (2006).
Muñoz-Pedreros, A.H. & Yáñez-Valenzuela, J.L. Mamíferos de Chile (CEA Eds., 2009).
Rozzi, R. & Sherriffs, M. E. visón (Mustela vison Schreber, Carnivora: Mustelidae), un nuevo mamífero exótico para la Isla Navarino. Anales del Instituto de la Patagonia (Chile) 31, 97–104 (2003).
Schüttler, E., Klenke, R., McGehee, S., Rozzi, R. & Jax, K. Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol. Conserv. 142(7), 1450–1460 (2009).
Jiménez, J. E. et al. Potential impact of the alien American mink (Neovison vison) on Magellanic woodpeckers (Campephilus magellanicus) in Navarino Island, southern Chile. Biol. Invasions 16, 961–966 (2014).
Schüttler, E., Carcamo, J. & Rozzi, R. Diet of the American mink Mustela vison and its potential impact on the native fauna of Navarino Island, Cape Horn Biosphere Reserve, Chile. Revista Chilena de Historia Nat. 81(4), 585–598 (2008).
Fasola, L., Zucolillo, P., Roesler, I. & Cabello, J.L. Carnívoro Exótico: el Caso del Visón Americano (Neovison vison) en América del Sur (CAPES-UC Eds., 2021).
Iriarte, A. & Jaksic, F.M. Los Carnívoros de Chile (Santiago, Chile: CAPES/Flora & Fauna, 2022).
Jaksic, F. & Martínez, D. Historical account and current knowledge of the southernmost Chiropterofauna in the world: The Magellanic/Fuegian bats. Stud. Neotrop. Fauna Environ. 59(3), 842–853 (2023).
QGIS.org. QGIS Geographic Information System. Open-Source Geospatial Foundation Project (2021). http://qgis.org.
Pisano-Valdés, E. Fitogeografía de Fuego-Patagonia chilena. I.-Comunidades vegetales entre las latitudes 52 y 56º S. Anales del Instituto de la Patagonia 8, 121–250 (1977).
Johnson, A. W. & Goodall, J. D. The Birds of Chile and Adjacent Regions of Argentina, Bolivia and Perú (Platt, 1965–1978).
Iriarte, A., Rivas-Fuenzalida, T. & Jaksic, F.M. Las Aves Rapaces de Chile (CAPES/Flora & Fauna, 2023).
Schüttler, E. et al. New records of invasive mammals from the sub-Antarctic Cape Horn Archipelago. Polar Biol. 42(6), 1093–1105 (2019).
Schüttler, E., Saavedra-Aracena, L. & Jiménez, J. E. Domestic carnivore interactions with wildlife in the Cape Horn Biosphere Reserve, Chile: husbandry and perceptions of impact from a community perspective. PeerJ 6, e4124 (2018).
Couve, E., Vidal, C.F. & Ruiz, J. Aves de Chile, Sus Islas Oceánicas y Península Antártica (FS Editorial, 2016).
Summers, R. W. The life cycle of the Upland Goose Chloëphaga picta in the Falkland Islands. Ibis 125(4), 524–544 (1983).
Weller, M. W. Ecology and behaviour of steamer ducks. Wildfowl 27, 45–53 (1976).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. (Vienna, 2023). https://www.R-project.org/.
Liljesthröm, M. et al. Kelp geese (Chloephaga hybrida) and flightless steamer-ducks (Tachyeres pteneres) in the Beagle Channel: The importance of islands in providing nesting habitat. Wilson J. Ornithol. 125(3), 583–591 (2013).
Schüttler, E., Ibarra, J. T., Gruber, B., Rozzi, R. & Jax, K. Abundance and habitat preferences of the southernmost population of mink: Implications for managing a recent island invasion. Biodivers. Conserv. 19, 725–743 (2010).
Medina-Vogel, G., Barros, M., Organ, J. F. & Bonesi, L. Coexistence between the southern river otter and the alien invasive North American mink in marine habitats of southern Chile. J. Zool. 290(1), 27–34 (2013).
Crego, R. D., Jimenez, J. E. & Rozzi, R. Potential niche expansion of the American mink invading a remote island free of native-predatory mammals. PLoS ONE 13(4), e0194745 (2018).
Tobar, C. et al. Dieta del pato quetru no volador Tachyeres pteneres en isla Guapiquilán, Chiloé, sur de Chile. Boletín Chileno de Ornitología 17(2), 103–108 (2011).
Mainwaring, M. C. et al. Climate change and nesting behaviour in vertebrates: A review of the ecological threats and potential for adaptive responses. Biol. Rev. 92(4), 1991–2002 (2017).
Laaksonen, T., Ahola, M., Eeva, T. A., Väisänen, R. & Lehikoinen, E. Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos 114(2), 277–290 (2006).
Jaureguiberry, P. et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 8(45), eabm9982 (2022).
Eichholz, M. W. & Elmberg, J. Nest site selection by Holarctic waterfowl: A multi-level review. Wildfowl 4, 86–130 (2014).
Liljesthröm, M., Fasola, L., Valenzuela, A., Rey, A. R. & Schiavini, A. Nest predators of flightless steamer-ducks (Tachyeres pteneres) and flying steamer-ducks (Tachyeres patachonicus). Waterbirds 37(2), 210–214 (2014).
Fasola, L. & Valenzuela, A. E. J. Invasive carnivores in Patagonia: defining priorities for their management using the American mink (Neovison vison) as study case. Ecol. Austral 24, 173–182 (2014).
Acknowledgements
We are very grateful to the Center of Applied Ecology and Sustainability (CAPES) for the opportunity to carry out this research. We thank Xenabeth A. Lázaro for reading and commenting on the manuscript regarding the language.
Funding
This research was funded by the Chilean National Agency for Research and Development through grants ANID PIA/BASAL FB0002, ANID/BASAL FB210018, and ANID Master’s grant 22220927.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. Material preparation, data collection, and analysis were performed by Valeria Gómez-Silva. The first draft of the manuscript was written by Valeria Gómez-Silva and Elke Schüttler; all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gómez-Silva, V., Jaksic, F.M., Crego, R.D. et al. Adaptive response in waterbirds after mink introduction in subantarctic ecosystems. Sci Rep 15, 15147 (2025). https://doi.org/10.1038/s41598-025-98920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98920-1