Abstract
Little is known about modifiable risk factors for young-onset ovarian cancer, except for obesity and nulliparity. We investigated the association between non-alcoholic fatty liver disease (NAFLD) and the risk of young-onset ovarian cancer. A total of 2,376,482 women aged 20–39 years who underwent national health screening under the Korean National Health Insurance Service between 2009 and 2012 were included in this nationwide cohort study and followed-up until December 2022. The fatty liver index was used as a diagnostic biomarker for NAFLD. The risk was estimated using multivariable Cox proportional hazards models after adjusting for potential confounders. During 26.8 million person-years of follow-up (median: 11.5 years), 6,319 young women were newly diagnosed with young-onset ovarian cancer. The cumulative incidence probability was significantly higher for those with NAFLD than for those without (log-rank P < 0.01). NAFLD was associated with an increased risk of young-onset ovarian cancer (adjusted hazard ratio [aHR], 95% confidence interval [CI]: 1.30, 1.16–1.45). As the severity of NAFLD increased, the risk of young-onset ovarian cancer tended to increase (aHR, 95% CI: Moderate and severe NAFLD; 1.26, 1.12–1.41 and 1.45, 1.22–1.72, respectively; P for trend < 0.01). NAFLD was independently associated with an increased risk of young-onset ovarian cancer. As NAFLD is modifiable, our findings may benefit the next generation by reducing premature morbidity and mortality associated with young-onset ovarian cancer.
Similar content being viewed by others
Introduction
Ovarian cancer is highly lethal and the fifth most common cause of cancer-related death among women in high-income countries1,2. The overall incidence of ovarian cancer in women over 40 years of age has decreased significantly possibly due to the increased use of oral contraceptives3,4. However, the incidence of young-onset ovarian cancer remained stable in most developed countries4,5,6. In Asia, an increase in the incidence of ovarian cancer was observed, especially among the young population4,5,6,7.
Young women with ovarian cancer face unique challenges throughout the course of their treatment, such as a diagnostic delay, iatrogenic menopause, infertility, incontinence, and sexual dysfunction8,9,10,11,12,13. In addition, young patients with ovarian cancer are at high risk for financial toxicity caused by the disease and its treatments, with very few enrollments in clinical trials14,15. Although preventing young-onset ovarian cancer is crucial, limited data exists on its modifiable risk factors, except for obesity or nulliparity16,17.
Ample evidence suggests that obesity increases ovarian carcinogenesis through persistent inflammation and oxidative stress18,19,20,21. However, the effect of non-alcoholic fatty liver disease (NAFLD) on the risk of young-onset ovarian cancer remains unclear. NAFLD is a multisystemic disease associated with chronic inflammation and oxidative stress22,23,24. Moreover, the prevalence of NAFLD among young adults in developed countries is 20–24%, with a 2.5-fold increase over the last three decades25,26. To the best of our knowledge, only one small study suggested the association between NAFLD and the risk of ovarian cancer in middle-aged women (median age, 54 years; 19 cases of ovarian cancer cases in the NAFLD group)27.
Therefore, we conducted a nationwide population-based cohort study to investigate the longitudinal association between NAFLD and the subsequent risk of young-onset ovarian cancer. We followed up more than 2.3 million young women aged 20–39 years for ten years.
Patients and methods
Data source
We used data from the South Korean National Health Insurance Service (KNHIS) database. The KNHIS is a government-run universal national health insurance program covering approximately 97% of the population. The KNHIS database includes demographic information, medical treatments, procedures, diagnoses (based on the International Classification of Diseases, 10 th Revision, Clinical Modification [ICD-10-CM]), hospital utilization, and national health screening results. The KNHIS provides a regular National Health Screening Program every two years to all insured individuals aged ≥ 20 years or employees, regardless of age. Approximately 76% of the target population participates in this program28. This national health screening consists of anthropometric measures, laboratory testing, and a self-administered questionnaire regarding past medical history and lifestyle factors.
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. SMC2021-05–066) and the KNHIS Big Data Steering Department (NHIS-2021–1–711). The requirement for written informed consent was waived because the KNHIS dataset was compiled after anonymization in accordance with stringent confidentiality regulations. This study adhered to the criteria outlined in the Declaration of Helsinki.
Study population
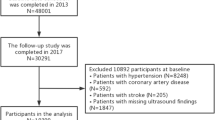
The selection process for the study population is illustrated in Fig. 1. A total of 2,755,790 women aged 20–39 years who underwent a national health examination between January 1, 2009, and December 31, 2012 were included. To minimize the impact of pre-existing diseases and avoid bias, we excluded participants who had been diagnosed with any cancer except non-melanoma skin cancer before cohort entry (n = 11,971) and those who developed cancer except non-melanoma skin cancer or died within the first year of follow-up (n = 5,965). In addition, based on the definition of NAFLD, we excluded individuals with heavy alcohol consumption (≥ 20 g of alcohol per day; n = 62,269), liver cirrhosis, and hepatitis (ICD-10-CM codes K70.3 and B15–B19, respectively) (n = 141,500). We excluded participants with missing data (n = 157,603). Ultimately, 2,376,482 women were included in this study. The participants were followed up until December 31, 2022, the development of young-onset ovarian cancer, or death, whichever occurred first.
Anthropometrics and laboratory measurements
Health professionals evaluated the following characteristics during the national health screening. Wearing lightweight clothing, the participants’ height, weight, and waist circumference were measured. Abdominal obesity was defined as a waist circumference ≥ 85 cm for females, according to Asian standards29. The body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m2). According to Korean standards, obesity was defined as a BMI ≥ 25 kg/m22,30. Abdominal obesity was defined as waist circumference ≥ 85 cm for women, according to Asian standards29.
Systolic and diastolic blood pressures were recorded in a seated position after a minimum of five minutes of rest. After overnight fasting, blood samples were collected and examined for glucose, creatinine, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and γ-glutamyl transferase (GGT). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study Eq31.. Chronic kidney disease was defined as an eGFR < 60 mL/min/1.73 m2.
Definition of non-alcoholic fatty liver disease
According to the European Association for the Study of the Liver’s international guidelines, noninvasive biomarkers such as the fatty liver index are the preferred methods for assessing NAFLD in large-scale population-based studies32,33,34. In the young, asymptomatic general population, liver biopsies or imaging studies are not feasible. We therefore utilized one of the best-validated fatty liver prediction models: The fatty liver index32,33.
The fatty liver index has good predictive value for detecting fatty liver35,36. This index ranges from zero to 100 and is computed as follows: (e0.953×Ln (triglycerides) +0.139×BMI+0.718×Ln (GGT) +0.053×waist circumference±15.745)/(1 + e0.953×Ln (triglycerides) +0.139×BMI+0.718×Ln (GGT) +0.053×waist circumference±15.745) × 10037. The optimal cut-off value of the fatty liver index to detect ultrasonography-diagnosed fatty liver was previously validated at ≥ 30 with an area under the receiver operating characteristic curve of 0.82 (0.81–0.84) in the general population of Korea35,38. Participants were categorized into three groups based on their NAFLD status: none (fatty liver index < 30); moderate (30 ≤ fatty liver index < 60); and severe (fatty liver index ≥ 60)35,36,39.
Definition of young-onset ovarian cancer
The primary endpoint of this study was the occurrence of ovarian cancer diagnosed in women aged 20 to 49. Young-onset ovarian cancer was determined by the ICD-10-CM code for ovarian cancer (C56) during hospitalization and the special reimbursement code for cancer (V193). Since 2006, the KNHIS policy has reduced co-payments for cancer-related examinations and treatments by 5%. Cancer diagnoses must therefore be certified by physicians and medical institutions using a specific reimbursement code (V193). This code adds all patients with a confirmed cancer diagnosis to the KNHIS national registry.
Clinical variables
During the national health screening, the participants respond to a standard self-administered questionnaire regarding alcohol consumption, smoking status, and level of physical activity. First, average daily alcohol consumption was determined by calculating the frequency of alcohol consumption per week and amount of alcohol consumed per occasion. Participants were then categorized as non-drinkers, light-to-moderate drinkers (< 20 g of alcohol per day), or heavy drinkers (≥ 20 g of alcohol per day). Each participant was classified as a never smoker, former smoker, or current smoker, based on their smoking history. Regular physical activity was defined as ≥ 20 min of vigorous-intensity physical activity ≥ 3 times per week, or ≥ 30 min of moderate-intensity physical activity ≥ 5 times per week. Those in the lowest quintile of the required insurance fee or those who received free medical care were categorized as having low-income status.
Diabetes was defined as a fasting glucose level ≥ 126 mg/dL or a minimum of one claim per year for an antidiabetic medication prescription (oral and/or injectable antidiabetic medication) under ICD-10-CM codes E11–E14. Dyslipidemia was determined based on total cholesterol levels ≥ 240 mg/dL or prescriptions for lipid-lowering drugs under ICD-10-CM code E78. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or antihypertensive drug prescription under ICD-10-CM codes I10–I13 and I15. The ICD-10-CM codes were used to define pelvic inflammatory disease (N73.9), endometriosis (N80), tubal ligation (R4341-R4345), and polycystic ovary syndrome (E28.2).
Statistical analysis
Baseline characteristics were analyzed using analysis of variance for continuous variables and the chi-square test for categorical variables. The incidence rates of young-onset ovarian cancer were computed by dividing the number of incident cases by the total follow-up duration and are expressed per 100,000 person-years. The log-rank test was used to compare the differences between Kaplan–Meier curves depicting the cumulative incidence probability of young-onset ovarian cancers in women with and without NAFLD. Using multivariate Cox proportional hazards regression models, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the associations between NAFLD in young women and young-onset ovarian cancer. Model 1 was not adjusted. Model 2 was adjusted for age. Model 3 was adjusted for age, smoking status, alcohol consumption, physical activity, BMI, diabetes, pelvic inflammatory disease, tubal ligation, endometriosis, and polycystic ovary syndrome. In addition, we examined the risk of young-onset ovarian cancer according to NAFDL severity. The proportional hazards assumption was tested through Schoenfeld residual and log–log plot. The test result was considered to satisfy the proportional hazards assumption. We also performed a sensitivity analysis, defining the outcome as ovarian cancer diagnosed before the ages of 30 and 45 years, respectively. Study participants were censored at the time they reached 30 or 45 years of age during follow-up. The level of statistical significance was set at P < 0.05, using two-sided tests. All statistical analyses were performed using the SAS software (version 9.3; SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the study population
During the 26,786,501 person-years of follow-up, 6,319 young-onset ovarian cancer cases were diagnosed, and the total number of deaths was 9,361 (mean ± SD age, 29.8 ± 5.2 years). The median follow-up duration was 11.5 years (interquartile range 10.4–12.2).
Table 1 shows the baseline characteristics of the study population based on the presence or absence of young-onset ovarian cancer. Women with young-onset ovarian cancer were older and had higher BMIs (all P < 0.01) than those without the disease. Additionally, women with young-onset ovarian cancer were more likely to have diabetes, hypertension, dyslipidemia, obesity, and endometriosis (all P < 0.01).
Association between non-alcoholic fatty liver disease and the risk of young-onset ovarian cancer
The Kaplan–Meier curve in Fig. 2 demonstrates that the cumulative incidence probability of young-onset ovarian cancer was consistently higher in women with NAFLD than in those without NAFLD during the follow-up period (log-rank P < 0.01). Table 2 also shows that the incidence of young-onset ovarian cancer was higher among women with NAFLD than among those without NAFLD (P < 0.01).
As shown in Table 2, NAFLD was associated with an increased risk of young-onset ovarian cancer in all models (all P < 0.01). After adjusting for potential confounding variables including obesity (Model 3), NAFLD remained significantly associated with an increased risk of young-onset ovarian cancer (hazard ratio [HR], 95% CI: 1.30, 1.16–1.45). The risk of young-onset ovarian cancer tended to increase with increasing severity of NAFLD (P for trend < 0.05). Moderate NAFLD and severe NAFLD were associated with an increased risk of young-onset ovarian cancer (Model 3; HR, 1.26; 95% CI, 1.12–1.41 and HR, 1.45; 95% CI, 1.22–1.72, respectively).
Supplementary Table 1 shows the results of ending the follow-up at the age of 45 years. The HR for young-onset ovarian cancer occurring before age 45 was 1.32 (95% CI, 1.10–1.58) in women with NAFLD after adjusting for potential confounders (Model 3). Supplementary Table 2 presents the findings when follow-up was restricted to age 30 years. In this analysis, the HR for young-onset ovarian cancer diagnosed before 30 years was 1.21 (95% CI, 0.60–2.44) in women with NAFLD (Model 3).
Discussion
In this nationwide cohort study of more than 2.3 million women aged 20–39 years, we found that NAFLD in young women was independently associated with an increased risk of young-onset ovarian cancer. The risk of developing young-onset ovarian cancer tended to increase as the severity of NAFLD increased.
Previously, a cohort study including women with a median age of 54 years reported that individuals with NAFLD had a higher incidence rate ratio of ovarian cancer than age-matched controls27. The study identified 19 cases of ovarian cancer in the NAFLD group, which was defined by diagnostic codes. In addition, a meta-analysis of observational studies found positive association between NAFLD and gynecological cancers, including those of the ovary, uterus, and cervical region (pooled random effects HR, 1.62; 95% CI, 1.13–2.32)40.
We provide new evidence that NAFLD in young women may be a modifiable risk factor for young-onset ovarian cancer, independent of obesity. Increasing physical activity, reducing fructose and cholesterol intake, and losing weight are recommended as management strategies for NAFLD41,42,43,44. Our findings present a significant potential opportunity to lower the risk of young-onset ovarian cancer, as there are few known risk factors in this age group.
The following mechanisms may explain the association between NAFLD and the risk of young-onset ovarian cancer. Individuals with NAFLD are more likely to have chronic low-grade inflammation, which may promote ovarian cancer development22,45. The hepatic production and systemic release of multiple proinflammatory cytokines and pro-oxidative mediators may promote ovarian carcinogenesis in NAFLD by promoting cell proliferation, anti-apoptosis, angiogenesis, and the production of free radicals that damage DNA and facilitate tumor initiation and development22,46. Moreover, individuals with chronic liver diseases, including NAFLD, have high estrone and estradiol levels, which is attributed to the increased peripheral conversion of androgens to estrogen in both males and females47,48. Ample evidence suggest that estrogen is associated with an increased risk of ovarian cancer49,50,51. Thus, the increased estrogen associated with NAFLD may be involved in ovarian carcinogenesis. In addition, NAFLD-associated microbiome alterations may be associated with ovarian carcinogenesis through altered host immunologic response and modulation of cell proliferation52,53,54,55.
Our study has several strengths. First, this nationwide cohort study was based on data from a large sample size of over 2.3 million young women, with a median follow-up duration of 11.5 years. Hence, we identified NAFLD as a modifiable risk factor for young-onset ovarian cancer, which has a significant disease burden but a relatively low incidence. Second, anthropometric measurements, blood test results, lifestyle factors, and extensive medical records from a nationwide database were used. After cohort entry, the clinical course was reliably monitored using the KNHIS database. Third, analyses were performed after controlling for several potential confounding variables including BMI, alcohol consumption, smoking status, physical activity, diabetes, and pelvic inflammatory disease. Fourth, we used both ICD-10-CM diagnostic codes (C codes) and national registration codes (V codes) to identify patients with young-onset ovarian cancer.
This study also has several limitations. First, we used the previously validated fatty liver index to evaluate NAFLD, and no liver biopsies or imaging data were included. Given the impracticality of performing liver biopsies or imaging tests on over 2.3 million young women, the fatty liver index is a useful diagnostic biomarker for NAFLD. Second, although the national health screening is free, it is voluntary, with a participation rate of approximately 76%. Individuals participating in national health screening are likely to represent a generally healthy population, as those with underlying symptoms or diseases are more likely to require hospital testing than a national health screening. Third, we could not include information on the participants’ familial cancer history, parity, hormonal contraceptive use, or the stage or histological subtype of ovarian cancer due to the lack of data in the KNHIS. Epithelial tumors account for over 90% of ovarian cancer in women over 30 years, while germ cell tumors predominate between 20 and 30 years15. Fourth, the Korean NHIS database does not have information on cases of loss of follow-up due to immigration. However, the number of immigrants every year is less than 0.001% of all Koreans. Fifth, self-reported information on alcohol consumption, smoking status, and physical activity levels may have been subject to recall bias.
In conclusion, this nationwide cohort study of more than 2.3 million women aged 20–39 years revealed that NAFLD in young women was significantly associated with an increased risk of young-onset ovarian cancer. Our findings suggest that NAFLD may be a modifiable risk factor for young-onset ovarian cancer. Additional research in other study populations is warranted to reduce premature morbidity and mortality associated with young-onset ovarian cancer among the next generation. Further investigation is also required to elucidate the precise mechanism by which NAFLD contributes to the carcinogenesis of young-onset ovarian cancer.
Data availability
This database is accessible to medical researchers whose study protocols have been approved by the Korea National Health Insurance Sharing Service Institutional Data Access/Ethics Committee (https://nhiss.nhis.or.kr/).
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NHIS:
-
National Health Insurance Service
- ICD-10-CM:
-
International Classification of Diseases, 10th Revision, Clinical Modification
- BMI:
-
Body mass index
References
Lheureux, S., Gourley, C., Vergote, I. & Oza, A. M. Epithelial ovarian cancer. Lancet 393, 1240–1253. https://doi.org/10.1016/s0140-6736(18)32552-2 (2019).
Cronin, K. A. et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 128(4251), 4284. https://doi.org/10.1002/cncr.34479 (2022).
Schrijver, L. H. et al. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: An international cohort study. Am. J. Obstet. Gynecol. 225(51), e51-51.e17. https://doi.org/10.1016/j.ajog.2021.01.014 (2021).
Cabasag, C. J. et al. Ovarian cancer today and tomorrow: A global assessment by world region and human development index using GLOBOCAN 2020. Int. j. cancer 151(1535), 1541. https://doi.org/10.1002/ijc.34002 (2022).
di Martino, E. et al. Incidence trends for twelve cancers in younger adults-a rapid review. Br. J. Cancer 126, 1374–1386. https://doi.org/10.1038/s41416-022-01704-x (2022).
Zhang, Y. et al. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 19, 984. https://doi.org/10.1186/s12885-019-6139-6 (2019).
Kim, S. I. et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J. Gynecol. Oncol. https://doi.org/10.3802/jgo.2016.27.e5 (2016).
Gonçalves, V. Long-term quality of life in gynecological cancer survivors. Curr. Opin. Obstet. Gynecol. 22, 30–35. https://doi.org/10.1097/GCO.0b013e328332e626 (2010).
Health-Related Quality of Life and Living Conditions et al. Long-Term gynecological cancer survivors in Côte d’Or. Oncologist 24, e490–e500. https://doi.org/10.1634/theoncologist.2018-0347 (2019).
Dunberger, G. et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int. j. gynecol. cancer: Off. J. Int. Gynecol. Cancer Soc. 20, 449–460. https://doi.org/10.1111/IGC.0b013e3181d373bf (2010).
Lind, H. et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: A population-based cohort study. Br. J. Cancer 105, 737–745. https://doi.org/10.1038/bjc.2011.315 (2011).
Dunberger, G. et al. Self-reported symptoms of faecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur. J. cancer (Oxford, England: 1990) 46(606), 615. https://doi.org/10.1016/j.ejca.2009.10.023 (2010).
Fruscio, R. et al. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br. J. Cancer 115, 641–648. https://doi.org/10.1038/bjc.2016.254 (2016).
Aviki, E. M. et al. Risk factors for financial toxicity in patients with gynecologic cancer. Am. J. Obstet. Gynecol. 226(817), e811-817.e819. https://doi.org/10.1016/j.ajog.2021.12.012 (2022).
Lockley, M., Stoneham, S. J. & Olson, T. A. Ovarian cancer in adolescents and young adults. Pediatr. Blood Cancer 66, e27512. https://doi.org/10.1002/pbc.27512 (2019).
Engeland, A., Tretli, S. & Bjørge, T. Height, body mass index, and ovarian cancer: A follow-Up of 1.1 million norwegian women. JNCI J. Natl. Cancer Inst. 95, 1244–1248. https://doi.org/10.1093/jnci/djg010 (2003).
Toufakis, V., Katuwal, S., Pukkala, E. & Tapanainen, J. S. Impact of parity on the incidence of ovarian cancer subtypes: A population-based case-control study. Acta Oncol. 60, 850–855. https://doi.org/10.1080/0284186x.2021.1919754 (2021).
Urbute, A., Frederiksen, K. & Kjaer, S. K. Early adulthood overweight and obesity and risk of premenopausal ovarian cancer, and premenopausal breast cancer including receptor status: Prospective cohort study of nearly 500,000 Danish women. Ann. Epidemiol. 70, 61–67. https://doi.org/10.1016/j.annepidem.2022.03.013 (2022).
Ma, X. et al. Anthropometric measures and epithelial ovarian cancer risk among Chinese women: Results from the Shanghai women’s health study. Br. J. Cancer 109, 751–755. https://doi.org/10.1038/bjc.2013.384 (2013).
World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and ovarian cancer, https://www.wcrf.org/wp-content/uploads/2021/02/ovarian-cancer-report.pdf (2018).
Ness, R. B. & Cottreau, C. Possible role of ovarian epithelial inflammation in ovarian cancer. JNCI: J. Natl. Cancer Inst. 91, 1459–1467. https://doi.org/10.1093/jnci/91.17.1459 (1999).
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. lancet. Gastroenterol. Hepatol. 6, 578–588. https://doi.org/10.1016/s2468-1253(21)00020-0 (2021).
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 158, 1851–1864. https://doi.org/10.1053/j.gastro.2020.01.052 (2020).
Delli Bovi, A. P. et al. Oxidative stress in non-alcoholic fatty liver disease an updated mini review. Front. Med. (Lausanne) 8, 595371. https://doi.org/10.3389/fmed.2021.595371 (2021).
Doycheva, I., Watt, K. D. & Alkhouri, N. Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. Hepatology (Baltimore, MD) 65, 2100–2109. https://doi.org/10.1002/hep.29068 (2017).
Abeysekera, K. W. M. et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. The lancet. Gastroenterology & hepatology 5, 295–305. https://doi.org/10.1016/s2468-1253(19)30419-4 (2020).
Allen, A. M., Hicks, S. B., Mara, K. C., Larson, J. J. & Therneau, T. M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J. Hepatol. 71, 1229–1236. https://doi.org/10.1016/j.jhep.2019.08.018 (2019).
Cheol Seong, S. et al. Data resource profile: The national health information database of the national health insurance service in South Korea. Int. j. epidemiol. 46(799), 800. https://doi.org/10.1093/ije/dyw253 (2017).
WHOE Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163. https://doi.org/10.1016/S0140-6736(03)15268-3 (2004).
Seo, M. H. et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 28, 40–45. https://doi.org/10.7570/jomes.2019.28.1.40 (2019).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 130, 461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002 (1999).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402. https://doi.org/10.1016/j.jhep.2015.11.004 (2016).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. https://doi.org/10.1016/j.jhep.2020.03.039 (2020).
Park, J. H. et al. Increased risk of young-onset digestive tract cancers among young adults Age 20–39 years with nonalcoholic fatty liver disease: A nationwide cohort study. J. clin. Oncol.: off. J. Am. Soc. Clin. Oncol. 41, 3363–3373. https://doi.org/10.1200/jco.22.01740 (2023).
Cho, E. J. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics (Basel, Switzerland) https://doi.org/10.3390/diagnostics11122233 (2021).
Kim, J. H., Kwon, S. Y., Lee, S. W. & Lee, C. H. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int.: Off. J. Int. Assoc. Study Liver 31, 1600–1601. https://doi.org/10.1111/j.1478-3231.2011.02580.x (2011).
Bedogni, G. et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33. https://doi.org/10.1186/1471-230x-6-33 (2006).
Han, E. et al. Fatty liver & diabetes statistics in Korea: Nationwide data 2009 to 2017. Diabetes Metab. J. 47, 347–355. https://doi.org/10.4093/dmj.2022.0444 (2023).
Kim, K. S., Hong, S., Han, K. & Park, C. Y. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: Nationwide population based study. BMJ 384, e076388. https://doi.org/10.1136/bmj-2023-076388 (2024).
Mantovani, A. et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 71, 778–788. https://doi.org/10.1136/gutjnl-2021-324191 (2022).
Kim, D. & Kim, W. R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 15, 474–485. https://doi.org/10.1016/j.cgh.2016.08.028 (2017).
Jin, Y. J. et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: Analysis of biopsies of living liver donors. J. Gastroenterol. Hepatol. 27, 1341–1347. https://doi.org/10.1111/j.1440-1746.2012.07165.x (2012).
Kwak, M. S., Kim, D., Chung, G. E., Kim, W. & Kim, J. S. The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int.: Off. J. Int. Assoc. Study Liver 37, 919–926. https://doi.org/10.1111/liv.13332 (2017).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology (Baltimore, MD) 67, 328–357. https://doi.org/10.1002/hep.29367 (2018).
Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet 397, 2212–2224. https://doi.org/10.1016/s0140-6736(20)32511-3 (2021).
Ding, D. N. et al. Insights into the role of oxidative stress in ovarian cancer. Oxid. Med. Cell. Longev. 2021, 8388258. https://doi.org/10.1155/2021/8388258 (2021).
Vaishnav, B. et al. Study of gonadal hormones in males with liver cirrhosis and its correlation with Child-Turcotte-Pugh and model for End-Stage liver disease scores. Cureus 15, e34035. https://doi.org/10.7759/cureus.34035 (2023).
Khalili, M. Endocrine-manifestations of cirrhosis and liver disease. Int. J. Pediatr. 2, 16–16. https://doi.org/10.22038/ijp.2014.2454 (2014).
Petrick, J. L. et al. Menopausal hormone therapy use and risk of ovarian cancer by race: The ovarian cancer in women of African ancestry consortium. Br. J. Cancer 129, 1956–1967. https://doi.org/10.1038/s41416-023-02407-7 (2023).
Trabert, B. et al. Circulating estrogens and postmenopausal ovarian cancer risk in the women’s health initiative observational study. Cancer Epidemiol. Biomark. Prev. 25, 648–656. https://doi.org/10.1158/1055-9965.Epi-15-1272-t (2016).
Cunat, S., Hoffmann, P. & Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 94, 25–32. https://doi.org/10.1016/j.ygyno.2004.03.026 (2004).
Chalif, J. et al. The microbiome and gynecologic cancer: Cellular mechanisms and clinical applications. Int. j. gynecol. cancer: Off. J. Int. Gynecol. Cancer Soc. https://doi.org/10.1136/ijgc-2023-004894 (2023).
Łaniewski, P., Ilhan, Z. E. & Herbst-Kralovetz, M. M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 17, 232–250. https://doi.org/10.1038/s41585-020-0286-z (2020).
Dhingra, A., Sharma, D., Kumar, A., Singh, S. & Kumar, P. Microbiome and development of ovarian cancer. Endocr. Metab. Immune. Disord Drug Target. 22, 1073–1090. https://doi.org/10.2174/1871530322666220509034847 (2022).
Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425. https://doi.org/10.1038/nrgastro.2016.85 (2016).
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea (2022R1I1 A1 A01054327) and by the Bio&Medical Technology Development Program of the NRF funded by the Korean government (MSIT) (No. RS-2023–00222838). The funders played no role in the study’s design, conduct, or reporting.
Author information
Authors and Affiliations
Contributions
Joo-Hyun Park: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Writing – Original Draft, Writing – Review & Editing. Jung Yong Hong: Conceptualization, Data Curation, Investigation, Methodology, Writing – Original Draft, Writing – Review & Editing. Kyungdo Han: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology Writing – Review & Editing. Wonseok Kang: Investigation, Methodology, Writing – Review & Editing. Jay J. Shen: Investigation, Methodology, Writing – Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, JH., Hong, J.Y., Han, K. et al. Increased risk of young-onset ovarian cancer in women with non-alcoholic fatty liver disease: A nationwide cohort study of 2.3 million women aged 20–39 years. Sci Rep 15, 14463 (2025). https://doi.org/10.1038/s41598-025-99093-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99093-7