Abstract
This study investigates the effects of continuous versus intermittent hydrocortisone administration on septic shock patients. Sixty patients were randomized into two groups: one receiving intermittent doses of 50 mg of hydrocortisone every 6 h and the other a continuous infusion of 200 mg/day. After a 7-day treatment period and a 28-day follow-up, we observed no significant differences in the duration of sustained shock, hospital, and ICU stays between the groups. However, those in the continuous infusion group experienced shorter periods of mechanical ventilation and vasopressor use, with significant improvements in hemodynamic stability. Both treatment approaches improved arterial pressure and lactate clearance, with no significant differences in heart rate or cortisol levels between the groups at the end of the treatment. Notably, shock reversal rates were higher and 28-day mortality rates were lower in the continuous infusion group. These results suggest that continuous hydrocortisone infusion may be more effective for managing septic shock, potentially leading to better patient outcomes without an increase in adverse reactions. This method could be considered for broader clinical implementation in septic shock treatment strategies.
Similar content being viewed by others
Introduction
Sepsis is a life-threatening organ dysfunction resulting from a host’s dysregulated response to infection, characterized by a malignant onset and rapid progression that can cause multi-organ dysfunction in a short period1. Septic shock refers to patients who require vasopressors to maintain a mean arterial pressure (MAP) > 65 mmHg and/or have lactate levels > 2 mmol/L despite adequate fluid resuscitation, and whose condition, if not rapidly reversed, can lead to poor recovery or death2,3,4. Previous research has shown5 that cortisol plays a crucial role in maintaining metabolic, vascular, and immune system homeostasis during the early stages of sepsis, reducing inflammation in organs and minimizing organ dysfunction6. Additionally, cortisol helps restore blood volume, enhance vasoconstriction, and improve blood pressure responses to α-1 agonists. Research from 2018 has indicated7 that hydrocortisone treatment in mechanically ventilated patients with septic shock can shorten the duration of shock resolution and ICU stays, but it has shown no significant effect on the 90-day mortality rate8. Despite the benefits of hydrocortisone use in most patients with septic shock, the impact of the drug may vary depending on the mode of administration due to its differences in pharmacokinetics and pharmacodynamics9. Consequently, this study aims to compare the effects and prognostic impacts of continuous versus intermittent hydrocortisone administration in this patient population.
Materials and methods
Study design
This was a randomized, double-blind, placebo-controlled trial designed to compare the effects of continuous versus intermittent hydrocortisone infusion on adult patients with septic shock. The study focused on various clinical outcomes, with the primary endpoint being 28-day mortality.
Selection of patients, inclusion and exclusion criteria, and randomization
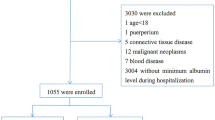
From January 2022 to January 2024, a total of 60 patients diagnosed with septic shock were enrolled. Inclusion criteria required patients to be diagnosed with septic shock according to the guidelines, needing vasopressors to maintain a mean arterial pressure (MAP) ≥ 65 mmHg and arterial lactate concentrations > 2 mmol/L despite adequate fluid resuscitation. Participants had to be ≥ 18 years old with a Sequential Organ Failure Assessment (SOFA) score ≥ 2 points. Exclusion criteria included known allergies or contraindications to hydrocortisone, psychiatric abnormalities, coagulation disorders, long-term corticosteroid use, current infectious shock under corticosteroid treatment, and expected survival of less than 24 h or imminent death from non-infectious causes. Patients were randomized in a 1:1 ratio using a random digit table that matched patient identifiers to treatment modalities, ensuring unbiased allocation. This table was managed by a designated individual who was not involved in patient assessments or treatments. Upon patient enrollment, researchers communicated with the randomization manager to determine the treatment modality for each patient based on the random digit table.
Clinical data and patient characteristics
The demographic and baseline clinical characteristics of each group were recorded, including age, gender, Body Mass Index (BMI), and underlying conditions such as severe pneumonia, biliary tract infections, septicemia, and acute peritonitis.
Methods and interventions
Both groups received standard treatment for sepsis and septic shock, including aggressive antibiotic therapy, intravenous fluid resuscitation, transfusion of blood products, and enhanced nutritional support. Additional care included prophylaxis for deep vein thrombosis, support for tracheal dysfunction including mechanical ventilation, and Continuous Renal Replacement Therapy (CRRT). Vasopressors were optimized based on clinical need, and insulin intervention was adjusted according to blood glucose levels. The control group received intermittent intravenous hydrocortisone at a dose of 50 mg every 6 h, while the intervention group received a continuous infusion of hydrocortisone at 200 mg/day.
Outcome measures
Primary and secondary outcomes were clearly defined. The primary outcome was 28-day mortality, and secondary outcomes included shock reversal, length of ICU stay, and safety outcomes assessed by monitoring infection rates and adverse reactions to steroids. Additional physiological data such as mean arterial pressure, heart rate, lactate clearance rates, and cortisol levels were collected to assess treatment response.
Blinding
To ensure the integrity of outcome assessments, the study was double-blinded. Both the clinicians administering the treatments and the staff responsible for assessing outcomes were unaware of group assignments. Blinding was achieved by administering hydrocortisone in identically appearing infusion pumps or syringes prepared by an independent pharmacist. These devices, externally identical in size, color, and labeling, operated in such a manner that did not reveal the method of administration. The pharmacist prepared these delivery systems ensuring that neither the method of administration (continuous vs. intermittent) was discernible to the clinical staff or the patients. Each device was placed in opaque, tamper-evident packaging that prevented any visual inspection until the point of administration, further maintaining the blinding integrity. This method ensures that all personnel involved in administering the medication or assessing patient outcomes had no knowledge of the specific treatment approach being used. This robust approach to blinding can be replicated in other healthcare settings, ensuring that treatment modalities are presented in a manner that is externally indistinguishable.
Power analysis and sample size consideration
A power analysis was conducted prior to the study initiation, aiming for a sample size sufficient to detect a statistically significant difference in 28-day mortality between the two groups. Although 120 patients were initially estimated to achieve an 80% power at a 5% significance level, the study proceeded with 60 patients due to recruitment challenges, acknowledging the potential impact on the power to detect smaller effect sizes.
Ethical considerations
The study protocol was approved by the institutional ethics committee, and informed consent was obtained from all patients or their legal guardians. In accordance with ethical guidelines and institutional policies, the ICU allowed for limitation of care procedures when medical futility was determined by a consensus of the treating team. To minimize bias related to end-of-life decisions, all cases where limitation of care was considered were reviewed by an independent ethics committee not involved in the direct care of the study participants. This committee was responsible for ensuring that decisions were made based on predefined clinical criteria and were consistent across all patients, irrespective of their group assignment in the study. These measures were intended to minimize the potential influence of subjective decision-making on mortality outcomes.
Statistical analysis
Statistical analysis was conducted using SPSS 24.0. Categorical data were analyzed with the χ2 test and presented as frequencies and percentages, while continuous data were analyzed with the t-test and presented as mean ± standard deviation. A p-value < 0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
We included a total of 60 patients in the analysis, with 30 in the treatment group and 30 in the control group. The age, gender, BMI, and underlying conditions of these patients aligned with the criteria outlined previously, ensuring a consistent demographic and clinical profile across both groups. Additionally, both groups were comparable in terms of severity scores at baseline, including SOFA and APACHE II scores, which reflect the patients’ critical status upon admission. See Table 1 for details.
Primary outcomes
Comparison of treatment outcomes
No significant differences were observed between the two groups in terms of the duration of sustained shock, hospital, and ICU stays (P > 0.05). However, the duration of mechanical ventilation and vasopressor use was significantly shorter in the treatment group compared to the control group (P < 0.05). See Table 2 for details.
In handling ICU and hospital stays for deceased patients, we performed a censored analysis for patients who died during the study period. This approach adjusted for the shorter potential duration of stay in these cases, ensuring that mortality did not skew the results significantly.
Secondary outcomes
Long-term outcomes and drug safety
The 7-day shock reversal rate was significantly higher in the treatment group (P < 0.05), and the 28-day mortality rate was significantly lower (P < 0.05). The incidence of adverse effects such as hypokalemia and hypernatremia was not significantly different between the groups (P > 0.05). All patients included were able to perform per protocol, ensuring full compliance with the study requirements. See Table 3 for details.
Additional physiological data
Hemodynamic parameters and catecholamine index
Significant improvements were noted in mean arterial pressure and heart rate from Day 1 to Day 7 in both groups (P < 0.05). We selected Day 1 and Day 7 for these measurements based on clinical standards, which are crucial for assessing early and sustained responses to septic shock treatment. This approach allows for a comprehensive evaluation of the immediate effects post-intervention and the longer-term stability under the treatment regimen. The catecholamine index, shock withdrawal, and the duration of use of elevated pressure medications are included, indicating comparable management of these physiological metrics under both treatment regimens. Details are provided in Table 4.
Discussion
In this study, we compared the effects of different administration routes of hydrocortisone on the prognosis of septic shock patients. The results demonstrated that there were no significant differences in the duration of shock, hospital, and ICU stays between the two groups (P > 0.05); however, the treatment group showed shorter durations of mechanical ventilation and vasopressor use post-treatment (P < 0.05), suggesting that hydrocortisone can improve outcomes, with continuous infusion being particularly effective.
The significant reduction in 28-day mortality rate observed in the treatment group may be attributed to the benefits of continuous hydrocortisone infusion on vascular permeability and immune response modulation. Continuous infusion of hydrocortisone effectively reduces fluctuations in blood sugar levels and avoids complications such as hypernatremia11. Previous research has shown that glucocorticoid use can lead to elevated blood sugar levels, with persistent hyperglycemia being an independent risk factor affecting patient outcomes12. Continuous hydrocortisone infusion can mitigate the body’s stress response, inhibit coagulation, endothelial cell damage, and apoptosis, thereby stabilizing vital signs, reducing cortisol levels, and enhancing lactate clearance13,14.
Both groups showed improved mean arterial pressure and lactate clearance rates after 1 and 7 days of treatment compared to before treatment (P < 0.05); heart rate and cortisol levels were also lower after treatment (P < 0.05). There were no significant differences between the groups in terms of mean arterial pressure, heart rate, cortisol levels, and lactate clearance rates after 1 and 7 days of treatment (P > 0.05). This suggests that both administration routes were effective, but continuous infusion provides additional benefits in stabilizing vital signs and improving lactate clearance rates.
Regarding the large differences observed in shock withdrawal rate and 28-day mortality rate, while it is tempting to attribute these differences solely to variations in blood glucose and electrolyte management, it is plausible that other underlying factors are at play. It is important to consider the potential for variations in patient adherence to treatment protocols, differences in baseline patient health statuses not fully captured by the study parameters, or even unmeasured environmental or procedural factors within our single-center setting that could influence these outcomes.
Despite these promising findings, several limitations of this study should be noted. Firstly, the smaller than initially calculated sample size, due to recruitment challenges, may have reduced the statistical power necessary to detect smaller but clinically significant differences between the treatment groups. This limitation is particularly relevant given the observed trends, and it warrants cautious interpretation of the mortality and shock reversal outcomes. The limited power might obscure true effects or exaggerate apparent differences; thus, the results should be viewed as preliminary. Additionally, as a single-center study, the generalizability of our results may be limited. The findings of this study need to be validated in larger, multicenter trials that could more robustly ascertain the effects observed here and potentially uncover additional insights that were not detectable in our study due to limited sample diversity and size. Future research should aim to replicate and extend these findings under more varied conditions and with broader populations to confirm the benefits and generalize the results to a wider patient population.
Administering low-dose, short-course glucocorticoids to septic shock patients can reduce the dosage of vasopressors needed and enhance the sensitivity of vascular smooth muscle to catecholamine receptors, thereby effectively correcting shock15,16. Moreover, continuous infusion of hydrocortisone can also stabilize the exogenous cortisol concentration, exerting a strong anti-shock effect and maintaining metabolic balance17. Recent meta-analyses have further supported the use of continuous infusion, demonstrating a trend towards improved survival rates without significant increases in adverse events, suggesting an optimized protocol for glucocorticoid administration in septic shock could further improve patient outcomes18,19.
Conclusions
In this study, patients receiving continuous hydrocortisone infusion exhibited a significantly higher shock reversal rate after 7 days (P < 0.05) and a notably reduced 28-day mortality rate compared to the control group treated with intermittent doses (P < 0.05). These findings suggest that continuous administration of hydrocortisone not only maintains a favorable safety profile but also significantly improves shock reversal rates and decreases long-term mortality.
Additionally, continuous hydrocortisone infusion was associated with shorter durations of mechanical ventilation and vasopressor support, enhanced hemodynamic stability, and overall better treatment outcomes in septic shock patients, all without an increase in adverse drug reactions.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author, Feng Xu, on reasonable request. The data that support the findings of this study are kept confidential and are protected under patient privacy regulations. Interested researchers who meet the criteria for access to confidential data may contact Dr. Feng Xu at the Department of Emergency Medicine, the First Affiliated Hospital of Soochow University.
Abbreviations
- BMI:
-
Body Mass Index
- CRRT:
-
Continuous Renal Replacement Therapy
- HPA:
-
Hypothalamic-Pituitary-Adrenal Axis
- ICU:
-
Intensive Care Unit
- MAP:
-
Mean Arterial Pressure
- RCT:
-
Randomized Controlled Trial
- SOFA:
-
Sequential Organ Failure Assessment
- SPSS:
-
Statistical Package for Social Sciences
References
Ji, B. et al. Effects of early bundle therapy on prognosis of patients with sepsis and septic shock. Chin. J. Emerg. Med.28 (2), 151–155 (2019).
Ji, B. et al. Effect of fluid balance state after bundle treatment on prognosis in patients with sepsis. Chongqing Med.48 (9), 1531–1534 (2019).
Vincent, J. L. & De Backer, D. Circulatory shock. N Engl. J. Med.369 (18), 1726–1734. https://doi.org/10.1056/NEJMra1208943 (2013).
Annane, D., Bellissant, E. & Cavaillon, J. M. Septic shock. Lancet365 (9453), 63–78. https://doi.org/10.1016/S0140-6736(04)17667-8 (2005).
Zhu, C. L. et al. Effects of CRRT at different time points on inflammatory indicators, hemodynamics and prognosis in sepsis patients. Hebei Med.25 (11), 1825–1829 (2019).
Marik, P. E. et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American college of critical care medicine. Crit. Care Med.36 (6), 1937–1949. https://doi.org/10.1097/CCM.0b013e31817603ba (2008).
Hou, X. et al. Glaucocalyxin A alleviates LPS-mediated septic shock and inflammation via inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol.81 (2), 106271 (2020).
Rhodes, A. et al. Surviving Sepsis campaign: international guidelines for management of Sepsis and septic shock: 2016. Intensive Care Med.43 (3), 304–377. https://doi.org/10.1007/s00134-017-4683-6 (2017).
Xu, W. et al. Effects of continuous intravenous micropump infusion of hydrocortisone combined with thymosin Α1 injection on blood glucose fluctuation and inflammatory factors in patients with septic shock. J. Difficult Dis.18 (11), 1101–1106 (2019).
Li, W. et al. Analysis of the prognostic predictive value of cTnT in patients with cardiogenic shock receiving V-AECMO treatment: a 5-year retrospective study. Chin. Crit. Care Med.32 (9), 1089–1093 (2020).
Annane, D. et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl. J. Med.378 (9), 809–818. https://doi.org/10.1056/NEJMoa1705716 (2018).
Tang, X. G. et al. Clinical value of dynamic changes in serum PCT and LPH levels in evaluating condition and prognosis of sepsis patients. Lab. Med. Clin.17 (15), 2178–2182 (2020).
Tian, X. G. et al. Meta-analysis of the effect of polymyxin B hemoperfusion on prognosis in patients with sepsis and septic shock. Chin. J. Respir Crit. Care Med.19 (1), 67–72 (2020).
Hotchkiss, R. S., Monneret, G. & Payen, D. Immunosuppression in sepsis: A novel Understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis.13 (3), 260–268. https://doi.org/10.1016/S1473-3099(13)70001-X (2013).
Georgakopoulou, A. et al. Role of inherited thrombophilic profile on survival of patients with sepsis. J. Investig Med.67 (8), 1068–1074 (2019).
Venkatesh, B. et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl. J. Med.378 (9), 797–808. https://doi.org/10.1056/NEJMoa1705835 (2018).
Zhou, Y. H. et al. Effects of Esmolol on left ventricular function and prognosis in patients with septic shock. Chin. J. Emerg. Med.28 (1), 71–74 (2019).
Salhotra, R., Sharahudeen, A., Tyagi, A., Rautela, R. S. & Kemprai, R. Effect of continuous infusion vs bolus dose of hydrocortisone in septic shock: A prospective randomized study. Indian J. Crit. Care Med.28 (9), 837–841. https://doi.org/10.5005/jp-journals-10071-24793 (2024).
Rygård, S. L. et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med.44 (7), 1003–1016. https://doi.org/10.1007/s00134-018-5197-6 (2018).
Acknowledgements
The authors would like to extend their sincere appreciation to the staff and colleagues at the Department of Emergency Medicine, the First Affiliated Hospital of Soochow University, Department of Emergency, Lianyungang Second People’s Hospital Affiliated to Kangda College of Nanjing Medical University, and the Department of Emergency, Nantong Third People’s Hospital, for their support and assistance throughout the duration of this study. Special thanks are also given to all the participating patients and their families, who made this research possible through their invaluable contributions and patience.
Funding
This study was supported by the project of Nantong City Natural Science Foundation (JCZ2024008), and the grants from Key Research and Development Program of Lianyungang Science and Technology Bureau (SF2308).
Author information
Authors and Affiliations
Contributions
L.J. and Z.L. conceived and designed the study, acquired data, drafted the manuscript, and revised it critically for important intellectual content. Z.L., J.Q., and W.L. analyzed and interpreted the data and revised the manuscript critically for important intellectual content. F.X. led the conception and design of the study, was involved in the analysis and interpretation of data, drafted the manuscript, and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Nantong Third People’s Hospital, Affiliated Nantong Hospital 3 of Nantong University. All procedures involving human participants were reviewed and approved by the ethics committee prior to the commencement of the study. Written informed consent was obtained from all individual participants included in the study, or from their legal guardians in cases where participants were unable to provide consent themselves. Participants were assured of their anonymity and the confidentiality of their data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, L., Li, Z., Qian, J. et al. Comparative efficacy and prognostic impact of continuous versus intermittent hydrocortisone administration in septic shock patients. Sci Rep 15, 14339 (2025). https://doi.org/10.1038/s41598-025-99198-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99198-z