Abstract
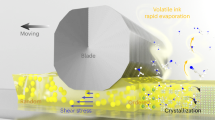
The isothermal precipitation kinetics of perovskite crystals was studied to clarify the mechanism for the influence of the steel slag content on the shape transformation of perovskite crystals. The results show that the growth index n were about 1.5, 2.5, and 2.5 when the contents of steel slag were 15, 25, and 35%, indicating that the precipitation of perovskite crystals was one-dimensional, three-dimensional, and three-dimensional growth. Thus the shapes of the perovskite crystals were dendritic, spherical, and spherical when the contents of steel slag were 15, 25, and 35%. An increase in the content of steel slag can promote the transformation of perovskite crystals into sphere, which is beneficial to the settling of perovskite crystals in molten slag. It realizes the conversion of perovskite from lean ore to rich ore, and avoids subsequent beneficiation process.
Similar content being viewed by others
Introduction
China has a large number of titanium resources, mainly in the form of vanadium-titanium magnetite. Its total reserve volume is about 10 billion tons, which mainly are situated in the Panzhihua regions. After blast furnace ironmaking, the titanium components in the vanadium-titanium magnetite enters the slag to generate titanium-bearing blast furnace slag (10–25% TiO2). Due to the dispersed distribution of titanium components in different fine-grained mineral phases, traditional beneficiation methods can not effectively separate the titanium-bearing phases from the gangue. Thus, the comprehensive utilization of titanium-containing blast furnace slag has always been a technical challenge in China.
To achieve the comprehensive utilization of titanium-bearing blast furnace slag, a large amount of related research has been conducted. On the basis of the classification of research products, methods can be parted into the following types: titanium pigment1, titanium-containing alloy2, photocatalyst3, construction materials4, TiCl45, CaTiO36, anosovite7, and synthetic rutile8,9,10,11,12,13,14,15. Though the aforementioned ways can facilitate the comprehensive utilization of titanium-bearing blast furnace slag, they typically entail drawbacks such as high energy consumption and elevated cost. Until now, titanium-bearing blast furnace slag can only be piled up in slag heaps, leading to the waste of titanium resources and environmental pollution.
To address the aforementioned issues, a new technology for the comprehensive utilization of Ti-bearing blast furnace slag was proposed by Zhang Li et al.16,17. This technology has the advantages of low energy consumption, low cost, and high efficiency. The core of this technology is to realize the settling of perovskite crystals by controlling the addition amount of steel slag. The previous research17 has achieved the settling of perovskite crystals through specific experiments and related theories, and concluded that the settling speed of spherical perovskite crystal is the largest. However, the mechanism for the influence of the steel slag content on the shape transformation of perovskite crystals is still unclear.
In this paper, the isothermal precipitation kinetics of perovskite crystals was firstly used to clarify the mechanism for the influence of the steel slag content on the shape transformation of perovskite crystals. Therefore, this research not only has a certain degree of innovation, but also lays a theoretical foundation for the industrial application of the new technology.
Materials and methods
Materials
In this study, Ti-bearing blast furnace slag and vanadium-bearing steel slag were used as raw materials. The chemical compositions and XRD patterns of Ti-bearing blast furnace slag are shown in (Table 1; Fig. 1), respectively. The chemical compositions of steel slag are shown in (Table 2).
As shown in Fig. 1, Ti-bearing blast furnace slag mainly contains perovskite, melilite, Ti-bearing diopside, and spinel. As illustrated in (Tables 1 and 2), the content of TiO2 in Ti-bearing blast furnace slag was 16.10%, and the content of CaO in vanidium-bearing steel slag was 48.23%.
Experimental procedures
The oxidation gas of modified experiments was air. 400 g of Ti-bearing blast furnace slag and a certain amount of vanadium-bearing steel slag were placed into a corundum crucible at 1470 °C for 60 min. As illustrated in (Table 3), the added amounts of vanadium-bearing steel slag were 15, 25, and 35%, respectively, and the calculation was shown in Eq. (1). Then air was blown into molten slag at a flow ratio of 2 L/min. The oxidation time was 4 min. Whereafter, molten slag was quickly cooled to a specified temperature (1360, 1330, 1300, and 1270 °C) at a cooling rate of 10 °C/min and then held temperature. A corundum tube was used to take samples at a regular interval (5, 4, and 3 min). Then the samples were quickly put into cold water for quenching treatment. The crystallization situation was observed and photographed under a scanning electron microscope. With the help of related software, 15 photos with different fields were selected to count the volume fraction of perovskite crystals.
Where w2 was the added amount of vanadium-bearing steel slag; m2 was the added mass of vanadium-bearing steel slag; m3 was the added mass of Ti-bearing blast furnace slag.
Characterization
The chemical constituents of these samples were analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES, PerkinElmer Optima 4300DV). The phase constituents of these samples were identified by X-ray diffraction (XRD, X’PERT PROMPD/PW3040, PANalytical B.V. Corp., The Netherlands) using Cu Kα radiation for 10 min from 10 to 90°. The micromorphology and element distribution in these samples were performed by scanning electron microscope (SEM, TESCAN VEGA III) equipped with an energy dispersive spectrometer (EDS, INCA Energy 350). The density was determined by the buoyancy method based on Archimedes principle.
Results and discussion
Influence of the content of vanadium-bearing steel slag on perovskite shape and perovskite settling
According to the experimental program in (Table 3), modification experiments were carried out. The microscopic morphology of samples at the upper and lower parts of the modified slag was observed, and then 15 images with different fields were selected to count the volume fraction of perovskite crystal. The illustration of the sample selection ___location is shown in (Fig. 2). The results are shown in (Figs. 3, 4 and 5). The apparent density of the quenched slag was measured, and the results are shown in (Table 4).
Volume fraction of the perovskite crystals in the upper and lower parts of the modified slag under the different experimental scheme (Table 3).
As shown in Figs. 3 and 4, when the content of vanadium-bearing steel slag increased from 15 to 35%, the morphology transformation of perovskite crystal: dendritic → spherical. Based on previous research17, the settling speed of spherical crystal > the settling speed of dendritic crystal. Therefore, the perovskite settling becomes more and more significant with an appropriate increase in the content of steel slag. Moreover, as shown in (Fig. 5), the modified slag is composed of three phases: perovskite (white phase in Fig. 3), melilite (dark grey phase in Fig. 3), and magnesium aluminum spinel (black phase in Fig. 3). As shown in (Table 4), the apparent density of the quenched slag was about 3.00 g/cm3. There is a significant difference in density compared to perovskite (3.98 g/cm3). This is another major reason for the settling of perovskite in molten slag.
Influence of the content of vanadium-bearing steel slag on the isothermal precipitation kinetics of perovskite
Theoretical derivation of the isothermal precipitation kinetics equation of perovskite
The precipitation kinetics of perovskite crystal can be studied by JMAK18,19,20 Eq. (2).
Where k is a coefficient related to the nucleation rate of the new phase and the crystal growth rate; n is the crystal growth index; R is the transition fraction.
Taking the logarithm of Eq. (2):
Taking the logarithm of Eq. (3):
Then a straight line was drawn according to Eq. (4). The n and k can be determined by the slope and intercept of the straight line.
Substituting the ln(k) values obtained at different temperatures into the Arrhenius equation can obtain the crystallization activation energy.
The Arrhenius21,22 equation is shown in Eq. (5).
Taking the logarithm of Eq. (5):
Here \({k_0}\) is constant; R is gas constant; E is the activation energy of crystallization.
The transformation fraction of perovskite crystal can be calculated using Eq. (7).
Where \(f\left( {T,t} \right)\) is the volume fraction of perovskite crystal at temperature T; \(f\left( {T,eq} \right)\) is the volume fraction of perovskite crystal under equilibrium condition.
Experimental study on the isothermal precipitation kinetics of perovskite
Through high temperature quenching experiments, the relationships between perovskite transformation fraction and time at four temperatures (1360, 1330, 1300, and 1270 °C) under different vanadium-bearing steel slag content (15, 25, and 35%) were obtained. The results are as shown in (Figs. 6, 7 and 8).
As illustrated in Figs. 6, 7 and 8, the transformation fraction of perovskite crystals first increased rapidly and then tended to be flat with an increase in time. In the meantime, the lower the temperature, the higher the transformation fraction of perovskite crystals. This is because the lower the precipitation temperature, the greater the degree of supercooling and the greater the supersaturation of CaTiO3, which is more conducive to the precipitation of perovskite crystals. In addition, with an increase in the content of vanadium-bearing steel slag, the time when the transformation fraction of perovskite crystals reached the maximum became shorter and shorter, indicating that an increase in the content of vanadium-bearing steel slag can promote the rapid precipitation of perovskite crystals. This is because an increase in the content of vanadium-bearing steel slag makes the supersaturation of CaTiO3 larger, thereby promoting the precipitation of perovskite crystals.
According to Eq. (4), a straight line can be obtained, and then the data was linearly fitted by Origin software. The linear fitting line and fitting parameters are shown in (Figs. 9, 10 and 11; Tables 5, 6 and 7).
It can be seen from Figs. 9, 10 and 11; Tables 5, 6 and 7 that the correlation coefficient R2 was greater than 98.00% when the contents of vanadium-bearing steel slag were 15, 25, and 35%. Thus ln[-ln(1-R)] and ln(t) satisfied the linear relationship, indicating that the isothermal precipitation kinetics of perovskite crystals can be described by JMAK Eq. (2). In addition, it can be seen from Tables 4, 5 and 6 that when the content of vanadium-bearing steel slag was 15%, the growth index n was about 1.5, indicating that perovskite precipitation was one-dimensional growth. Thus the shapes of peroskite crystals were dendritic. When the content of vanadium-bearing steel slag was 25%, the growth index n was about 2.5, indicating that perovskite precipitation was three-dimensional growth. Therefore the shapes of perovskite crystals were mostly spherical. When the content of vanadium-bearing steel slag was 35%, the growth index n was about 2.5, indicating that peroskite precipitation was three-dimensional growth. Thus the shapes of perovskite crystals were mostly spherical. The above results fully prove the influence of the vanadium-bearing steel slag content on the shape transformation of perovskite crystals in the Sect. 3.1. Moreover, The perovskite crystal showed one-dimensional or three-dimensional growth, which might be affected by the chemical composition. The effect of vanadium-bearing steel slag is to provide CaO for titanium-bearing blast furnace slag. At high temperature, CaO reacts with TiO2 in titanium-bearing blast furnace slag (Oxidation process turns low valent titanium oxides into TiO2) to form CaTiO3. In other words, the addition of vanadium-bearing steel slag increases the concentration of CaTiO3. The increase of CaTiO3 concentration leads to the increase of nucleation rate and the decrease of growth rate, which promotes the transformation of perovskite from one-dimensional growth to three-dimensional growth.
According to Eq. (6), a straight line can be obtained, and the data was linearly fitted by origin software. The linear fitting line and fitting parameters are shown in (Figs. 12, 13 and 14; Table 8).
It can be seen from Figs. 12, 13 and 14; Table 8 that the correlation coefficient R2 was greater than 97.00% when the contents of vanadium-bearing steel slag were 15, 25, and 35%. Thus ln(k) and 1/T met the linear relationship, indicating that the activation energy for the precipitation of perovskite crystals can be calculated by Arrhenius Eq. (6). As shown in (Table 8), the isothermal precipitation activation energy (absolute value) of perovskite crystal gradually decreased with an increase in the content of vanadium-bearing steel slag, indicating that an increase in the content of vanadium-bearing steel slag is conducive to the precipitation of perovskite crystals. In addition, it can be seen from Table 8 that the isothermal crystallization activation energy of perovskite crystals was negative. This is because the coefficient k related to the nucleation rate of new phase and the crystal growth rate increased with a decrease in temperature, resulting in the activation energy was negative.
Conclusions
-
(1)
While the content of vanadium-bearing steel slag increased from 15 to 35%, the shape transformation of perovskite crystals: dendritic → spherical.
-
(2)
The isothermal precipitation kinetics of perovskite crystals can be described by JMAK equation.
-
(3)
When the contents of vanadium-bearing steel slag were 15, 25, and 35%, the growth index n was about 1.5, 2.5, and 2.5, respectively, indicating that the precipitation of perovskite crystals was one-dimensional, three-dimensional, and three-dimensional growth. Thus the shapes of perovskite crystals were dendritic, spherical, and spherical.
-
(4)
The isothermal precipitation activation energy (absolute value) of perovskite crystals gradually decreased with an increase in the content of vanadium-bearing steel slag, indicating that an increase in the content of vanadium-bearing steel slag is conducive to the precipitation of perovskite crystals.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Fang, Y., Chun, L. & Bin, L. A two-step sulfuric acid leaching process of Ti-bearing blast furnace slag. Chin. J. Process. Eng. 3, 413–417 (2006).
Zhou, X. & li Lu Xiong gang. Preparation of titanium alloy by direct reduction of Ti-bearing blast furnace slag. Chin. J. Nonferrous Met. 20 (09), 1829–1835 (2010).
Lei Xue fei, Xue Xiang xin. Effect of sulfate on photocatalytic activity of titanium-bearing blast furnace slag. Mater. Rep. 23 (02), 63–66 (2009).
Yang He, Z. Comprehensive utilization of BF slags in Building materials. Conserv. Utilization Mineral. Resour. 1, 47–51 (2004).
Gao, Y. Li ci Ying, Li Ya Wei. Analysis of carbothermal reduction of TiO2 and extraction of titanium carbonitride from the blast furnace slag bearing Titania. J. Wuhan Univ. Sci. Technol. 30 (01), 5–9 (2007). (in Chinese).
Li, Y. Lou Tai ping, Sui Zhi Tong. Selective enrichment of Ti component in Ti-bearing blast furnace slag and precipitation behavior of perovskite phase. Chin. J. Nonferrous Met. 5, 719–722 (2000). (in Chinese).
Li, J. et al. The influence of SiO2 on the extraction of Ti element from Ti-bearing blast furnace slag [J]. Steel Res. Int. 82 (6), 607–614 (2011).
Zhang, L. et al. Thermodynamic analysis of extraction of synthetic rutile from modified slag. Ind. Eng. Chem. Res. 52 (13), 4924–4931 (2013).
Zhang, W. et al. Crystallization and coarsening kinetics of rutile phase in modified Ti-bearing blast furnace slag. Ind. Eng. Chem. Res. 51 (38), 12294–12298 (2012).
Zhang, W. et al. An environmental procedure to extract titanium components and metallic iron from Ti-bearing blast furnace slag [J]. Green. Process. Synth. 4 (4), 307–316 (2015).
Zhang, W. et al. Crystallization behavior and growing process of rutile crystals in Ti-bearing blast furnace slag. High Temp. Mater. Processes (Lond.) 35 (8), 787–797 (2016).
Li, J., Wang, X. D. & Zhang, Z. T. Crystallization behavior of rutile in the synthesized Ti-bearing blast furnace slag using single hot thermocouple technique. ISIJ Int. 51 (9), 1396–1402 (2011).
Sun, Y. Q. et al. The effect of P2O5 on the crystallization behaviors of Ti-bearing blast furnace slags using single hot thermocouple technique. Metall. Mater. Trans. B. 45 (4), 1446–1455 (2014).
Du, Y. et al. Selective precipitation and in situ separation of rutile crystals from titanium bearing slag melt in a super-gravity field. CrystEngComm 20 (27), 3868–3876 (2018).
Du, Y. et al. Recovery of rutile from Ti-bearing blast furnace slag through phase transformation and super-gravity separation for dielectric material. Ceram. Int. 46 (7), 9885–9893 (2020).
Zhang, J. H., Han, J. Q., Chen, X., Zhang, J. & Zhang, L. Experimental study on titanium-containing mixed slag. Light Met. 09, 40–44 (2021).
Zhang Jiahao. Research on Calcium Titanium Mineralization and Sedimentation Technology of Titanium Components in Titanium-containing Mixed Slag [D] (Northeastern University, 2021).
Lorenzo, A. T. et al. DSC isothermal polymer crystallization kinetics measurements and the use of the avrami equation to fit the data: guidelines to avoid common problems. Polym. Test. 26 (2), 222–231 (2007).
Kalu, P. N. & Waryoba, D. R. A JMAK-microhardness model for quantifying the kinetics of restoration mechanisms in inhomogeneous microstructure. Mater. Sci. Eng. A. 464 (1–2), 68–75 (2007).
Li, J. J. et al. Comparison of Johnson-Mehl-Avrami-Kologoromov (JMAK) kinetics with a phase field simulation for polycrystalline solidification. Acta Mater. 55 (3), 825–832 (2007).
Petrowsky, M. & Frech, R. Salt concentration dependence of the compensated arrhenius equation for alcohol-based electrolytes. Electrochim. Acta. 55 (4), 1285–1288 (2010).
Chen, H. & Liu, N. Application of non-Arrhenius equations in interpreting calcium carbonate decomposition kinetics: revisited. J. Am. Ceram. Soc. 93 (2), 548–553 (2010).
Funding
This work was supported by Funding Projects for General Project of Fundamental Science Research in Higher Education Institutions in Jiangsu Province (24KJB450001), Jiangsu Vocational College of Electronics and Information Natural Science Research Project (JSEIY2023002), Huai’an Fundamental Research Program Project (HABZ202320), Huai’an Fundamental Research Program Project (HAB2024068), Huai’an Innovation Service Capacity Building Plan Project (HAP202303), Huai’an Natural Science Research Program Project (HABZ202222), and Jiangsu Universities Excellent Science and Technology Innovation Team Funding Project, Jiangsu Vocational College of Electronics and Information Youth Fund Project (JSEISYB202409).
Author information
Authors and Affiliations
Contributions
Jiqing Han, Li Zhang, and Hongmei Yin designed the experiments; Jiqing Han and Qiuping Feng conducted the experiments and collected the data; Jiqing Han and Hongsheng Zhang analyzed the data; Jiqing Han wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, J., Zhang, L., Yin, H. et al. Mechanism research for influence of steel slag on shape transformation of perovskite crystals. Sci Rep 15, 13961 (2025). https://doi.org/10.1038/s41598-025-99209-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99209-z