Abstract
This study explored the relationship between cognitive function and brain structure in lung cancer (LCs) patients without brain metastases and healthy controls (HCs). A cohort of 75 chemotherapy-naive LCs without brain metastases and 29 age-, sex-, and education-matched HCs underwent cognitive assessments and structural MRI. The MRI focused on cortical thickness, surface area, and volume of subcortical structures. We examined the relationships among these parameters. The volume of twelve subcortical structures was significantly reduced in patients with advanced-stage lung cancer (aLCs) compared to HCs (p < 0.05). In aLCs, cortical thickness decreased in one brain region and surface area in five regions (p < 0.05). Patients with early-stage lung cancer (eLCs) exhibited increased cortical thickness in three regions. When comparing eLCs to aLCs, there was a notable decrease in cortical thickness and surface area (p < 0.05). Visuospatial/executive and delayed memory functions were impaired in aLCs and worsened with disease progression. These impairments correlated positively with the thickness of several cerebral cortices and the surface area and volume of subcortical structures (p < 0.05). Structural brain changes and cognitive dysfunction are evident in aLC patients, independent of metastasis. Since none of the patients received chemotherapy, the observed abnormalities in aLCs, absent in eLCs, are likely attributable to the disease itself rather than chemotherapy effects.
Similar content being viewed by others
Introduction
LC ranks among the world’s most prevalent cancers1. It often eludes early detection due to the lack of distinct symptoms in its initial stages, leading to late diagnoses at advanced stages with poor prognoses2. The treatment for LC encompasses various therapies, including chemotherapy, radiation, immunotherapy, and targeted therapy. These treatments are selected based on cancer characteristics and patient-specific factors3. An additional consideration is the potential side effects or complications of treatments, which can impact patients’ quality of life and functioning in both the short and long term. In advanced LC stages (III or IV), where cure is improbable, the treatment objective shifts to prolonging and maintaining quality of life rather than tumor elimination. Consequently, patients’ awareness of the tumor’s impact on cognitive function is crucial in choosing a suitable and personalized treatment plan, especially to avoid therapies that might exacerbate cognitive impairment.
Recent attention has focused on“chemical brain”or"chemical fog,"phenomena thought to affect patients’ attention, executive function, and memory significantly4,5. These cognitive changes, commonly known as"cancer-related cognitive impairment"(CRCI)6, have been observed in LC patients following chemotherapy, particularly affecting executive function, fluency, and verbal memory7,8,9,10. However, cognitive changes in cancer patients might also stem from the cancer itself. Brain metastases from LC can impair cognitive functions. Furthermore, as LC progresses, primary tumors can release cytokines or form circulating tumor cells (CTCs), which invade the brain’s microcirculation11,12and disrupt the blood–brain barrier (BBB). This disruption allows harmful substances, such as pro-inflammatory cytokines (e.g., TNF-α, IL-6), tumor-derived exosomes, and circulating cell-free DNA, into the brain, potentially affecting cognitive function and brain structure12,13. Yet, research on the direct cognitive impacts of LC itself, including various stages and types, is limited. Thus, it remains challenging to attribute cognitive impairment post-chemotherapy solely to the cancer’s progression or the chemotherapeutic agents.
CRCI can manifest at various stages of cancer, including at diagnosis, during treatment, and even years after treatment has concluded. Research indicates that some cancer patients, such as those with breast cancer, blood cancer, gynecologic cancer, brain tumors, and childhood cancer6,14,15, experience cognitive impairment prior to any treatment. Numerous factors may contribute to this, including the cancer itself, the type of treatment, individual differences, and psychosocial factors. Consequently, research on CRCI must take these factors into account to better comprehend and manage cognitive impairment in cancer patients.
While most studies have concentrated on the cognitive effects of conventional chemotherapy in LC patients, the direct impact of LC on cognition and brain structure remains less understood. There is a scarcity of research examining cognitive performance in untreated LC patients across different stages. This gap is significant because LC progression involves the secretion of cytokines and the formation of CTCs in the brain’s microcirculation, which can damage the BBB. This damage allows harmful substances to enter the brain, leading to cognitive dysfunction. To bridge this gap, our study employed MRI scans on LC patients without brain metastases at various stages. Our goal was to investigate the correlation between changes in the volume of the cerebral cortex and subcortical structures, cognitive function, and the progression of LC.
Materials and methods
Participant selection
This cross-sectional study received ethical approval from the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University (NO. SLKYLX202118). All participants provided informed consent prior to participation.
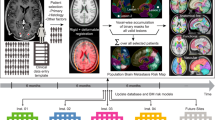
Between August 2021 and February 2022, 75 untreated LCs from the Department of Thoracic Surgery at the same hospital, and 29 HCs, recruited through advertisements and matched for age, sex, and education level, were enrolled. All subjects were right-handed. Inclusion criteria for LC patients included a confirmed diagnosis of LC, being older than 18 years, and no prior treatment (surgery, radiotherapy, chemotherapy, or immunotherapy). Exclusion criteria for all participants encompassed prophylactic cranial irradiation, brain metastases, history of stroke, cranial trauma, epilepsy, Alzheimer’s disease, Parkinson’s disease, other acute mental or neurological disorders, severe medical conditions (e.g., anemia, serious heart, thyroid, liver, or kidney dysfunction), and significant visual or hearing impairments. The Montreal Cognitive Assessment (MoCA) assessed neuropsychological status and general cognitive function16. According to TNM (tumor-node-metastasis) criteria17, early-stage LC was classified as stage I, and Advanced-stage LC (aLC) includes stages II–IV per TNM classification. None of the patients exhibited brain metastases; however, stage IV patients had distant metastases in organs such as bone, liver, or adrenal glands. The study’s flowchart is depicted in Fig. 1.
MRI acquisition
MRI data was obtained using 3.0 T MRI scanners (Discovery MR750, GE Healthcare). A 21-channel head and neck array coil facilitated parallel acquisition. Head movement was minimized with firm foam pads, and earplugs were used to reduce scanner noise. Participants lay with closed eyes, stayed awake, and were instructed to think of nothing specific. Imaging sequences included T1- and T2-weighted thick-slice and FLAIR imaging to rule out brain lesions or metastases, and high-resolution T1-weighted 3D axial anatomical imaging (BRAVO, TR = 8.6 ms, TE = 3.3 ms, FOV 25.6 × 25.6, 256 × 256 matrix, 1 mm voxel size, 31.25 kHz bandwidth, 12° FA, acceleration 2, 2 averages, 4 min 29 s scan time). Data with excessive head movement, incomplete images, or scanning errors were excluded after quality control.
Data preprocessing
Whole brain volumes and T1-weighted anatomical images were processed using FreeSurfer software (version 7.2)18. FreeSurfer’s morphometric analysis, known for its test–retest reliability19,20, involved skull stripping, talairach transformations, atlas registration, and spherical surface mapping and parcellation based on an unbiased in-patient template (Desikan-Killiany atlas21). Cortical thickness, area of 34 cortical regions, and volume of 16 bilateral subcortical brain regions were calculated. Brain volumes were normalized to total intracranial capacity (TIV) to adjust for head size variations22.
The blood collection and processing
Blood samples were collected through venipuncture in the fasting state in the early morning, and EDTA was used as an anticoagulant. Samples were centrifuged within 2 h of collection, plasma/serum was separated, and stored at −80 °C until analysis.
The blood samples were analyzed by electrochemical immunoassay (EIA) to detect tumor markers. We selected Carcinoembryonic antigen (CEA), Neuron-specific enolase (NSE), Cytokeratin 19 fragment (CYFRA21-1), and Squamous cell carcinoma antigen (SCC) as markers because they are closely related to the progression and prognosis of lung cancer.
Statistical analysis
Statistical analyses were conducted using R (version 4.0.5). The Shapiro–Wilk test assessed the normal distribution of continuous variables. Parametric statistics were applied to normally distributed data, and nonparametric statistics to non-normally distributed data. For normally distributed outcomes, ANOVA with least significant difference (LSD) pairwise tests were used. For non-normal outcomes, the Kruskal–Wallis test followed by Bonferroni post-hoc analysis was employed. A p-value of less than 0.05 was deemed statistically significant.
Results
Demographic and clinical characteristics
In this study, 75 LCs (39 eLCs and 36 aLCs) and 29 HCs (recruited via online advertisements) participated. The study was conducted at the Department of Thoracic Surgery, Third Affiliated Hospital, Kunming Medical University, China, from May 2021 to December 2021. The participants varied in age and gender. Eleven individuals were excluded due to excessive head motion, allergies to contrast agents, or scanning errors. Figure 1 illustrates the participant selection and exclusion process.
Table 1 provides a comprehensive summary of the participants’ demographic data, histologic diagnoses, and tumor stages. There were no significant differences in age, education, sex, smoking habits, KPS scores, or pathology types among eLC, aLC, and HC groups (P > 0.05). However, MoCA scores were significantly lower in the aLC group compared to both eLC and HC groups (25.42 ± 2.48 vs. 27.49 ± 1.65 and 27.45 ± 1.66, respectively, P < 0.001). No notable difference was observed in MoCA scores between eLC and HC (P = 0.936). Tumor diameters were smaller in the eLC group than in the aLC group (P < 0.001). Levels of tumor markers including CEA, NSE, CYFRA21-1, and SCC were higher in the aLC group compared to the eLC group (P < 0.05).
Data are expressed as mean ± SD, n (%) or interquartile range (P25, P50, P75) expressed. a P values were determined using the χ2 test. b P values were determined using the one-way test ANOVA. c P values were determined using the two-sample t test. d NA is not statistically analyzed. e P values were determined using the Kruskal–Wallis test. *P < 0.05, **P < 0.01, ***P < 0.001. II (no metastases), III (lymph node), IV (distant metastases, liver = 5, bone = 4, adrenal = 3).
CEA (carcinoembryonic antigen), NSE (neuron-specific enolase), CYFRA21-1 (cytokeratin 19 fragment), SCC (squamous cell carcinoma antigen).
Cerebral cortex alterations in LC
Cortical thickness in eLC patients increased in the bilateral cuneus and left insula compared to HCs (P < 0.05), while aLC patients exhibited decreased thickness in the superior temporal region (P < 0.05). Further, aLC patients showed a reduction in cortical thickness in various regions compared to eLC patients (P < 0.05) (Figs. 2A, 3, and Supplementary tables S1, S2). In aLC patients, the surface area of several cortical regions decreased relative to HCs (P < 0.05), while eLC patients exhibited an increase in the area of the left supramarginal gyrus (P < 0.05). Significant differences were also observed between eLC and aLC groups in various cortical areas (P < 0.05) (Figs. 2B, 4, and Supplementary tables S3, S4).
The distribution of different subcortical volumes and cortical thicknesses in the brain (different colors represent different brain structures). A-D is the axial image, E-I is the coronal image, and J-K is the sagittal image. lh, left hemisphere; rh, right hemisphere; left-Inf-Lat-Vent: left temporal horn of the lateral ventricle.
Subcortical volume, normalized as a percentage of TIV, decreased in several regions in aLC patients compared to HCs (P < 0.05), with an increase in the volume of the left temporal horn of the lateral ventricle. Except for the bilateral ventral diencephalon, no significant differences were observed in eLC patients. The volumes of certain structures were smaller in aLC than in eLC patients (P < 0.05) (Figs. 2C, 3, and Supplementary tables S5, S6).
Cognitive function alterations
Cognitive impairments in aLC patients, particularly in visuospatial/executive and delayed memory functions, were significantly different compared to HCs (P < 0.001; P = 0.031, respectively). No notable difference was found between eLC patients and HCs in these domains (Figs. 2D, and Supplementary tables S7, S8).
A Cortical Thickness Differences: eLC exhibited increased cortical thickness in the bilateral cuneus and left insula compared to healthy controls (HCs) (P < 0.05). aLC showed decreased cortical thickness in the superior temporal region compared to HCs (P < 0.05). When comparing aLC patients to eLC patients, there was a reduction in cortical thickness in various regions (P < 0.05).
B Cortical Surface Area Differences: aLC patients had a reduction in cortical surface area in several regions compared to HCs (P < 0.05). eLC patients showed an increase in the area of the left supramarginal gyrus compared to HCs (P < 0.05). Significant differences in various cortical areas were also observed between eLC and aLC groups (P < 0.05).
C Normalized Brain Structure Volume Ratio Differences: aLC patients showed a decrease in the volume ratio of several regions compared to HCs (P < 0.05), with an increase in the volume of the left temporal horn of the lateral ventricle. Except for the bilateral ventral diencephalon, no significant differences were observed in eLC patients. The volumes of certain structures were smaller in aLC than in eLC patients (P < 0.05).
D Cognitive Function Differences: aLC patients had significant differences in visuospatial/executive and delayed memory functions compared to HCs (P < 0.001; P = 0.031, respectively). No notable differences were found between eLC patients and HCs in these domains.
P < 0.05 is considered significant. * P < 0.05, ** P < 0.01, *** P < 0.001.
Correlation analyses
Brain structure and cognitive function
The study analyzed the correlation between differential cortical thickness, surface area, volume, and cognitive function in eLC, aLC, and HCs. Visual-spatial/executive function showed positive correlations with certain brain regions (P < 0.05). Delayed recall was positively correlated with volumes of various subcortical structures and negatively with right choroid plexus volume (P < 0.05) (Fig. 5A and Supplementary tables S9).
Correlations between brain structure and cognitive functions. A show the correlation between cognitive impairment and brain regions in patients with LCs and HCs (n = 104). B shows the correlation between tumor diameter, serum tumor markers, cognitive impairment, and brain structure in patients with LCs (n = 75).
Tumor characteristics, brain structure, and cognitive function
The relationship between tumor diameter, serum tumor markers, brain structure, and cognitive function in eLC and aLC patients was explored. Visuospatial/executive performance was negatively correlated with tumor diameter and CYFRA211 levels (P < 0.05). Delayed recall showed a negative correlation with tumor diameter (P = 0.002) (Fig. 5B and Supplementary tables S10). Tumor diameter and serum markers exhibited correlations with various brain structures and cognitive functions, with both positive and negative associations observed (P < 0.05) (Fig. 5B and Supplementary tables S10-11).
Discussion
Our research indicates that structural brain changes and cognitive dysfunctions are evident in LC patients without brain metastases, particularly in advanced stages. These findings suggest that these abnormalities may be due to the disease itself rather than its treatment, contributing to the understanding of the direct impact of LC on cognition and brain structure prior to treatment. The results highlight the need for treatment of advanced LC to consider potential damage to brain structure and cognitive function.
There is growing evidence that cancer therapies, even those not targeting the central nervous system, can have acute and long-term cognitive effects, affecting educational and occupational goals, and overall quality of life6,14,23,24. However, studies on the direct impact of cancer on cognition and its relation to brain structure before treatment are limited. Understanding these cognitive changes is essential for patients to make informed treatment decisions, as inappropriate choices may exacerbate cognitive impairments and reduce quality of life.
Our study investigated brain structure and cognitive function differences among participants with eLC, aLC, and HC. Key findings include that all LCs were free of brain metastases, and there were no significant differences in KPS, smoking, or tumor pathology types, enhancing the study’s reliability. The results showed reduced subcortical volume and cortical surface area in aLC, while eLC patients exhibited a slight increase in cortical thickness and surface area. This increase in cortical volume in early-stage lung cancer patients could be due to neuroplastic changes and compensatory mechanisms, as the brain may respond to the tumor by increasing synaptic connections or the volume of certain brain regions to maintain or enhance cognitive function as a protective response25. Furthermore, visuospatial/executive reasoning and delayed recall functions were impaired in aLC patients, worsening with disease progression and correlating positively with cerebral cortex thickness, surface area, and subcortical structure volumes, suggesting that LC itself may impact brain function during its progression.
The development of lung cancer involves a complex interplay of factors over many years3. Tumor cells secrete growth factors, chemokines, and cytokines, and CTCs formed by local infiltration enter the bloodstream, reaching the cerebral microcirculation13. As LC progresses, the number of CTCs increases26, and they may undergo epithelial-mesenchymal transition27, altering their gene expression profiles and increasing their invasive and migratory potential. Concurrently, various factors can alter the BBB, facilitating the invasion of surviving CTCs into brain tissue28,29. In early-stage LC, compensatory mechanisms might be triggered to maintain normal brain function25, but as the disease progresses, BBB dysfunction and increased permeability allow more CTCs and harmful substances to invade the brain, damaging its structure and function30. This is reflected in reduced cortical thickness, surface area, and subcortical volume, accompanied by cognitive impairment.
LC frequently metastasizes the brain, primarily affecting the frontal lobe, followed by the parietal lobe, cerebellum, occipital lobe, and temporal lobe31. Our study noted a reduction in cortical thickness and surface area in frontal, parietal, and temporal lobes in advanced LC patients without overt brain metastases. These structural changes may reflect early microvascular or inflammatory effects of CTCs or cytokines on the brain parenchyma, as CTCs can disrupt the BBB and induce neuroinflammation even in the absence of macroscopic metastases12,13,30. Similar patterns of cortical thinning have been observed in preclinical models of systemic cancer, where tumor-derived factors promote neuronal damage (Shi et al., 2021).
Cognitive impairment in cancer patients raises concerns about the increased risk of dementia32,33. Our findings indicate significant impairments in visuospatial/executive function and delayed memory in aLC patients, exacerbated by disease progression. These impairments may be linked to the structural changes in specific brain regions described above. When treating LC, it is crucial to consider these cognitive impairments and avoid treatment plans that could worsen them, thereby affecting the patient’s quality of life.
The strengths of this study include the absence of recruitment bias and the short interval between clinical assessment and neuroimaging, typically within a week. However, its limitations include a small sample size, restricting the generalizability of the findings. As a cross-sectional study, it precludes the assessment of disease evolution. Moreover, the MoCA, while a simple and accessible cognitive screening tool, may lack sensitivity in certain areas.
Conclusions
This study uncovers the direct impact of lung cancer on brain structure and cognitive function, particularly in advanced-stage patients. It underscores the importance of considering potential brain and cognitive effects when devising treatment plans, steering clear of therapies that might worsen cognitive impairments. The study’s contribution to the existing body of knowledge is that it demonstrates the direct effects of lung cancer, independent of treatment side effects, on the brain. It also underscores the correlation between disease progression and changes in the brain.
For future research, this study suggests new avenues, such as investigating biomarkers, refining treatment strategies, and developing preventive interventions. In terms of patient care, it advocates for personalized treatment plans and underscores the importance of cognitive rehabilitation services and interdisciplinary collaboration between oncology, neurology, and cognitive science.
Data availability
The data that support the findings of the current study may be requested from the corresponding author upon reasonable request.
Abbreviations
- LC:
-
Lung cancer
- eLC:
-
Early lung cancer
- aLC:
-
Advanced lung cancer
- HC:
-
Healthy controls
- CRCIs:
-
Cancer-related cognitive impairment
- CTCs:
-
Circulating tumor cells
- BBB:
-
Blood–brain barrier
- MoCA:
-
Montreal Cognitive Assessment
- TNM:
-
Tumor-node-metastasis
- TIV:
-
Total intracranial capacity
- CEA:
-
Carcinoembryonic antigen
- NSE:
-
Neuron-specific enolase
- CYFRA21-1:
-
Cytokeratin 19 fragment
- SCC:
-
Squamous cell carcinoma antigen
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. https://doi.org/10.3322/caac.21442 (2018).
Hirsch, F. R. et al. Lung cancer: current therapies and new targeted treatments. Lancet 389, 299–311. https://doi.org/10.1016/s0140-6736(16)30958-8 (2017).
Haaland, G. S., Falk, R. S., Straume, O. & Lorens, J. B. Association of warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern. Med. 177, 1774–1780. https://doi.org/10.1001/jamainternmed.2017.5512 (2017).
Joly, F., Rigal, O., Noal, S. & Giffard, B. Cognitive dysfunction and cancer: which consequences in terms of disease management?. Psychooncology 20, 1251–1258. https://doi.org/10.1002/pon.1903 (2011).
Lange, M. et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol 30, 1925–1940. https://doi.org/10.1093/annonc/mdz410 (2019).
Joly, F. et al. Impact of Cancer and Its treatments on cognitive function: Advances in research from the paris international cognition and cancer task force symposium and update since 2012. J. Pain Symptom Manage 50, 830–841. https://doi.org/10.1016/j.jpainsymman.2015.06.019 (2015).
Grosshans, D. R., Meyers, C. A., Allen, P. K., Davenport, S. D. & Komaki, R. Neurocognitive function in patients with small cell lung cancer : effect of prophylactic cranial irradiation. Cancer 112, 589–595. https://doi.org/10.1002/cncr.23222 (2008).
Komaki, R. et al. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int. J. Radiat. Oncol. Biol. Phys. 33, 179–182. https://doi.org/10.1016/0360-3016(95)00026-u (1995).
Welzel, T. et al. Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am. J. Neuroradiol. 29, 379–383. https://doi.org/10.3174/ajnr.A0797 (2008).
Whitney, K. A. et al. Is“chemobrain” a transient state? A prospective pilot study among persons with non-small cell lung cancer. J. Support Oncol. 6, 313–321 (2008).
Spano, D., Heck, C., De Antonellis, P., Christofori, G. & Zollo, M. Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 22, 234–249. https://doi.org/10.1016/j.semcancer.2012.03.006 (2012).
Carpenter, P. M. et al. Migration of breast cancer cell lines in response to pulmonary laminin 332. Cancer Med. 6, 220–234. https://doi.org/10.1002/cam4.957 (2017).
Shi, J. Y. et al. Alternative splicing events in Tumor Immune Infiltration in colorectal cancer. Front. Oncol. 11, 583547. https://doi.org/10.3389/fonc.2021.583547 (2021).
Ahles, T. A., Root, J. C. & Ryan, E. L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol. 30, 3675–3686. https://doi.org/10.1200/jco.2012.43.0116 (2012).
Ruiz-Casado, A., Álvarez-Bustos, A., de Pedro, C. G., Méndez-Otero, M. & Romero-Elías, M. Cancer-related Fatigue in Breast Cancer Survivors: A Review. Clin. Breast Cancer 21, 10–25. https://doi.org/10.1016/j.clbc.2020.07.011 (2021).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Detterbeck, F. C., Boffa, D. J., Kim, A. W. & Tanoue, L. T. The Eighth Edition Lung Cancer Stage Classification. Chest https://doi.org/10.1016/j.chest.2016.10.010 (2017).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: A robust approach. Neuroimage 53, 1181–1196. https://doi.org/10.1016/j.neuroimage.2010.07.020 (2010).
Han, X. et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32, 180–194. https://doi.org/10.1016/j.neuroimage.2006.02.051 (2006).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418. https://doi.org/10.1016/j.neuroimage.2012.02.084 (2012).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021 (2006).
Whitwell, J. L., Crum, W. R., Watt, H. C. & Fox, N. C. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am. J. Neuroradiol. 22, 1483–1489 (2001).
Deprez, S. et al. International Cognition and Cancer Task Force Recommendations for Neuroimaging Methods in the Study of Cognitive Impairment in Non-CNS Cancer Patients. J. Natl. Cancer Inst. 110, 223–231. https://doi.org/10.1093/jnci/djx285 (2018).
Wefel, J. S., Vardy, J., Ahles, T. & Schagen, S. B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12, 703–708. https://doi.org/10.1016/s1470-2045(10)70294-1 (2011).
Enciu, A. M. et al. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 11, 75. https://doi.org/10.1186/1471-2377-11-75 (2011).
Xie, Z., Gao, X., Cheng, K. & Yu, L. Correlation between the presence of circulating tumor cells and the pathologic type and staging of non-small cell lung cancer during the early postoperative period. Oncol. Lett. 14, 5825–5830. https://doi.org/10.3892/ol.2017.6910 (2017).
Stovold, R. et al. Biomarkers for small cell lung cancer: neuroendocrine, epithelial and circulating tumour cells. Lung Cancer 76, 263–268. https://doi.org/10.1016/j.lungcan.2011.11.015 (2012).
Reijm, E. A. et al. An 8-gene mRNA expression profile in circulating tumor cells predicts response to aromatase inhibitors in metastatic breast cancer patients. BMC Cancer 16, 123. https://doi.org/10.1186/s12885-016-2155-y (2016).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584. https://doi.org/10.1126/science.1228522 (2013).
Verheggen, I. C. M. et al. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 42, 1751–1764. https://doi.org/10.1007/s11357-020-00282-1 (2020).
Wu, S. G. et al. Distribution of metastatic disease in the brain in relation to the hippocampus: a retrospective single-center analysis of 6064 metastases in 632 patients. Oncotarget 6, 44030–44036. https://doi.org/10.18632/oncotarget.5828 (2015).
Heck, J. E., Albert, S. M., Franco, R. & Gorin, S. S. Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. J. Am. Geriatr. Soc. 56, 1687–1692. https://doi.org/10.1111/j.1532-5415.2008.01848.x (2008).
Lovelace, D. L., McDaniel, L. R. & Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Womens Health 64, 713–724. https://doi.org/10.1111/jmwh.13012 (2019).
Acknowledgements
This study is a joint effort of many investigators and staff members, and their contribution is gratefully acknowledged. We especially thank all patients who participated in this study.
Funding
The Outstanding Youth Science Foundation of Yunnan Basic Research Project (202101 AW070001). Innovation Team of Kunming Medical University (CXTD202110). The Applied Basic Research Projects of Yunnan Province (202301 AT070128). The Applied Basic Research Projects of Education Department of Yunnan Province (2023Y0762). High-level Scientific and Technological Talents and Innovation Teams of Yunnan Province(202405 AS350016). Yunnan Provincial Science and Technology Department Kunming Medical University Joint Special Project, (202401 AY070001-354).
Author information
Authors and Affiliations
Contributions
Dafu Zhang: Conceptualization, Methodology, Software, Formal analysis, Visualization, Writing – original draft. Funding acquisition Huan Ma: Formal analysis, Visualization, Writing – review & editing Zhenhui Li: Conceptualization, Writing – review & editing, Supervision, Funding acquisition Wenting Cao: Conceptualization, Visualization, Investigation, Writing – review & editing Zhiping Zhang, Yifan Liu: Methodology, Software Jing Ai, Zongsheng Pu: Data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We declare that the work described has been carried out in accordance with the Declaration of Helsinki of the World Medical Association revised in 2013 for experiments involving humans Consent for publication. The study involving human participants were reviewed and approved by the ethics committee of the third affiliated Hospital of Kunming Medical University (NO: SLKYLX202118). The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, DF., Ma, H., Zhang, ZP. et al. Brain structural alterations and cognitive dysfunction in lung cancer patients without brain metastasis. Sci Rep 15, 16366 (2025). https://doi.org/10.1038/s41598-025-99326-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99326-9