Abstract
Declining soil fertility due to unstable cultivation practices is a major challenge, particularly under water-limited conditions, leading to reduced crop productivity. Identifying sustainable soil amendments that enhance plant resilience and improve soil properties is crucial for ensuring food security in arid and semi-arid regions. Among these, activated acacia biochar has shown potential in improving soil quality. A pot-scale experiment was conducted to evaluate the effects of four different soil amendments i.e., biochar amended soil (BAS), vermicompost amended soil (VCAS), perlite amended soil (PAS), activated biochar amended soil (ABAS) on wheat physiology and yield under moisture stress conditions. Soil amendments were incorporated at 2.5% and 5% (dry weight basis) by through mixing. Two water regimes were applied from booting to grain filling: (i) optimal moisture (80% of field capacity, FC) (ii) moisture stress (50% of FC). The highest improvement in water holding capacity was observed in soil amended with 5% ABAS which increased by 39.31% (16.3 ± 0.72) compared to non-amended soil (NAS). Under 50% FC, plants grown in 2.5% and 5% ABAS exhibited significantly higher total chlorophyll content (11%), stomatal area (42%), stomatal aperture (29%), and guard cell area (44%) than NAS. The maximum increase in photosynthetic rate (threefold and 4.2-fold higher) and transpiration rates (threefold and 3.2-fold higher) were observed in plants of 2.5% BAS and 5% ABAS, respectively than NAS. However, the highest improvement in water use efficiency (52%) was observed in the plants of 2.5% ABAS than NAS. Leaf nutrient assessment showed a 10.4% and 7.6-fold increase in percent weight of carbon and phosphorus in the plants of BAS, 75% higher sodium in PAS, 139% higher calcium in VCAS, and 97.5% higher nitrogen and 58% higher magnesium in ABAS than NAS. Additionally, the highest accumulation of metabolites including amides, polysaccharides, lignin and waxes, was recorded in plants grown in amended soil, particularly in ABAS. Regarding yield attributes, plants grown in 2.5% and 5% ABAS had significantly (p < 0.05) higher leaf area (84–85%), spike length (89–93%), spikelet per spike (139–166%), and 1000 grain weight (133–157%) under 50% FC compared to NAS. In conclusion, activated biochar considerably enhances plant physio-biochemical attributes and yield under water stress conditions. Its applications may be recommended for farmers in arid regions to mitigate the adverse effects of water scarcity.
Similar content being viewed by others
Introduction
An expanding worldwide population requires an increase in food production, but intensive agricultural practices are deteriorating the soil quality1. Soil degradation reduces soil fertility, water retention capacity, and productivity2. Furthermore, higher water demands for irrigation due to excessive evapotranspiration in arid places could be one of the worst repercussions of climate change on agricultural production, exacerbating the issue of water scarcity3. Therefore, enhancing the soil’s capacity to retain moisture, improving its quality, and promoting plant growth even in situations where moisture is low are the primary difficulties in irrigated and rain-fed agronomic soils4. It is also necessary to enhance food production in order to feed the world’s rapidly expanding population5. Moreover, the focus should be on increasing crop yield to its greatest potential rather than increasing the amount of land under cultivation6.
The recurrent drought conditions have had an impact on the global wheat production industry7,8. Climate-resilient farming is a growing trend that aims to mitigate the harmful effects of climate change for future generations. Managing agricultural soils and implementing new cropping techniques are two key components of a climate-resilient farming system9. Therefore, an on-farm soil management strategy seems more attractive to improve water retention and utilization. The organic soil conditioners, such as vermicompost and biochar, appear more promising because they can increase soil productivity and fertility.
The organic inputs like compost, biochar and vermicompost are the natural soil amendments, improving fertility, water-holding capacities, and microbial activities, thereby making them crucial for sustainable agriculture10. They become particularly relevant due to the problems of soil degradation and climate change caused by the overuse of synthetic fertilizers11. The Farm to Fork (F2F) Strategies of the European Union emphasize organic matter to reduce fertilizer dependency and improve sustainable food production; by 2030, 25% of agricultural land aimed to be covered by organic farming12. Likewise, it also impacts multiple SDGs by improving the food security, reducing runoff water, and sequestering carbon13. It is, therefore, quite essential to adopt it to achieve resilient and climate-smart agricultural systems.
The biochar is a long-lasting organic soil amendment14. It is produced by the anaerobic pyrolysis of organic waste in oxygen free environment. Vermicompost is the product of the biodegradation of organic waste, aided by earthworms as well as microorganisms. Its high porosity allows it to retain substantial moisture and feed the soil with nutrients, which can improve soil health15,16. In addition to the aforementioned advantages, the high humus contents of vermicompost aid in drought resistance and improve crop growth15,17. Perlite is a silica-rich, amorphous hydrated glass formed after a volcanic eruption. It is exceptionally lightweight, porous, and low in density, and it has a high capacity to retain moisture. Its use in the soil can improve water retention, aeration, and drainage18. Perlite is an excellent soil additive due to its high moisture-carrying capacity and thermal insulation19, which protect the soil from excessive evapotranspiration. Activated biochar is another highly effective organic amendment for sustainable agriculture. Its enhanced adsorption capacity, attributed to higher surface area and numerous unclogged micropores, improves soil properties and moisture and nutrient retention20. Multiple studies have documented that biochar with a larger surface area, micropore volume, and pore-specific surface area exhibits the enhanced capacity to enhance soil water retention capabilities21,22,23. Several research studies examined their effects on wheat cultivar growth as well as soil health. Activated biochar, for example, boosted photosynthetic efficiency and improved the tolerance towards stress24.
Currently, physical or chemical methods are the most common for activating biochar. However, these agents have limitations because of their high cost and chemical waste generation. Conversely, there is a growing interest in organic processes for biochar activation due to their environmentally favorable and cost-effective characteristics25. The high content of organic material and microorganisms in vermicompost makes it a useful agent for effective activation. The microorganisms found in it function as a reservoir for extracellular enzymes, which in turn interact with the surfaces of biochar, augmenting its porosity26.
Soil organic matter, water holding capacity, and nutrient levels are important indicators of soil health27,28,29. The agronomic soils with biochar amendments can hold sufficient amount of water for longer durations, which may reduce the irrigation requirements30. While by improving surface area and micropore volume, which in turn enhanced soil water holding capacity, activated biochar maintains appropriate moisture levels in the root zone of agricultural soils, and its significance increases extensively when it is accessible to crop plants during periods of stress27. So, it’s very important to look into how activated biochar affects the interactions between it and soil, especially how it changes the physicochemical and nutrient makeup of the soil. The primary aim of the present study was to assess the efficacy of fresh biochar, vermicompost, perlite, and activated biochar, as well as to investigate the physical and chemical properties of the soil in relation to these amendments. Gaining a thorough understanding of the physiological, biochemical, morphological, anatomical, and yield aspects of plants under stressful conditions can be extremely beneficial in determining the influence of stress protective strategies on plants31. Many plant metabolites, especially amino acids, sugars, and antioxidant enzymes, increase during stress. These metabolites are essential for inducing tolerance in plants to stressful conditions. Several studies have revealed a relationship between osmolyte accumulation (proline, sugar, proteins, etc.) and stress exposure in plants. Since osmolytes play a vital role in plant stress responses, acting as key regulators of antioxidant defense mechanisms and signaling pathways32. Given their significance, this study aimed to investigate the impact of soil amendments including vermicompost, biochar, perlite, and activated biochar on the physio-biochemical and yield responses of wheat under both optimal and water stressed conditions. We hypothesized that, due to its superior physicochemical properties, would significantly enhance photosynthetic efficacy, stomatal regulation, secondary metabolite accumulation, and, most importantly, yield attributes. Furthermore, we expected that under moisture stress conditions, activated biochar would outperform other soil amendments in mitigating drought-induced damage and improving overall plant performance.
Material and methods
Biochar production and activation
Wood shavings from Acacia nilotica underwent a series of processing steps for biochar production. Initially, the shavings were subjected to heating in an oven at 70 ℃ to decrease their moisture content. Subsequently, slow pyrolysis was carried out at 450 ℃ for a duration of three hours using a temperature-controlled biochar production unit. The resulting biochar was further subjected to organic activation by combining it with vermicompost and perlite in a 1:1:1 ratio. Additionally, a solution containing molasses was introduced as a catalyst to facilitate the activation process. The mixture was manually agitated on a daily basis for a period of three weeks until excess moisture evaporated, resulting in the transformation of the product into glossy, dust-free activated biochar. The saturation percentage was 56, 51, 67, and 62%, pH was 7.61, 8.12, 7.32 and 7.82, and EC was 0.105, 0.189, 0.292 and 0.141 mSm-1 for the fresh biochar, vermicompost, perlite, and activated biochar as given in Jahan et al.33.
Soil analysis
A total of three soil samples were taken from each treatment at a depth of 10 cm, and then combined to obtain a representative sample. After heating at 80 ℃, the soil was subjected to grounding and sieved by utilizing a 2 mm sieve for physicochemical testing.
The soil pH was assessed by a pH meter (Infitek BEP-12DW, China), ISO 10,390 (2021). A 50 g soil sample was combined with distilled water (250 mL), and the suspension was stirred gently with a glass stirrer. After one hour, the suspension was re-stirred, and pH readings were taken in triplicate with a pH meter. The method used to determine electrical conductivity (EC) was based on the approach of Rayment and Higginson34. The soil suspension (50 g soil in 250 mL H2O) was filtered until the transparent filtrate was attained and shifted into a plastic bottle (50 mL). Subsequently, EC was recorded by immersing the probe of the conductivity meter in the bottle.
The soil’s field capacity was determined using the approach of Samarah35. The potting soil was inundated with water, and the pots were subsequently covered with a plastic sheet in order to limit evaporation, while holes at the bottom allowed to drain off excess water. After 72 h, the soil’s moisture level was noted using a moisture meter (Lutron, model: PMS-714). The moisture reading was designated as the 100% moisture of field capacity and was further used to calculate 80% and 50% moisture of field capacity, i.e., 17.6% and 11%.
To measure soil organic matter, 1 g of soil was added to a beaker with 20 mL of H2SO4 (Conc.) and 10 mL of 1N K2Cr2O736. After incubation (30 min), Conc. H3PO4 (10 mL) was added to the beaker with distilled water (200 mL). Subsequently, the mixture was subjected to cooling. Afterward, diphenylamine indicator (15 drops) was added and titration was performed with ferrous ammonium sulfate (0.5 M) until the appearance of green color. The subsequent formulas were used to calculate the oxidizable organic carbon (OOC), total organic carbon (TOC), and organic matter (OM) content.
Experimental set up
A pot-scale experiment has been carried out at the Botanical Garden, University of Gujrat (32.64° N, 74.16° E) from November, 2020 to April, 2021 (mean temperature was 63°F and the average humidity level was 71%) to determine the most effective soil amendment. Wheat seeds (Zincol-16) were obtained from NARC, Islamabad. The experiment followed a Completely Randomised Design (CRD) with three replications. Plastic pots (30 cm in diameter, 21.60 cm in length, and 15.2 cm in width) were filled with 10 kg of soil, and 5 seeds were sown in each pot at a depth of 2–3 cm. The determination of field capacity involved fully saturating the pot soil while it was covered from the top to prevent evaporation. Moisture readings were recorded after a period of 72 h. The moisture level at field capacity was 18%. For soil moisture levels of 80%, the field capacity was maintained at 14.4%, and for levels of 50% moisture, the field capacity was maintained at 9%.
Experimental treatments
The sandy loam soil was amended with 2.5% and 5% of biochar, vermicompost, perlite, and activated biochar. The experimental treatments are summarized in Table 1.
Water Stress imposition and sampling
Water stress was applied for 30 days from 50% booting (60 days after sowing; DAS) to 50% grain filling stages (90 DAS) by monitoring moisture with soil moisture meter (Model: PMS-714, Lutron) on daily basis. Sampling was done before optimal irrigation at 30th day of stress imposition and three samples were taken from each treatment.
Physio-biochemical analysis
The method employed to determine the relative water content (RWC) of wheat leaves was that proposed by Wheatherley37. The aforementioned relation was used to ascertain the relative water content of the leaf.
The electrolyte leakage of the membrane of wheat leaf was determined, following the revisions by Sairam,38 on the method of Premchandra et al.39. The washed leaf disc (10 mg) was heated for 30 min in a water bath (at 40 °C). The electrical conductivity (C1) was measured with the conductivity meter probe after removing the test tube from the water bath. The same samples were retested for electrical conductivity (C2) after being subjected to a water bath (100 °C) for 10 min. The formula for calculating membrane stability index (MSI) is as follows:
The chlorophyll content was quantified by Arnon’s (1949) method, and the carotenoid content of wheat leaf was assessed using the procedure of Lichtenthaler and Wellburn40. The determination of soluble sugar content was conducted by taking 0.5 g of crushed leaf material and mixed with 10 mL of distilled water41. The measurement of proline content was conducted using Bates et al.42 method. The assessment of leaf protein content was carried out using the method outlined by Bradford43. The estimation of lipid peroxidation was based on the approach of Prochazkova et al.44. Malondialdehyde equivalents (indicating the level of lipid peroxidation) were determined by the following relation45.
The molar absorbance for sucrose solutions of 1–10 mM concentration was recorded to be 8.6 and 149 at 532 nm and 440 nm.
Measurement of photosynthetic attributes
Photosynthetic parameters were recorded (96 DAS) by a portable Infra-Red Gas Analyzer (IRGA) having a fixed leaf chamber (LI-COR Inc., Lincoln, NE, USA). The investigation schedule was 9:30 a.m. to 4:30 p.m. on 18th February 2022. The 4th fully developed leaf was inserted inside the leaf chamber, and three readings were recorded from each replicate for the sub-stomatal CO2 level (Ci), the rate of photosynthesis (A), gaseous diffusion through stomata (gs) and the rate of transpiration (E), and water use efficiency (WUE). The temperature of the leaf chamber (Tch) was adjusted within the range of 32 to 36 °C, while the maximum photosynthetically active radiation at the leaf surface (Qleaf) reached 950 units. Concurrently, the atmospheric pressure was maintained at 986 millibars.
Antioxidant enzyme assay
Leaf material (0.5 g) was crushed using a mortar while being placed in an ice bath. The crushed leaf material was homogenized with 50 mM phosphate buffer (5 mL) and centrifuged (at 13,000 g and 4 °C) for 20 min. Enzyme activity was assessed using the supernatant.
For, superoxide dismutase determination, the reaction mixture comprises of a 50 mM phosphate buffer, 0.075 mM Nitro blue tetrazolium,13 mM methionine, 0.2 mL Triton X, 0.002 mM riboflavin, and enzyme extract. After UV-irradiating the mixture for 15 min, the readings were taken at 560 nm46.
Here, IU represents the international unit for enzyme activity.
The methodology of Teranishi et al.47 was employed to determine catalase activity. The reaction mixture comprising 0.2 mL of enzyme extract, 15 mM of hydrogen peroxide, and 50 mM of potassium phosphate buffer. The inhibition of the reaction was achieved by the addition of 2 mL of titanium reagent after 5 min of reaction. After centrifugation, absorbance values were recorded at 410 nm by spectrophotometer. The calculation of catalase activity was determined using the provided formula.
The peroxidase activity was analyzed using technique of Vetter et al.48, with modifications by Gorin and Heidema49. The reaction mixture consists of enzyme extract (0.2 mL), 3 mM H2O2, 1% w/v ρ-phenylenediamine aqueous solution, and 100 mM phosphate buffer. The absorbance variations were measured using a dual-beam spectrophotometer at 485 nm wavelength for 3 min. Peroxidase activity was calculated by the relation given below:
Analysis of secondary metabolites in leaves
The KBr (potassium bromide) method was used to record FT-IR spectra (by Nicolet iS5 Thermo Scientific, USA) of powdered leaf samples50. The scanning of the leaf samples was performed between 4000 to 400 cm−1 (resolution = 1–4 cm−1) to record their IR spectra. The spectra were subjected to additional processing and analysis using Origin Pro 2020.
Analysis of leaves nutrient composition by SEM–EDX
The leaf samples were dried in an electric oven at 70 °C for 72 h and then grounded. The nutrient composition of the samples was determined by Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM–EDX) (JEOL, Japan, Model: JSM910). Total five scans were performed for each sample to determine its chemical composition by energy dispersive X-ray analysis.
Morphological and anatomical parameters
For the purpose of measuring absolute growth, plants were sampled in triplicates at maturity. The plants were separated into shoots, leaves, and roots. The fresh weights of these components were then recorded. Afterward, shoot length, root length, and leaf area was measured by ImageJ software (v. 1.51, USA). The number of leaves per plant was counted manually. The dry weights of the roots, stem, and leaves were measured subsequent to a drying process in an oven (at 70 °C) for 72 h. For stomatal observation, a small incision (after rinsing and cleaning) was made on the abaxial surface of the leaf with a scalpel, and gently removed the epidermal peel. Stomatal anatomy was studied from leaf peal at 160 × magnification by light microscope (Olympus CX21). Then area of stomata, area of guard cells, stomatal aperture was recorded from images taken from microscope by ImageJ software (v. 1.51, USA). The stomatal index was calculated by the following formula:
where s is indicating the number of stomata per unit area and e is the number of epidermal cells per unit area.
Yield Related parameters
After seed ripening, plants were harvested, and yield parameters were recorded. number of spikes per pot, number of spikelets/spikes, number of grains/spikelets, 1000-grain weight.
Statistical analysis
Two-way analysis of variance (ANOVA) was conducted using MINITAB 19 software. The determination of treatment significance was conducted using the Fisher’s LSD test (95% confidence level). The FTIR spectra were analyzed and presented using the Origin 19 software (Version 19b).
Results
Physicochemical properties of soil
The ANOVA based comparison (Table 2) showed that water holding capacity in 2.5% and 5% vermicompost (VCAS), perlite (PAS), biochar (BAS), and activated biochar amended soil (ABAS) was considerably (p < 0.05) improved by 14.5–25.4%, 7.61–16.4%, 7.24–16.38%, and 28.2–38.7%, than non-amended soil (NAS). The application of 2.5% BAS and 5% ABAS resulted in significant improvements in soil organic matter content, from 12.64% to 34.14% and 40% to 78%, compared to NAS soil. The EC of soil modified with 2.5%, and 5%, BAS as well as ABAS increased significantly (p < 0.05). The EC values were found to be 49–130% higher in the case of BAS, and 48.4–65.7% higher in ABAS than in NAS. All soil amendments (except 5% perlite) caused an improvement in total oxidizable carbon and oxidizable carbon (Table 2) and highest improvement was detected in 5% ABAS i.e., 65% and 60% higher in contrast to NAS.
Physio-biochemical attributes of wheat plants
The influence of 80% and 50% FC on physiological and biochemical attributes of wheat was highly significant (p < 0.001) for NAS and soil amended with different treatments, i.e., BAS, VCAS, PAS and ABAS as shown in Table 3 and 4. Highest RWC was detected, under 80% FC, in the plants of 2.5% BAS and 2.5% and 5% ABAS. Under 50% FC, RWC (%) in the leaves significantly (p < 0.05) enhanced by 36.9–39.3% in ABAS as compared to the plants of NAS. Under 50% FC, a significantly higher improvement in the leaf’s MSI was observed in both concentrations of BAS and ABAS i.e., 43% and 50% than NAS. The leaf proline content in the wheat treatments facing water stress was increased by 57% than plants receiving optimum water quantity. However, considerably lower proline content was traced in plants from 5% BAS, VCAS, PAS and ABAS and lowest proline content (− 20%) was recorded in 5% PAS, then the plants of NAS under water stress. Sugar content was 2-folds higher in NAS under 50% FC. However, sugar content was considerably lower in all treatments except 5% VCAS then to plants of NAS facing water stress (Table 3). Water stress imposition caused a considerable enhancement in lipid peroxidation of leaves in NAS by 7-folds, then to NAS under 80% FC. However, lipid peroxidation was decreased in the plants from amended soil with lowest lipid peroxidation was found in 2.5% BAS soil by 36.4%, then to NAS under 50% FC (Table 3).
The results of the inter-treatment comparison (Table 3) indicate that the application of 50% FC, resulted in a notable rise in catalase activity in the leaves of NAS. This increase amounted to 2-folds than plants of NAS that were receiving 80% moisture. Catalase activity decreased by 42–43% in 2.5% and 5% ABAS, then to plants of NAS under 50% FC. Peroxidase activity was onefold higher in the plants of NAS under 50% FC then to plants of NAS under 80% FC. However, peroxidase activity significantly lower in all treatments and lowest peroxidase activity was observed in 2.5% ABAS and 2.5% BAS (i.e., 22.7% and 33.3% lower), than to NAS under 50% FC. Water stress caused an enhancement in superoxide dismutase activity in the plants of NAS by 133% then to the plants of NAS under 80% FC. While superoxide dismutase activity was reduced by 34–38% and 30.7–31% in 2.5% and 5% BAS and ABAS, then to the plants of NAS under 50% FC (Table 3).
Water stress caused a severe decline in photosynthetic pigments in the plants of NAS under 50% FC. However, 8–13.5% higher chlorophyll a content was recorded in the plants of 2.5% and 5% ABAS, in contrast to the plants of NAS under 50% FC. Highest chlorophyll b content was assessed in the plants of 2.5% ABAS i.e., 13.4%, then to the plants of NAS (Table 4). Under 50% FC, total chlorophyll content was increased by 10% in 2.5% PAS and by 11% in the plants of 5% ABAS, in contrast to plants of NAS under 50% FC. Carotenoid content under 50% FC was increased by 89% in the plants of NAS then to plants of NAS under 80% FC. However, the significantly (p < 0.05) lower carotenoid content was observed in the plants of BAS, VCAS, PAS and ABAS under 50% FC (Table 4).
Influence on photosynthetic parameters and stomatal anatomy
Water stress caused a significant decline in the rate of photosynthesis (A) in the plants of NAS by 77% than in the plants of NAS under 80% FC (Table 4). However, A in the plants of 2.5% and 5% ABAS was assessed to be 2.4–fourfold higher than to the plants of NAS facing water stress. The rate of transpiration (E) in the leaves of NAS was reduced by 74% after facing water stress than the plants of NAS under 80% FC. The E in the plants of 2.5% and 5% BAS was recorded to be 1.5–3.2-fold higher, respectively, and in 2.5% and 5% ABAS was 126–405% higher, respectively, in contrast to plants grown in NAS under 50% FC. Water stress caused a reduction in gaseous diffusion through stomata (gs) in the plants of NAS by 94% than in the plants of NAS under 80% FC. However, gs in the plants of all soil amendments was higher than plants of NAS. Water stress decreased the water use efficiency (WUE) of leaves in NAS by 15%, then to NAS under 80% FC (Table 4). However, under 50% FC, maximum improvement of WUE by 52% was recorded in the plants of 5% ABAS in contrast to plants of NAS under 50% FC.
The stomatal anatomy is presented in Fig. 1 and Supplementary Fig. S1 online. The two-way analysis of variance indicated highly significant (p < 0.001) influence of moisture content, amendments and their interaction on area of stomata, stomatal aperture, number of stomata, number of epidermal cells, area of guard cells. The stomatal area (Fig. 1a) was recorded to be 38–42% higher in 2.5% and 5% ABAS than NAS under water stress. Similarly, maximum improvement in stomatal aperture (Fig. 1b) and area of guard cells (Fig. 1c) was recorded in the plants of 2.5% and 5% ABAS under 50% FC i.e., 23–29% and 41–44% higher, than to the plants of NAS. The stomatal index (Fig. 1d) was assessed to be 112–122%, 89–94%, 72–81%, and 46–69% higher in 2.5% and 5% BAS, VCAS, PAS, and ABAS, then to the stomatal index in the plants of NAS.
Showing a) Area of stomata b) Stomatal aperture c) Area of guard cells d) Stomatal index of wheat (Triticum aestivum L.) plants grown in non-amended, 2.5% and 5% biochar, 2.5% and 5% vermicompost, 2.5% and 5% Perlite and 2.5% and 5% activated biochar amended soil under 80% moisture under 50% moisture. Note: Where, T0 = non-amended soil, T1 = 2.5% biochar amended soil, T2 = 5% biochar amended soil, T3 = 2.5% vermicompost amended soil, T4 = 5% vermicompost amended soil, T5 = 2.5% perlite amended soil, T6 = 5% perlite amended soil, T7 = 2.5% activated biochar amended soil, T8 = 5% activated biochar amended soil
Influence on nutrient status of wheat leaves
Nutrient composition of the plant leaves grown under 80% and 50% FC is presented in Fig. 2a-i and Fig. 3a-i. Under 50% FC, the percent weight of carbon in the leaves was enhanced significantly in all treatments and maximum percent weight of carbon and phosphorus was traced in 5% BAS i.e., 10.4% and 7.6-fold higher than NAS. Maximum percent weight of nitrogen and magnesium was traced in ABAS increased from 6.8%-97.5% and 54–58% in 2.5% and 5% ABAS. The percent weight of sodium in leaves was found to decline in both concentrations of ABAS. However, the highest percent weight of sodium was found in 2.5% PAS i.e., 75% higher than NAS. The percent weight of magnesium, oxygen, silicon, sulfur, chloride, and calcium was found to enhance in the plants of all soil amendments and their maximum percent weight was recorded in 5% VCAS i.e., 139%, 17%, 21.2%, 121%, 309% and 28% than to plants of NAS. Highest percent weight of potassium was found in the plants of VCAS (56.7%) and PAS (45.3%) amended soil than NAS. Under 50% FC, the percent weight of calcium was reduced in plants of all amendments except plants of 5% VCAS (Fig. 4).
Nutrient analysis by SEM–EDX of biochar, vermicompost, perlite and activated biochar amendment on wheat (Triticum aestivum L.) leaves Where, a) = non-amended soil + 80% moisture of FC, b) = non-amended soil + 50% moisture of FC, c) = 2.5% biochar amended soil + 80% moisture of FC, d) = 2.5% biochar amended soil + 50% moisture of FC e) = 5% biochar amended soil + 80% moisture of FC, f) = 5% biochar amended soil + 50% moisture of FC, g) = 2.5% vermicompost amended soil + 80% moisture of FC, h) = 2.5% vermicompost amended soil + 50% moisture of FC, i) = 5% vermicompost amended soil + 80% moisture of FC.
Nutrient analysis by SEM–EDX of biochar, vermicompost, perlite and activated biochar amendment on wheat (Triticum aestivum L.) leaves Where, a) = 5% vermicompost amended soil + 50% moisture of FC, b) = 2.5% perlite amended soil + 80% moisture of FC, c) = 2.5% perlite amended soil + 50% moisture of FC, d) = 5%perlite amended soil + 80% moisture of FC e) = 5% perlite amended soil + 50% moisture of FC, f) = 2.5% activated biochar amended soil + 80% moisture of FC, g) = 2.5% activated biochar amended soil + 50% moisture of FC, h) = 5% activated biochar amended soil + 80% moisture of FC, i) = 5% activated biochar amended soil + 50% moisture of FC.
Weight percentage (%) of different elements in the leaves of wheat plants grown in non-amended soil, biochar, vermicompost, perlite and activated biochar amended soil under 80% and 50% moisture of FC. Where, T1 = non-amended soil under 80% moisture of FC, T2 = non-amended soil under 50% moisture of FC, T3 = 2.5% biochar amended soil under 80% moisture of FC, T4 = 2.5% biochar amended soil under 50% moisture of FC, T5 = 5% biochar amended soil under 80% moisture of FC, T6 = 5% biochar amended soil under 50% moisture of FC, T7 = 2.5% vermicompost amended soil under 80% moisture of FC, T8 = 2.5% vermicompost amended soil under 50% moisture of FC, T9 = 5% vermicompost amended soil under 80% moisture of FC, T10 = 5% vermicompost amended soil under 50% moisture of FC, T11 = 2.5% perlite amended soil under 80% moisture of FC, T12 = 2.5% perlite amended soil under 50% moisture of FC, T13 = 5% perlite amended soil under 80% moisture of FC, T14 = 5% perlite amended soil under 50% moisture of FC, T15 = 2.5% activated biochar amended soil under 80% moisture of FC, T16 = 2.5% activated biochar amended soil under 50% moisture of FC, T17 = 5% activated biochar amended soil under 80% moisture of FC, T18 = 5% activated biochar amended soil under 50% moisture of FC.
Influence on morphological attributes
The maximum improvement in the shoot length, shoot fresh and shoot dry weight was recorded in 2.5% and 5% ABAS i.e., 73–92%, 66–72% and 116–134% higher, than the shoot lengths in NAS under 50% FC (Table 4). Root length (cm) in NAS after facing water stress was 67% higher under 50% FC then to root length (cm) in NAS under 80% FC. However, root length (cm) was improved in all types of soil amendments used in the experiment and maximum root length was observed in 2.5% BAS, than in the plants grown in NAS under 50% FC (Table 5). In 2.5% and 5% VCAS and ABAS, a significant (p < 0.05) improvement was observed in root fresh weight (63% and 87%) and root dry weights (45% and 69%) after water stress exposure, in contrast to NAS. The maximum number of leaves (93–121% and 129–136%), leaf fresh weight (96–106% and 97–99%) and leaf dry weight (60.6–61% and 51–62%) was observed in 2.5% and 5% VCAS and ABAS under 50% FC in comparison to the NAS under 50% FC (Table 5). Leaf area was significantly higher in the plants cultivated from all types soil amendments and maximum leaf area (cm2) was observed in 2.5% and 5% ABAS i.e., 82–138% higher, respectively, than the leaf area in NAS (Table 5).
Influence on secondary metabolites of leaves
The FTIR spectra of leaves from NAS, 2.5% BAS, 2.5% VCAS, 2.5% PAS, and 2.5% ABAS is given in Fig. 5a. Compared to plants grown in NAS, the band intensity of carbohydrates and cellulose at 1239–1300 cm−1 was 17.7%, 8.4%, 5.6%, and 23.4% higher in plants of 2.5% BAS, 2.5% VCAS, 2.5% PAS and 2.5% ABAS. At 2917–2997 cm−1 the peak intensity of lipids was 7.78%, 9.61%, 12.9%, and 7.8% higher in plants of 2.5% BAS, 2.5% VCAS, 2.5% PAS and 2.5% ABAS. The FTIR spectra of wheat leaves after facing drought stress grown under 2.5% BAS, VCAS, PAS, and ABAS are given in Fig. 5b. Compared to plants grown in NAS, the band intensity of carbohydrates and cellulose at 1239–1300 cm−1 was 12%, 11.78%, 11.73%, and 14.9% higher in plants of BAS, VCAS, PAS, and ABAS. The band intensity of proteins at 1631–1728 cm−1 was 7.2%, 6.2%, 3.3%, and 7.2% higher in plants of 2.5% BAS, VCAS, PAS and ABAS. The band intensity of lipids at 2991–2920 cm−1 was 11.3%, 7.6%, 2.3%, and 11.7% higher in plants of BAS, VCAS, PAS, and ABAS. The band intensity of lipids, fats and waxes at 3646-3302 cm−1 was 4.8%, 2.8%, 2.2%, and 5.3% high in plants of 2.5% BAS, VCAS, PAS, and ABAS.
FTIR analysis of secondary metabolites in wheat (Triticum aestivum L.) roots grown in non-amended, 2.5% biochar, 2.5% vermicompost, 2.5% Perlite and 2.5% activated biochar amended soil under a) 80% moisture b) 50% moisture, and non-amended, 5% biochar, 5% vermicompost, 5% Perlite and 5% activated biochar amended under c) 80% moisture d) 50% moisture.
The FTIR spectra of wheat leaves grown in 5% BAS, VCAS, PAS, and ABAS are given in Fig. 5c. In contrast to plants grown in NAS, a peak of starch and carbohydrates at 843–768 cm−1 was detected in BAS, VCAS, PAS, and ABAS. Compared to plants grown in NAS, the band intensity of carbohydrates at 1139–1029 cm−1 was increased by 82%, 85%, 104%, and 98% in plants of 5% BAS, VCAS, PAS, and ABAS. At 1200–1500 cm−1, the peak intensity of carbohydrate and cellulose was increased by 40%, 105%, 75%, and 113% in plants of 5% BAS, VCAS, PAS, and ABAS.Contrary to the plants of NAS, the band intensity of proteins and lignin at 1608–1735 cm−1 was increased by 25% and 23.4% in plants of 5% BAS and ABAS. The FTIR spectra of wheat plants after facing drought in 5% BAS, VCAS, PAS, and ABAS are shown in Fig. 5d. In contrast to plants grown in NAS, the band intensity at 1198–1027 cm−1 was improved by 1.78%, 0.94%, 1.16%, and 2.36% in 5% BAS, VCAS, PAS, and ABAS. Compared to plants grown in NAS, the band intensity of proteins at 1504–1408 cm−1 was improved by 7.6%, 6.3%, 7.3%, and 7.8% in 5% BAS, VCAS, PAS, and ABAS. Contrary to the NAS, the band intensity of proteins at 1637–1736 cm−1 was increased by 7.2%, 3.9%, 1.93%, and 6.9% in 5% BAS, VCAS, PAS, and ABAS. The band intensity of waxes at 3571–3287 cm−1 was 9.9%, 8.7%, 4.1%, and 9.4% higher in plants of 5% BAS, VCAS, PAS, and ABAS.
Yield attributes
The moisture %, amendments and amendments*moisture showed a highly significant (p < 0.01) influence on yield attributes. In contrast to NAS under 50% FC, the maximum improvement in spike length (cm) was observed in 2.5% and 5% ABAS i.e., 89–93% higher than the plants of NAS (Fig. 6a). Water stress causes a decline in the number of spikelets per spike (Fig. 6b) in NAS by 24.34% than the NAS under 80% FC. However, the number of spikelets per spike was considerably improved by 117–137%, 73–102%, 42–78%, and 139–166% in the plants of 2.5% and 5% BAS, VCAS, PAS, and ABAS, in contrast to the NAS under 50% FC. Similarly, the number of seeds per spikelet (Fig. 6c) also significantly (p < 0.05) improved by 26–30%, 23–34%, 21–27%, and 66–66.2% in the plants of 2.5% and 5% BAS, VCAS, PAS, and ABAS, in contrast to the plants of NAS under 50% FC. The maximum improvement in thousand (1000) seed weight (g) was recorded in 2.5% and 5% ABAS i.e., 133–157% higher, in contrast to plants of NAS under 50% FC (Fig. 6d).
Showing a) spike length b) Number of spikelets per spike c) Number of seeds per spike d) thousand seed weight of wheat (Triticum aestivum L.) plants grown in non-amended (T0), 2.5% (T1) and 5% biochar (T2), 2.5% vermicompost (T3), 5% vermicompost (T4), 2.5% perlite (T5), 5% Perlite (T6) and 2.5% activated biochar (T7), and 5% activated biochar (T8) amended soil under 80% and 50% moisture of FC Note: Where, T0 = non-amended soil, T1 = 2.5% biochar amended soil, T2 = 5% biochar amended soil soil, T3 = 2.5% vermicompost amended soil, T4 = 5% vermicompost amended soil, T5 = 2.5% perlite amended soil, T6 = 5% perlite amended soil, T7 = 2.5% activated biochar amended soil, T8 = 5% activated biochar amended soil.
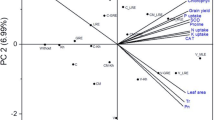
The Principal component analysis
The biplot of principle component analysis (PCA) showed that first two components share a total of 74.3% variance i.e., the PC-1 share a 59.4% variance while PC-2 share a total of 14.9% variance (Fig. 7). Further, it becomes also clear that both amendment levels of VCAS, PAS, BAS, ABAS under 80% FC and only ABAS under 50% FC are present on the right side of the plot and positively correlated with enhancement of chlorophyll a, chlorophyll b, total chlorophyll content, carotenoid content, membrane stability index, relative water content, photosynthetic rate (A), sub-stomatal CO2 (Ci), stomatal conductance (gs), transpiration rate (E), water use efficiency (WUE), protein content, shoot length, shoot fresh and dry weight, root fresh and dry weight, number of leaves, leaf area, leaf fresh and leaf dry weight, area of stomata, stomatal aperture, area of guard cell, stomatal index, seed per spikelet, and thousand seed weight of wheat plants. However, VCAS, PAS, BAS under 50% FC are present on left side the PCA plot and positively correlated with increase in sugar content, proline content, malondialdehyde content, superoxide dismutase, peroxidase, catalase, root length. From PCA analysis it becomes more evident that ABAS is linked with improvement in plant physiological, photosynthetic, biochemical and yield attributes even under water stress conditions.
Biplot of principle component analysis (PCA) showing the influence of non-amended soil (NAS), biochar amended soil (BAS), vermicompost amended soil (VCAS) and activated biochar amended soil (ABAS) on chlorophyll a, chlorophyll b, total chlorophyll content (Chl. a, Chl b, T Chl cont), carotenoid content (Caro cont), membrane stability index (MSI), relative water content (RWC), sugar content (sugar Cont), proline content (Pro Cont), malondialdehyde content (MDA Cont), photosynthetic rate (A), sub-stomatal CO2 (Ci), stomatal conductance (gs), transpiration rate (E), water use efficiency (WUE), protein content (prot Cont), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), shoot length (SL), shoot fresh and dry weight (SFW and SDW), root length (R Len), root fresh and dry weight (RFW and RDW), number of leaves (NL), leaf area (LA), leaf fresh and leaf dry weight (LFW and LDW), area of stomata (S Ar), stomatal aperture (SA), area of guard cell (AGC), stomatal index (SI), seed per spikelet (SPS), and thousand seed weight (TSW) of wheat plants grown under 80% and 50% moisture (M) of field capacity (FC).
Discussion
Physicochemical attributes of soil
The most significant results regarding electrical conductivity, organic carbon, nitrogen, organic matter, oxidizable carbon, phosphorus, and water-holding capacity were found in ABAS and BAS. However, the slightly higher electrical conductivity in soil with biochar amendments could be due to salts from ashes in biochar51. The BAS, VCAS, PAS, and ABAS have the potential to enhance the physicochemical properties of the soil. However, ABAS demonstrated the most promising results in terms of enhanced total organic carbon, and oxidizable carbon, and organic matter. The ABAS system played a significant role in enhancing soil fertility by increasing surface area, micro-pore volume and water-holding capacity of soil, thereby enhancing soil fertility by reducing nutrient leaching and augmenting nutrient and moisture retention52,53,54.
Physio-biochemical attributes
The stability of chlorophyll content is one of the vital defense mechanisms of plants because chlorophyll is the primary pigment of the chloroplast and is associated with the light-harvesting phase of photosynthesis, and it has a direct correlation with the rate of photosynthesis55,56. Water stress exposure causes a decline in chlorophyll a content by oxidative stress, which ultimately triggers photo-oxidation and degradation of chlorophyll pigments57. Hence, the number of chlorophyll pigments declined in the plants facing water stress, affecting the efficiency of photosystems58. Additionally, previous studies reported that water stress-induced hydrolysis of the chloroplast proteins reduces the formation of protein pigment complexes and affects the integrity of the chloroplast membrane (thylakoid membrane). All these modifications are associated with the overall decline of photosynthetic pigments59. The improved photosynthetic pigments in BAS, VCAS, PAS, and ABAS under limited supply of moisture might be due to the improved moisture retention capacity of these amendments as discussed above. In addition, organic amendments like activated BAS, biochar, and VCAS are more useful for sustainably enhancing soil fertility and moisture holding potential60,61. Plants of NAS accumulated high levels of carotenoid content; this might be due to the direct role of carotenoid content in the defense mechanism against oxidative stress posed by water stress as well as they can also dissipate the surplus light energy and offer protection to reaction centres against singlet oxygen species62,63,64. A relatively lower leaf carotenoid content from ABAS, BAS, VCAS, and PAS might be caused by increased water storage capacity of soil due to improved soil capillary pore volume and continuous provision of moisture to the plants65.
Water stress causes oxidative damage to lipids in cellular membranes and ultimately causing lipid peroxidation66,67. The increase in lipid peroxidation in the leaves of wheat plants grown in NAS under moisture-deficient conditions may have been caused by stomatal closure, which reduces CO2 concentrations for photosynthesis. Thus, NADP+ concentration may have decreased, resulting in ROS generation and lipid peroxidation68. In contrast, the wheat plants cultivated in BAS, VCAS, PAS, and ABAS exhibited reduced levels of lipid peroxidation. Under conditions of limited moisture availability, the observed reduction in lipid peroxidation in the leaves could potentially be attributed to their positive impact on the cation exchange capacity of the soil, as well as their enhanced capacity to retain nutrients and moisture69. Adequate moisture provision to plants by these amendments efficiently reduces lipid peroxidation by reducing oxidative damage to lipids70 which could help protect the plants from water stress. Latest research reports also indicated that BAS increases the gene expression in plants related to defense mechanisms and minimizes damages caused by water stress71; hence this might be the reason for the most significant results regarding lower lipid peroxidation in ABAS.
Moisture stress causes plasma membrane dysfunction and cytoplasm dehydration, which might be linked with increased proline content, lipid peroxidation, and increased electrolyte leakage in NAS plants72. As water stress increased, plants deposited proline, which protects cells from damage triggered by moisture deficit conditions57. However, the decreased proline content in wheat leaves grown in ABAS, BAS, VCAS, and PAS might be due to suitable moisture and nutrients in times of water stress73. The most significant results regarding low proline content were observed in the leaves from ABAS and BAS. Soil, particularly BAS and ABAS, have been found to decrease electrolyte leakage, proline content, and lipid peroxidation, this might be due to the ability of biochar to regulate water potential in plants, and improvement in RWC, and its ability to reduce oxidative stress74.
Under moisture limited conditions, there was an increase in leaf sugar content, likely attributed to elevated starch hydrolysis as a response to the stress. Large levels of soluble sugars may have maintained carbon supply even when CO2 fixation was limited, enabling speedier stress recovery. Moreover, enhanced quantities of specific sugars, like trehalose, have the potential to assist plants to deal with water shortages by minimizing cell membrane damage75. However, the sugar content was lower in wheat grown in BAS, PAS, and ABAS can be attributed to the timely availability of moisture and nutrients facilitated by these soil amendments76,77. However, under moisture stress, sugar content was increased in the plants of VCAS soil amendment might be due to their role in osmotic adjustment after facing water stress. Hence, the increased sugar content which may increase the leaf water potential78.
Under conditions of moisture stress, the plants of NAS exhibited enhanced activities of peroxidase, catalase, and superoxide dismutase. Increased antioxidant activities suggest that these enzymes play a critical role in securing leaf tissues against oxidative harm57. The observed increase in superoxide dismutase activity in wheat leaves subjected to moisture limitation may be attributed to their elevated catalytic capacity in converting superoxide anions to hydrogen peroxide79. Resultant hydrogen peroxide serves as a potent signaling molecule that can activate plants’ signal transduction and defense mechanism80,81. Catalase can potentially convert hydrogen peroxide into the water in peroxisomes, as catalase is linked with reducing the hydrogen peroxide levels in the cell and promoting membrane stability and CO2 fixation because several Calvin cycle enzymes within chloroplasts are susceptible to hydrogen peroxide82. Under limited moisture conditions, the increased peroxidase activity might be linked with enhanced cell wall protection against oxidative damage, lignification, and crosslinking83,84.
However, the catalase, peroxidase, and superoxide dismutase activities were lower in wheat grown in BAS, VCAS, PAS, and ABAS under limited moisture conditions. These amendments have the potential to decrease the activities of antioxidant enzymes in plants by enhancing soil moisture retention and providing hydration to plant roots in times of drought85. Furthermore, the lower enzyme activities were found in the plants of ABAS under limited moisture indicating less generation of ROS. This observation may be attributed to the high porosity of ABAS, which enables it to retain and supply moisture to plants during periods of stress86.
Under moisture-stress conditions, the RWC in the NAS plants decreased. The water stress caused a reduction in RWC and stomatal conductance (gs) in wheat plants, which can be attributed to a decrease in water uptake by plants due to the limited availability of soil water87. However, under limited moisture conditions, the RWC and gs were higher in the plants of BAS, VCAS, PAS, and ABAS. In the soil, the beneficial impact of BAS and VCAS amendments enhanced the availability of soil water, thereby reducing osmotic stress and preventing turgor loss in plants under limited moisture conditions88. The beneficial influence of these amendments on improved RWC, and gs, could be due to the better availability of macronutrients (N, P, and K) and moisture content89. The wheat plants exhibited an increase in K+ content and a decrease in Na+ content in BAS and ABAS, resulting in a better K+/Na+ ratio in soil under limited moisture which could also be a reason for improved physiological traits of plants. Furthermore, VCAS also reduces the harmful effects of moisture stress90 improves soil nutrient characteristics, and increases available moisture to plants91, resulting in improved RWC and gs92.
The decrease in leaf MSI of NAS under moisture stress could be attributed to the increased lipid peroxidation caused by ROS93. Furthermore, a drop in the MSI could be caused by an increase in ROS production, which could damage the cell membrane and cause the MSI to drop94,95. However, the MSI was increased in wheat grown in BAS, VCAS, PAS, and ABAS under optimum and limited moisture conditions; this might be linked with improvement in plant water status by these amendments, as seen by RWC21,96,97. Under stress conditions, ABAS and PAS effectively increased plant’s MSI, this increase may be attributed to a decrease in oxidative damage to lipids, as evidenced by a reduction in lipid peroxidation.
Photosynthetic, gaseous exchange and stomatal characteristics
Water stress negatively influences the photosynthetic attributes, primarily by disturbing all the significant components of thylakoid membranes including electron transport enzymes and carriers98. The results elucidated that water stress decreased photosynthetic rate, sub-stomatal CO2, and transpiration rate. Water stress severely hindered crop plants’ gaseous exchange factors, which could be linked with leaf area reduction, reduction in stomatal density, stomatal index, area of guard cells photosynthetic apparatus damage, oxidation of lipid constituents of the chloroplast, and alterations in pigment-protein complexes99. Plants exhibit a physiological response by closing their stomata to reduce transpiration during drought. Nevertheless, this phenomenon leads to a reduction in stomatal conductance and the levels of CO2 within the stomata, ultimately causing a decline in the rate of photosynthesis. This decline occurs due to the diminished the carbon dioxide supply to ribulose 1,5 bisphosphate (RUBP) and a subsequent decrease in its carboxylation efficiency100,101. On the contrary, the A, Ci, and E were improved in the wheat plants grown in BAS, VCAS, PAS, ABAS. During stress intervals, BAS and ABAS maintains the appropriate water potential in the soil and regulates stomatal functioning which may be linked to continued gs, E, and Ci levels, and this might be linked with enhanced stomatal index and density, area of guard cells, which enhances CO2 uptake and improved A of plants in BAS102.
Secondary metabolites of leaves
The sharp band at 1120–1000 cm−1 indicates C-H stretching, C-O stretching, C–O–C and P = O stretching of cellulose, hemicellulose, polysaccharides, and phosphodiester bonds of nucleic acid103,104,105. The smaller peak at 1200–1300 cm−1 in wheat plants (particularly plants grown in 2.5% ABAS) indicates the C-H stretching, C-N stretching, and O–H band of carbohydrates and cellulose that could explain the total sugar content in the plant leaves104, and highest band intensity of the leaves grown in ABAS indicated the presence of highest total sugar content. The band at 1600–1800 cm−1 in wheat plants (mainly grown in 2.5% BAS and 2.5% ABAS) indicates the C = C stretch and C = O of the amides of proteins106. The small band at 2900–3000 cm−1 represents the CH2 stretching vibration of aliphatic lipids107. The peak at 3700–3300 cm−1 represents the O–H stretch and N–H band of alcohols from lipids, fats, and wax108. After facing drought stress, a sharp band (more intense in plants of 2.5% VCAS and PAS) at 1160–1016 cm−1 indicates C-O–H stretching and C-H stretching of sugars, cellulose, lignin, hemicellulose, and polysaccharides109. The band at 1631–1728 cm−1 in wheat plants (particularly of 2.5% BAS and ABAS) indicates the C = C stretch and C = O of the protein110. The band at 2991–2920 cm−1 also represents the O–H stretch of aliphatic lipids107. The peak at 3300–3700 cm−1 represents the N–H band of lipids, fats, and wax111. The peak intensity was higher, particularly 2.5% BAS and 2.5% ABAS after facing drought stress.
A sharp band at 843–768 cm−1, particularly in plants of 5% ABAS, indicates C-H stretching, C-N stretching, and O–H band of starch and carbohydrates112. The more prominent peak at 1139–1029 cm−1 in wheat plants, mainly in 5% ABAS, indicates the C-H stretching, C-O stretching, C-N stretching, and S = O band of carbohydrates, cellulose, and hemicellulose 113. The band at 1409–1516 cm−1 in wheat plants (grown in 5% BAS, 5% VCAS, and 5% ABAS) indicates the C-O stretch, C = C stretch, C = O stretch, and N–H bending of the protein, cellulose, and lignin114. The band at 1608–1735 cm−1 (particularly in the plants of 5% amended ABAS soil) also represents the C = C, C = O, and C-N bending of the protein115. The peak at 3694–3321 cm−1 (particularly in plants of 5% ABAS) represents O–H, and N–H of lipids, fats, and wax116. The FTIR spectra of drought, 5% BAS, 5% VCAS, 5% PAS, and 5% ABAS are shown in Fig. 4d. A sharp band at 1198-1027 cm−1 indicates C-H, C-O, and C–O–C stretching117. The band at 1504–1408 cm−1 in wheat plants indicates the protein’s N–H and C-N stretch118. The small band at 1637–1736 cm−1 also represents the C = O, C = C, and C-N stretch of lipids119. The peak at 3571–3287 cm−1 represents O–H stretch and N–H of lipids, fats and wax108. The highest peak intensities of lipids, fats, waxes, amides, carbohydrates and polysaccharides under limited moisture were mostly detected in the leaves of BAS and ABAS. Fats and lipids mostly regulate the membrane stability and metabolic processes of plants because these compounds are also involved signaling events120. Waxes consist of aldehydes, fatty alcohols, fatty acids, alkanes, and wax esters. These components play a crucial role in safeguarding the plant from moisture stress conditions121.
The improved shoot length, and shoot fresh and dry weights of plants grown in BAS, VCAS, PAS, and ABAS; might be linked with enhanced improved moisture storage capacity of these amendments122. Hence, increased moisture content enhances water uptake and transpiration rate in plants123,124. Increased transpiration rates help in the increase of plant height due to improved plant growth.
Morphological and yield attributes
The wheat plants cultivated in a NAS with limited moisture experienced reduced root length, as well as decreased fresh and dry weight. The phenomenon of water stress induces a reduction in root length as well as fresh and dry weight because the plant roots absorb less water, so root cells have less enlargement due to loss of turgidity, and hence the root growth is retarded; they cannot profusely grow125. Contrarily, the root length, root fresh, and dry weight were maximum in plants grown in ABAS. The utilization of organic soil amendments has been found to enhance soil health, resulting in heightened crop production and improved root growth126. The utilization of organic soil amendments increases the density of microorganisms around roots and increases soil enzymes like phosphatase and catalase, and these factors might be responsible for enhanced root growth127.
Leaves serve a crucial function in exchanging gases between plants and their surroundings and are the major photosynthesizing organ of the plant body122. The number of leaves, leaf area, stomatal density, and leaf fresh and dry weight were higher in wheat grown in ABAS both under conditions of moisture stress and non-stress. The addition of ABAS enhanced the availability of moisture to plants and the availability of moisture increases the turgidity of guard cells which causes the stomatal opening, increasing the CO2 supply to plants, and enhancing A128. The increased A rate provides more assimilates for leaf growth, leading to an increase in leaf area, leaf fresh and dry weight, leaf number, and stomatal density122.
Water stress drastically reduced the number of spikes per pot, number of spikelets per spike, number of seed per spikelet, and 1000-seed wight in NAS. During anthesis, water stress negatively affects pollination, leading to a lower number of grains formed per spike. This ultimately results in a reduction of grain yield129. The reduction in the yield attributes in NAS observed at present may be linked to the low chlorophyll content and A rates in wheat plants under limited moisture. Plants maintain their internal moisture by stomatal closure under limited moisture availability, resulting in a decline of gaseous exchange and transpiration which eventually reduces grain yield130,131. However, the yield attributes were higher in wheat plants grown in ABAS under limited moisture. This increase in the yield attributes in ABAS under limited moisture conditions may be due to its potential to improves the moisture, nutrient, and carbon availability to plant roots; which results in improved metabolic functions in plants; hence it improves biomass production and growth of wheat132. Our research work provides evidence that ABAS most positively influences the number of spikes per pot in contrast to biochar, VCAS, and PAS due to its beneficial characteristics.
Conclusion
Water stress is the most critical dilemma faced by agricultural food production. The present work evidenced that water stress caused marked modifications in wheat morphological (such as shoot and root length, shoot–root fresh and dry wights and leaf area), anatomical features of stomata (stomatal index, area of guard cell, stomatal aperture, area of stomata) physiological (relative water content, membrane stability index, photosynthetic performance), and biochemical attributes (lipid peroxidation, proline, protein, and sugar content, catalase, and peroxidase, super oxide dismutase enzyme assays) which ultimately caused a considerable reduction in its yield. Among four types of soil amendments, most influential effects on soil quality and wheat growth under water stress were obtained in activated biochar as evident by considerable improvement in water holding capacity, organic matter, nitrogen and total oxidizable carbon percentage of soil and enhanced water use efficiency of wheat plants, photosynthetic rates, biochemical and yield attributes. Further, nutrient analysis of plants revealed highest nitrogen and magnesium content in the leaves of activated biochar amended soil, suggesting that these nutrients might play an important role in protecting plants from the adverse effects of water stress. Thus, the findings of the current work are revealing that activated biochar as a soil amendment may provide a suitable eco-friendly solution to improve the soil quality and wheat production water stress conditions.
Data availability
The data sets associated with present work are available from the corresponding authors on reasonable request.
References
Chen, K., Wang, X., Li, D. & Li, Z. Driving force of the morphological change of the urban lake ecosystem: A case study of Wuhan, 1990–2013. Ecol. Modell 318, 204–209 (2015).
Liu, Y. Landscape connectivity in soil erosion research: concepts, implication, quantification. Geogr Res. 1, 195–202 (2016).
Iglesias, A. & Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric Water Manag. 155, 113–124 (2015).
Khan, H. R., McDonald, G. K. & Rengel, Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arientinum L.). Plant Soil. 267, 271–284 (2004).
García-Oliveira, P., Fraga-Corral, M., Pereira, A. G., Prieto, M. A. & Simal-Gandara, J. Solutions for the sustainability of the food production and consumption system. Crit. Rev. Food Sci. Nutr. 62, 1765–1781 (2022).
Araus, J. L., Slafer, G. A., Royo, C. & Serret, M. D. Breeding for yield potential and stress adaptation in cereals. CRC Crit. Rev. Plant Sci. 27, 377–412 (2008).
Jabborova, D. et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 11, 1–9 (2021).
Khan, I. et al. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant 172, 696–706 (2021).
Ndede, E. O., Kurebito, S., Idowu, O., Tokunari, T. & Jindo, K. The Potential of Biochar to Enhance the Water Retention Properties of Sandy Agricultural Soils. Agronomy 12, 311 (2022).
Shu, X. et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 829, 154627 (2022).
Francaviglia, R., Almagro, M. & Vicente-Vicente, J. L. Conservation agriculture and soil organic carbon: Principles, processes, practices and policy options. Soil Syst 7, 17 (2023).
Wesseler, J. The EU’s farm-to-fork strategy: An assessment from the perspective of agricultural economics. Appl. Econ. Perspect Policy 44, 1826–1843 (2022).
Stefanovic, L. SDG performance in local organic food systems and the role of sustainable public procurement. Sustainability 14, 11510 (2022).
Gosling, P., Hodge, A., Goodlass, G. & Bending, G. D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst Environ. 113, 17–35 (2006).
Benaffari, W. et al. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 11, 393 (2022).
Lalander, C. H., Komakech, A. J. & Vinnerås, B. Vermicomposting as manure management strategy for urban small holder animal farms–Kampala case study. Waste Manag. 39, 96–103 (2015).
Hosseinzadeh, S. R., Amiri, H. & Ismaili, A. Effect of vermicompost fertilizer on photosynthetic characteristics of chickpea (Cicer arietinum L.) under drought stress. Photosynthetica 54, 87–92 (2016).
Güneri, E. & Yukselen-Aksoy, Y. Improvement of thermally durable soil material with perlite additive. Environ. Earth Sci. 81, 1–3 (2022).
Karaman, S., Karaipekli, A., Sarı, A. & Bic, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol Energy Mater Sol. Cells 95, 1647–1653 (2011).
Tian, S. Q. et al. Enhanced permanganate oxidation of sulfamethoxazole and removal of dissolved organics with biochar: formation of highly oxidative manganese intermediate species and in situ activation of biochar. Environ. Sci. Technol. 53, 5282–5291 (2019).
Basso, A. S., Miguez, F. E., Laird, D. A., Horton, R. & Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. Glob. Change Biol. Bioenergy. 5, 132–143 (2013).
Liu, Z., Dugan, B., Masiello, C. A. & Gonnermann, H. M. Biochar particle size, shape and porosity act together to inflluence soil water properties. PLoS ONE 12, e0179079 (2017).
Masiello, C. A. et al. Biochar effects on soil hydrology. In Biochar for Environmental Management Science, Technology and Implementation 2nd (eds. Lehmann, J. & Joseph, S.) 543–562 (Routledge, London, UK, 2015).
Ghazouani, H. et al. Integrative effect of activated biochar to reduce water stress impact and enhance antioxidant capacity in crops. Sci. Total Environ. 905, 166950 (2023).
Sanchez-Hernandez, J. C., Ro, K. S. & Díaz, F. J. Biochar and earthworms working in tandem: research opportunities for soil bioremediation. Sci. total Environ. 688, 574–583 (2019).
Sanchez-Hernandez, J. C. Biochar activation with exoenzymes induced by earthworms: a novel functional strategy for soil quality promotion. J. Hazard Mater 350, 136–143 (2018).
Mahmood, S., Nunes, M. R., Kane, D. A. & Lin, Y. Soil health explains the yield-stabilizing effects of soil organic matter under drought. Soil Environ Health. 1, 100048 (2023).
Nath, C. P. et al. Pulse crop and organic amendments in cropping system improve soil quality in rice ecology: Evidence from a long–term experiment of 16 years. Geoderma 430, 116334 (2023).
Raghavendra, M. et al. Soil health indicators: Methods and applications. Soil. Analysis: Recent Trends Appl. 2020, 221–253 (2020).
Liu, Z. et al. Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 533, 461–472 (2016).
Schönbeck, L. et al. Plant physiological indicators for optimizing conservation outcomes. Conservation Physiol. https://doi.org/10.1093/conphys/coad073 (2023).
Hadid, M. L. et al. Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot Horti Agrobo. 5, 1329 (2023).
Jahan, S., Ahmad, F., Rasul, F., Amir, R. & Shahzad, S. Physicochemical Analysis of Vermicompost-Perlite Based Activated Biochar and its Influence on Wheat (Triticum aestivum L.) Growth Under Water Stress. J. Soil Sci. Plant Nutr. 27, 1–7 (2023).
Rayment, G. E. & Higginson, F. R. Australian laboratory handbook of soil and water chemical methods. Inkata press Port Melbourne 330 (1992).
Samarah, N. H. Effects of drought stress on growth and yield of barley. Agron. Sust. Dev. 25, 145–149 (2005).
George, P. B. L. et al. Evaluation of mesofauna communities as soil quality indicators in a national-level monitoring programme. Soil. Biol. Biochem. 115, 537–546 (2017).
Wheatherley, P. E. Studies in water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol. 49, 81–87 (1950).
Sairam, R. K. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul. 14, 173–181 (1994).
Premchandra, G. S., Saneoka, H. & Ogata, S. Cell membrane stability, an indicator of drought tolerance. as affected by applied nitrogen in soybean. J. Agric. Sci. 115, 63–66 (1990).
Lichtenthaler, H. K. & Wellburn, W. R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11, 591–592 (1983).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil. 39, 205–207 (1973).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Prochazkova, D., Sairam, R. K., Srivastava, G. C. & Singh, D. V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 161, 765–771 (2001).
Du, Z. & Bramlage, W. J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 40, 1566–1570 (1992).
Beauchamp, C. & Fridovich, I. Superoxide dismutase. Improved assays and an assay applicable to acrylamide gel. Anal. Biochem. 44, 276–287 (1971).
Teranishi, Y., Tanaka, A., Osumi, M. & Fukui, S. Catalase activity of hydrocarbon utilizing candida yeast. Am. J. Agric. Biol. Sci. 38, 1213–1216 (1974).
Vetter, J. L., Steinberg, M. P. & Nelson, A. L. Quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 6, 39–41 (1958).
Gorin, N. & Heidema, F. T. Peroxidase activity in golden delicious apples as a possible parameter of ripening and senescence. J. Agric. Food Chem. 24, 200–201 (1976).
Celi, L., Schnitzer, M. & Nègre, M. Analysis of carboxyl groups in soil humic acids by wet chemical method, FTIR spectrometry and solution-state carbon-13 NMR. A comparative study. Soil Sci. 162, 189–197 (1997).
Muegue, L. C. D., González, J. C. A. & Mesa, G. P. Characterization and potential use of biochar for the remediation of coal mine waste containing efflorescent salts. Sustainability 9, 2100 (2017).
Li, Y., Feng, G. & Tewolde, H. Biochar derived from papermill factories improves soil physical and hydraulic properties in no-till cotton fields. Biochar 5, 35 (2023).
Joshi, R., Singh, J. & Vig, A. P. Vermicompost as an effective organic fertilizer and biocontrol agent: effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotechnol. 14, 137–159 (2015).
Ding, Y. et al. Biochar to improve soil fertility. A Rev Agron Sustain Dev. 36, 1–18 (2016).
Rahdari, P., Hosseini, S. M. & Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plant Res. 6, 1539–1547 (2012).
Farooq, M., Wahid, A., Kobayashi Nsma, F., Ma, D. B. S. & Sma, B. Plant drought stress: effects, mechanisms and management. in Sustainable agriculture 153–188 (Springer, Dordrecht, 2009). https://doi.org/10.1007/978-90-481-2666-8_12.
Anjum, S. A. et al. Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front. Plant Sci. 8, 1–12 (2017).
Fathi, A. & Tari, D. B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 10, 1–6 (2016).
Li, Y. T. et al. Dynamic light caused less photosynthetic suppression, rather than more, under nitrogen deficit conditions than under sufficient nitrogen supply conditions in soybean. BMC Plant Biol. 20, 1–13 (2020).
Scotti, R., Bonanomi, G., Scelza, R., Zoina, A. & Rao, M. A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. Soil. Sci. Plant Nutr. Soil. 15, 333–352 (2015).
Rasa, K. et al. How and why does willow biochar increase a clay soil water retention capacity?. Biomass. Bioenergy. 119, 346–353 (2018).
Campos, M. D. et al. Carrot plastid terminal oxidase gene (dcptox) responds early to chilling and harbors intronic pre-mirnas related to plant disease defense. Plant Gene. 7, 21–25 (2016).
Nagy, L. et al. Thermal effects and structural changes of photosynthetic reaction centers characterized by wide frequency band hydrophone: Effects of carotenoids and terbutryn. Photochem. Photobiol. 91, 1368–1375 (2015).
Santabarbara, S. et al. The requirement for carotenoids in the assembly and function of the photosynthetic complexes in Chlamydomonas reinhardtii. Plant Physiol. 161, 535–546 (2013).
Zong, Y., Wang, Y., Sheng, Y., Wu, C. & Lu, S. Ameliorating soil acidity and physical properties of two contrasting texture Ultisols with wastewater sludge biochar. Environ. Sci. Pollut Res. 25, 25726–25733 (2018).
An, Y. M., Song, L. L., Liu, Y. R., Shu, Y. J. & De Guo, C. H. novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 7, 931 (2016).
Zhang, H. H. et al. 2-Cys Prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. J. Plant Interact. 14, 119–128 (2019).
Hernandez, J. A., Jiménez, A., Mullineaux, P. & Sevilia, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 23, 853–862 (2000).
Abunyewa, A. A., Osei, C., Asiedu, E. K. & Safo, E. Y. Integrated manure and fertilizer use, maize production and sustainable soil fertility in sub humid zone of west africa. J. Agron. 6, 302–309. https://doi.org/10.3923/ja.2007.302.309 (2007).
Jia, W. et al. Mineral elements uptake and physiological response of Amaranthus mangostanus (L.) as affected by biochar. Ecotoxicol. Environ Saf. 175, 58–65 (2019).
Jaiswal, A. K. et al. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 10, 1–15 (2020).
Abdelaal, K. A. et al. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agron 10, 26 (2020).
Shao, H. B., Liang, Z. S. & Shao, M. A. Investigation on dynamic changes of photosynthetic characteristics of 10 wheat (Triticum aestivum) genotypes during two vegetative-growth stages at water deficit. Colloids Surf. B Biointerfaces 43, 221–227 (2005).
Hafez, Y. et al. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 10, 630 (2020).
Delorge, I., Janiak, M., Carpentier, S. & Van Dijck, P. Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front. Plant Sci. 5, 147 (2014).
Ali, S. et al. Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ. Sci. Pollut Res. 24, 12700–12712 (2017).
Danish, S., Younis, U., Nasreen, S., Akhtar, N. & Iqbal, M. T. Biochar consequences on cations and anions of sandy soil. J. Biodivers Environ. Sci. 6, 121–131 (2015).
Salehi, A., Tasdighi, H. & Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pac J. Trop Biomed. 6, 886–891 (2016).
Turkan, I., Bor, M., Özdemir, F. & Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 168, 223–231 (2005).
Aono, M., Kubo, A., Saji, H., Tanaka, K. & Kondo, N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 34, 129–135 (1993).
Marulanda, A., Porcel, R., Barea, J. M. & Azcón, R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb. Ecol. 54, 543 (2007).
Lotfi, R., Pessarakli, M., Gharavi-Kouchebagh, P. & Khoshvaghti, H. Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity. Crop J. 3, 434–439 (2015).
Cakmak, I. & Horst, W. J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant 83, 463–468 (1991).
Moussa, H. R. & Abdel-Aziz, S. M. Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust J. Crop Sci. 1, 31–36 (2008).
Liu, M., Wang, C., Liu, X., Lu, Y. & Wang, Y. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil. Ecol. 156, 103705 (2020).
Xie, T., Reddy, K. R., Wang, C., Yargicoglu, E. & Spokas, K. Characteristics and applications of biochar for environmental remediation: a review. Crit. Res. Environ. Sci. Technol. 45, 939–969 (2015).
Alvarez-Campos, O. et al. Biochar and mill ash improve yields of sugarcane on a sand soil in Florida. Agric. Ecosyst. Environ. 253, 122–130 (2018).
Abou, K. A. S., Elsoud, H. M., Hafez, E. M. & Ali, O. A. Integrated effect of nano-Zn, nano-Si, and drainage using crop straw-filled ditches on saline sodic soil properties and rice productivity. Arab. J. Geosci. 12, 471 (2019).
Akhtar, S. S., Li, G., Andersen, M. N. & Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 138, 37–44 (2014).
Di, W. et al. Biochar combined with vermicompost increases crop production while reducing ammonia and nitrous oxide emissions from a paddy soil. Pedosphere 1, 82–94 (2019).
Ibrahim, M. M., Mahmoud, E. K. & Ibrahim, D. A. Effects of vermicompost and water treatment residuals on soil physical properties and wheat yield. Int. Agrophys. 29, 157 (2015).
Doan, T., Thierry, H., Cornelia, R., Jean-Louis, J. & Pascal, J. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: a three year mesocosm experiment. Sci. Total Environ. 514, 147–154 (2015).
Moran, J. F. et al. Drought induces oxidative stress in pea plants. Planta 194, 346–352 (1994).
Iqbal, S. & Bano, A. Water stress induced changes in antioxidant enzymes, membrane stability and seed protein profile of different wheat accessions. Afr J Biotechnol 8, (2009).
Shanazari, M., Golkar, P. & Mirmohammady Maibody, A. M. Effects of drought stress on some agronomic and bio-physiological traits of Trititicum aestivum, Triticale, and Tritipyrum genotypes. Arch. Agron. Soil Sci. 64, 2005 (2018).
Novak, J. M. et al. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 174, 105–112 (2009).
Artiola, J. F., Rasmussen, C. & Freitas, R. Effects of a biochar-amended alkaline soil on the growth of romaine lettuce and bermudagrass. Soil Sci. 177, 561–570 (2012).
Allen, D. J. & Ort, D. R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends. Plant Sci. 6, 36–42 (2001).
Samarah, N. H., Alqudah, A. M., Amayreh, J. A. & McAndrews, G. M. The effect of late-terminal drought stress on yield components of four barley cultivars. J. Agron. Crop. Sci. 195, 427–441 (2009).
Flexas, J., Bota, J., Loreto, F., Cornic, G. & Sharkey, T. D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 6, 269–279 (2004).
Mathobo, R., Marais, D. & Steyn, J. M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 180, 118–125 (2017).
Abideen, Z. et al. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 22, 259–266 (2020).
Abdelkader, A. F., Hassanein, R. A., Abo-Aly, M. M., Attia, M. S. & Bakir, E. M. Screening the bio-safety of wheat produced from pretreated grains to enhance tolerance against drought using physiological and spectroscopic methods. Food Chem. Toxic 48, 1827–1835 (2010).
Nikalje, G. C., Kumar, J., Nikam, T. D. & Suprasanna, P. FT-IR profiling reveals differential response of roots and leaves to salt stress in a halophyte Sesuvium portulacastrum L. Biotechnol. Rep. 23, e00352 (2019).
Kaur, P., Ghildiyal, S. & Soni, S. Development and evaluation of hydrodynamically balanced system of tramadol hydrochloride by using chitosan and locust bean gum. J. Intern. Med. Emerg. Res. 1, 1–20 (2020).
Akyuz, S., Akyuz, T., Celik, O. & Atak, C. FTIR spectroscopy of protein isolates of salt-tolerant soybean mutants. J. Appl. Spectrosc. 84, 1019–1023 (2018).
Artz, R. R. et al. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 40, 515–527 (2008).
Legner, N., Meinen, C. & Rauber, R. Root differentiation of agricultural plant cultivars and proveniences using FTIR spectroscopy. Front. Plant Sci. 9, 748 (2018).
Din, M. I. et al. Eco Friendly Synthesis of Nickel Oxide Nanoparticles and Its Application on Pyrolysis of Calotropis Procera (AKH) Plant Roots. J. Optoelectron Biomed Mater. 13, 119–125 (2021).
Rewald, B., Meinen, C., Trockenbrodt, M., Ephrath, J. E. & Rachmilevitch, S. Root taxa identification in plant mixtures–current techniques and future challenges. Plant Soil 359, 165–182 (2012).
Suica-Bunghez, I. R. et al. Fitochemical and antioxidant characterization of Autumn Crocus (Colchicum autumnale) flowers and roots plant extracts. J. Sci. Arts 17, 539–546 (2017).
Sharma, N. et al. Microscopic, elemental and molecular spectroscopic investigations of root-knot nematode infested okra plant roots. Vacuum 158, 126–135 (2018).
Akoto, C. O. & Rainier, J. D. Harnessing glycal-epoxide rearrangements: the generation of the AB, EF, and IJ rings of adriatoxin. Angew. Chem. Int. Ed 47, 8055 (2008).
Namanusart, W., Saksirirat, W. & Tanthanuch, W. FTIR spectroscopic analysis of luminescent mushroom (Neonothopanus nambi Speg.) induced disease resistance against tomato root-knot nematode. Meloidogyne incognita Chitwood J. Pure Appl. Microbiol. 10, 923–935 (2016).
Streit, J., Meinen, C., Nelson, W. C. D., Siebrecht-Schöll, D. J. & Rauber, R. Above-and belowground biomass in a mixed cropping system with eight novel winter faba bean genotypes and winter wheat using FTIR spectroscopy for root species discrimination. Plant Soil. 436, 141–158 (2019).
Sharma, N. et al. Study of molecular and elemental changes in nematode-infested roots in papaya plant using FTIR, LIBS and WDXRF spectroscopy. Int. J. Spectrosc. 41, 110–118 (2020).
Acheampong, A., Okyem, S., Akoto, C. O. & Baah, A. B. Antioxidant, antimicrobial and FTIR analysis of methanol root extract of Cnestis ferruginea and ethanol root extract of Citrus limon. J. Pharmacogn. Phytochem. 7, 2938–2946 (2018).
Manley, M., Van Zyl, L. & Osborne, B. G. Using Fourier transform near infrared spectroscopy in determining kernel hardness, protein and moisture content of whole wheat flour. J. Near Infrared Spectrosc. 10, 71–76 (2002).
Zhao, X., Yang, X., Shi, Y., Chen, G. & Li, X. Protein and lipid characterization of wheat roots plasma membrane damaged by Fe and H2O2 using ATR-FTIR method. J. Biophyic. Chem. 4, 28489 (2013).
Ullah, S. et al. Targeted metabolomics reveals fatty acid abundance adjustments as playing a crucial role in drought-stress response and post-drought recovery in wheat. Front. Genet. 13, 972696 (2022).
Patwari, P. et al. Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J 98, 727–744 (2019).
Hosseinzadeh, S. R., Amiri, H. & Ismaili, A. Evaluation of photosynthesis, physiological, and biochemical responses of chickpea (Cicer arietinum L. cv. Pirouz) under water deficit stress and use of vermicompost fertilizer. J. Integr. Agric. 17, 2426–2437 (2018).
Atik, A. Effects of planting density and treatment with vermicompost on the morphological characteristics of oriental beech (Fagus orientalis Lipsky). Compost Sci. Util. 21, 87–98 (2013).
Ganjeali, A., Porsa, H. & Bagheri, A. Assessment of Iranian chickpea (Cicer arietinum L.) germplasms for drought tolerance. Agri Water Manag. 98, 1477–1484 (2011).
Iqbal, M. S., Singh, A. K. & Ansari, M. I. Effect of drought stress on crop production. New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, HB, Singh, AK, Singh, US, Fraceto, L., Eds 35–47 https://doi.org/10.1007/978-981-15-1322-0_3 (2020).
Bista, P., Ghimire, R., Machado, S. & Pritchett, L. Biochar effects on soil properties and wheat biomass vary with fertility management. Agron 9, 623 (2019).
Maji, D., Misra, P., Singh, S. & Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of (Pisum sativum). Appl. Soil Ecol. 110, 97–108 (2017).
Zafar-ul-Hye, M., Danish, S., Abbas, M., Ahmad, M. & Munir, T. M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agron 9, 343 (2019).
Ashraf, M. Y. photosynthetic efficiency of wheat under water stress conditions. Pak. J. Sci. Ind. Res. Ser. Biol. 41, 156–163 (1998).
Perdomo, J. A., Cap-Bau, S., Carmo-Silva, E. & Galms, J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 8, 490 (2017).
Zandalinas, S. I., Mittler, R., Balfagon, D., Arbona, V. & Mez-Cadenas, A. G. Plant adaptations to the combination of drought and high temperatures. Physiol.s plant 162, 2–12 (2018).
Keshavarz Afshar, R., Hashemi, M., DaCosta, M., Spargo, J. & Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 47, 743–752 (2016).
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R236), King Saud University, Riyadh, Saudi Arabia. The authors would like to acknowledge PARC, Islamabad, for providing the seeds of the wheat varieties used in this study.
Funding
Researchers supporting project number (RSP2025R236), King Saud University, Riyadh, Saudi Arabia. The study was supported by Higher Education Commission (HEC) of Pakistan under NRPU (National Research Program for Universities) Project, grant No. 20–16716.
Author information
Authors and Affiliations
Contributions
RA; Experimentation and Methodology, SJ; Supervision and Validation, MZ; Statistical analysis, MBN & SU; writing-original draft preparation, AAS; Resource acquisition and Investigation, MN & AAA; Conceptualization and writing-revised draft preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable. Our experiment follows with the relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amir, R., Jahan, S., Zafar, M. et al. Organic amendments optimize Triticum aestivum var Zincol-16 performance under water stress through enhancement in physio biochemical traits, secondary metabolites and yield. Sci Rep 15, 17995 (2025). https://doi.org/10.1038/s41598-025-99506-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99506-7