Abstract
The role of collagen and its receptor, discoidin ___domain receptor 1 (DDR1) in immune response of colorectal cancer (CRC) remains unclear. We identified DDR1 as a promising target of immunotherapy resistance using a pooled in vivo CRISPR/sgRNA screening in microsatellite stable (MSS) CRC mouse models. Our findings demonstrated that knockdown or inhibition of DDR1 could enhance infiltration of CD8+ T cells and sensitize MSS CRC to PD-1 blockade. Furthermore, DDR1 was found to facilitate kinase ___domain phosphorylation, upregulate EZH2, consequently elevating H3K27me3 levels at the CXCL10 promotor, which led to the suppression of CXCL10 transcription once bound to collagen in ECM. Lastly, DDR1 was found positively correlated with collagen I expression in MSS CRC specimens. These findings indicated that targeting DDR1 or its inhibitor 7rh might be potential strategy for overcoming immunotherapy resistance in MSS CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) remains a major public health issue worldwide, ranking as the third most common malignancy and the second leading cause of cancer-related mortality1.Over the past decades, cancer immunotherapy has shown remarkable therapeutic achievements across various cancers, including CRC2. Notably, immune checkpoint inhibitors (ICIs), specifically anti-CTLA4 and PD-1/PD-L1 blockade, have received substantial attention3,4.
Presently, only 10–40% of cancer patients can respond and develop rapid tumor shrinkage monotherapy of ICIs5. Tumors characterized by deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H) status are distinguished by their heightened production of tumor antigens, resulting in effective T cell recognition, activation and response to ICIs. However, only a small fraction (approximately 10–15%) of CRC patients exhibit dMMR/MSI-H status, while most (85–90%) are proficient mismatch repair (pMMR)/microsatellite stable (MSS) CRC, which are recognized for their limited responsiveness to ICIs6.Notably, in clinical trials such as KEYNOTE-0167 and KEYNOTE-1778, PD-1 blockade demonstrated an objective response rate (ORR) of 40% in dMMR CRC, whereas there was no response was observed in pMMR CRC patients. Therefore, it is essential to elucidate the underlying mechanism responsible for immunotherapy resistance in pMMR/MSS CRC to identify potential therapeutic targets.
The infiltration of immune cells, particularly CD8+ T cells, within the tumor is associated with improved response to PD-1 blockade in cancer patients9. Within the tumor microenvironment (TME), three distinct phenotypes have been delineated based on the distribution of CD8+ T cells10,11. Among these, the immune-excluded phenotype was characterized by the retention of immune cells within the fibrotic and collagen-rich stroma surrounding the tumor parenchyma. In this particular phenotype, T cells within the extracellular matrix (ECM) display signs of activation and proliferation, yet they fail to infiltrate the tumor even when subjected to ICI treatment12,13,14,15. Substantial evidence has underscored the crucial roles played by components of the ECM, including cancer-associated fibroblasts, cytokines, chemokines (such as CXCL12 and TGF-β), and desmoplasia (marked by tumor fibrosis), in contributing to resistance against cancer immunotherapy16,17,18.
Kinases play a major role in cellular activation process including signal transduction by diverse immunoreceptors. Given the roles in cell growth, cell death and production of inflammatory mediators, targeting kinases has proven to be an effective strategy for anti-cancer therapies and immune-mediated diseases. Hence, we chose kinase family for our study targets for they occupied vital roles in cellular process.DDR1, a receptor tyrosine kinase (RTK), possesses a structural composition that includes an N-terminal extracellular discoidin ___domain, specifically designed for binding to collagen, the most prevalent component of ECM and also features a transmembrane ___domain and a intracellular C-terminal tyrosine kinase ___domain19. Activation of DDR1 occurs upon the interaction of collagen with its extracellular ___domain (ECD), prompting the phosphorylation of the intracellular kinase ___domain through the involvement of Src20. DDR1 has demonstrated high expression levels in various human cancers, such as breast, ovarian, lung, brain, and esophageal cancer21,22,23,24,25. In CRC, DDR1 has been recognized as a collagen receptor playing a role in CRC liver metastasis and the associated stromal reaction26,27. Nevertheless, the precise role of collagen and its receptor in CRC immunotherapy remains unclarified.
In this study, we employed a pooled in vivo screening approach utilizing CRISPR-Cas9 technology in mouse models of MSS CRC to identify potential targets associated with resistance to immunotherapy, based on which DDR1 was found to be a promising target, indicating that it could be a potential marker for resistance to PD-1 blockade in MSS CRC.
Methods
Patients and tissue samples
26 human colon cancer samples were obtained from patients at Sun Yat-sen University Cancer Center from May 1st, 2016 to May 1st,2023. Informed consent was obtained from all the patients before tissue collection. All the patients were pathologically confirmed to have colon adenocarcinoma. And all the baseline information of patients was collected blindly, without knowing the expression.
Ethic Approval
Informed consent was obtained from all the patients before tissue collection. Studies involving human tissue samples were carried out in accordance with the guidelines approved by the Ethics Committees of Sun Yat-Sen University Cancer Center (IRB NO.B2023-230-01).
In vivo CRISPR/Cas9 screening
Murine CT26 CRC cells were first transfected with a Cas9 plasmid, enabling the generation of CT26-Cas9 cells with GFP for Flow cytometry-based selection. Subsequently, CT26-Cas9 cells were transduced with a sgRNA library to facilitate the identification of potential kinases associated with ICI resistance. This sgRNA library comprised a total of 2614 sgRNAs targeting 698 kinases. These sgRNAs were packaged in lentiviral vectors and organized into four distinct pools. The multiplicity of infection (MOI) was adjusted to 0.3 to ensure that each cell was transduced with only one sgRNA. Following transduction, the cells underwent puromycin selection (for CT26, 7 days with 10 ng/ml puromycin). Each of the four pools of sgRNA-transduced CT26-Cas9 cells (1 × 106 cells) was subcutaneously transplanted into individual BALB/c mice and categorized into four groups based on the different pools. Seven days after tumor transplantation, each group of mice was further divided into a treatment group and a control group. The treatment group received RMP1-14 (murine PD-1 blockade, BioxCell, #BE0146) at four doses, with each dose administered every three days (Fig. 3b, top). Depletion of the corresponding sgRNAs occurred upon the inactivation of ICI resistance genes, which were indicated within the pool of sgRNA-transduced CT26-Cas9 cells. The most significant reduction in tumor volume was observed in response to RMP1-14 (PD-1 blockade) treatment compared to the control saline treatment group. Seven days after the final dose of RMP1-14, the mice were sacrificed, and tumor DNA was extracted using QIAGEN (#69504), followed by amplification through nested PCR, following the established protocol. For analysis, three of the tumors were combined as sample 1, while the remaining two tumors constituted sample 2. Downregulated sgRNAs in both sample 1 and sample 2 were compared to the control group, and the top 8 candidate genes were selected based on the following criteria: (1) genes with between two and four downregulated sgRNAs in the comparisons; (2) genes with sgRNA negative rank values (determined using the RRA score calculated by Model-based Analysis of Genome-wide CRISPR-Cas9 Knockout(MAGeCK) within the top 20 in both samples28.
Cells, plasmids, siRNA, shRNA transfection
The mouse CRC cell line CT26 and human CRC cell lines HCT116, DLD1, and HT29 were purchased from Procell Life Science & Technology Company (Wuhan, Hubei,China). MC38 was a gift from the laboratory of Professor Penghui Zhou. SW620 cells were a gift from the laboratory of Professor Ranyi Liu. Cells (CT26, HCT116, DLD1 and SW620) were cultured and maintained in Roswell Park Memorial Institute-1640 (RPMI-1640, Gibco, Rockville, MD, USA) containing 10% heat-inactivated fetal bovine serum (FBS; WISENT [086-150], Pengchen Science&Technology Company, Guangzhou,Guangdong,China). MC38 cells were cultured and maintained in DMEM (10% FBS). HT29 cells were cultured in MyCo5A (10% FBS). All cells were incubated in 5% CO2 and 95% N2 at 37 °C and subjected to cell line authentication. Cells in the mid-log phase were used in the experiments. Small interfering RNA (siRNA) was used for gene silencing. siRNA targeting DDR1 (siDDR1 1-4) and negative control siRNA (siNC) were constructed by Tsingke Biotechnology Co., Ltd. (as presented in the Supplementary Table S1). A total of 3 × 105 cells were inoculated in each well of 6-well culture dishes, with one dish for each cell line. All transient transfections were performed with the TransIT-X2 Dynamic Delivery System (Mirus, #MIR6000, NeoBioscience Technology Co.,Ltd,Shenzhen,Guangdong,China) according to the manufacturer’s instructions. Specific target sequences are shown in Supplementary tables.
Generation of stable knockdown cell lines
To generate recombinant lentiviruses, human and mouse shRNAs targeting DDR1, as well as a negative control shRNA (detailed in the Supplementary tables), were transfected into 293 T cells along with lentiviral packaging plasmids. Subsequently, both human and mouse CRC cell lines with stable knockdown of DDR1 were established through lentiviral transduction followed by puromycin selection29,30. The specific target sequences used in this process are provided in Supplementary tables.
Reverse transcription and quantitative real-time reverse transcription polymerase chain reaction (RT‒PCR)
Total RNA extraction was performed using TRIzol reagent (Invitrogen, #15596018), and subsequently, reverse transcription was conducted using the Hifair III 1st Strand cDNA Synthesis SuperMix for qPCR (inclusive of gDNA digester plus, Yeasen Biotechnology [Shanghai] Co., Ltd., #11141ES60). Quantitative real-time PCR assays were performed following the protocol with the use of Hieff qPCR SYBR Green Master Mix (No Rox, inclusive of gDNA digester plus, Yeasen Biotechnology [Shanghai] Co., Ltd., #11201ES08). Detailed primer sequences can be found in Supplementary tables.
Western blotting analysis
Cell dishes were subjected to two washes with PBS and subsequently lysed in precooled RIPA lysis buffer (comprising 50 mmol Tris-HCl, pH 7.4, 150 mmol NaCl, 1 mmol dithiothreitol [DTT], 0.25% sodium deoxycholate, and 0.1% NP-40) enriched with 1 mmmol/L phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor for 30 min on ice. Protein extracts were obtained through centrifugation for 30 min at 4 °C. The protein concentration was assessed using a bicinchoninic acid assay (BCA) following the manufacturer’s guidelines. Western blotting was conducted following standard protocols, using the antibodies detailed in the Supplementary tables. β-Actin and GAPDH served as internal controls for the western blot analysis. Specific antibodies used in the study are listed in Supplementary table.
CCK-8, apoptosis and transwell assays
CRC cells transfected with each siRNA were seeded into 96-well plates at a density of 2000 cells per well. After 24, 48, 72, and 96 h of incubation, the cells were exposed to 10 μL of CCK-8 reagent (TargetMol, #C0005) and further cultured for 4 hours. The absorbance of cells at 450 nm was measured at different time points using a microplate reader (BIO-Tek EPOCH). Each sample consisted of 10 replicate wells, and we conducted three independent experiments. For apoptotic capacity determination, siRNA-transfected cells (at quantities of 3, 4 or 5 × 105 cells) were seeded into 6-well dishes and assessed using the CCK-8 assay at 96, 72, and 48 h. Apoptosis assays were performed via flow cytometry with the Annexin V Alexa Fluor 647/PI Apoptosis Detection Kit (#FXP023, 4 A Biotech Co., Ltd.), following standard protocol. FlowJo software (version 10.7) was used to analyze the apoptosis rate. Transwell assays were conducted utilizing transwell inserts (Corning, #353097) with 8 μm pore filters. Matrigel (Corning, #356234, 10 mg/ml) was applied to the upper surface of the insert at a concentration of 500 µg/ml. siRNA-transfected CRC cells (3 × 105) were placed in the upper chamber of the insert in a serum-free medium, while a complete medium containing 20% serum was added to the lower chamber. After a specific incubation period (HCT116: 30 hours; SW620: 36 hours; MC38: 30 h; CT26: 24 h), cells on the upper side of the membrane with Matrigel were removed. The cells on the lower side of the membrane were fixed with methanol, stained with crystal violet, and examined under a Nikon Eclipse Ni-U microscope at 100× magnification. Six random fields were photographed, and the average number of migrated and invaded cells was determined.
Immunofluorescence and Immunochemistry
Tumor specimens from both mouse models and patients were fixed in 10% neutral-buffered formalin, followed by paraffin embedding, sectioning and mounting onto slides. They were then subjected to standard staining procedures using antibodies against collagen I, DDR1, CD8 and DAPI (Supplementary tables), with the subsequent steps conducted in accordance with the manufacturer’s instructions for the immunofluorescence (IF) kit (PANOVUE #10203100050 and #10079100050). Images were captured using confocal laser scanning microscopy(LSM 980 Basic Operation, Carl Zeiss Microscope,German). Specific antibodies utilized in this study are detailed in the Supplementary tables. A total of 15 tumor tissues from patients with dMMR and 15 from those with pMMR CRC were used for immunohistochemistry (IHC) analysis. IHC staining and assessment were performed following a standardized protocol, and antibody dilution information can be found in the supplementary tables. The staining score was calculated as the product of staining intensity and percentage of positive cells. Percentage of positive cells was scored as 0,1,2 and 3 when the percentage of positive cells accounts for <5%, 5% ~ 25%, 26% ~ 50% and >50%, respectively. Intensity of IHC staining was scored as 0 for negative staining, 1 for weak, 2 for moderate and 3 for strong stainig. The total score was calculated as the product of positive cell percentage and intensity score.
Syngeneic mouse models
MC38 and CT26 cells (1 × 106) with stable knockdown of DDR1 or stable expression of negative control (shNC) were transplanted subcutaneously into C57B/L6 and BALB/c mice. Seven days after tumor inoculation, the mice were evenly sorted into a treatment group and a control group based on their tumor volume. In the treatment group, the mice received RMP1-14 (BioxCell, #BE0146, 10 mg/kg) every 3 days for a total of 4 doses (Fig. 3b).
For the mouse models receiving combination therapy of the DDR1 inhibitor 7rh and RMP1-14, the treatment group was given 7rh (MCE, # HY-U00444) at a dose of 12.5 mg/kg, administered orally three times with a three-day interval in both the CT26 and MC38 mouse models. In the CT26 mouse model, RMP1-14 was used, as previously mentioned, while in the MC38 mouse model, RMP1-14 was administered at a reduced dose of 5 mg/kg for each dose, totaling four doses. This adjustment was made to mitigate combined toxicity with RMP1-14 and explore the potential of 7rh in sensitizing MSI CRC cells to lower doses of RMP1-14 (Fig. 3f).
Mice were euthanized three days following the final dose of RMP1-14, and tumor samples were harvested. These tumor samples were subsequently dissociated into single cells using a tumor dissociation kit (Miltenyi Biotec, #130-096-730) following the provided protocol, in preparation for immune cell analysis.
Flow cytometry
Tumors obtained from the in vivo animal experiment were initially mechanically minced into 1 cm3 pieces, which were then digested into single cells by passing them through a 70-μm cell strainer (BD), adhering to the mouse tumor dissociation kit protocol (Miltenyi Biotec #130-096-730).
The filtered cells were blocked with anti-CD16/32 antibodies and stained with the indicated surface antibodies for 30 min on ice. Dead cells were marked using Fixable Viability Stain 700 (BD). Intracellular antibodies were added (see Supplementary tables) after fixation and permeabilization (BD, #562574) according to the manufacturer’s instructions. The anti-mouse fluorochrome-conjugated antibodies are listed in the Supplementary tables. Cell analysis was conducted using a Beckman Cyto FLEX cytometer. Initial analysis of cell profiles was performed using CytExpert, and subsequent data analysis was carried out using FlowJo (Version 10). Specific antibodies employed in this study are indicated in the Supplementary tables.
Enzyme-Linked Immunosorbent Assay(ELISA)
Supernatants, amounting to a volume of 1 ml, obtained from the culture of 1 × 107 cells were subjected to the analysis of CXCL10 levels. An enzyme-linked immunosorbent assay (ELISA) was conducted in accordance with the provided protocol, utilizing the Human IP-10/CXCL10 ELISA kit (Neobioscience, #EHC157).
ChIP‒qPCR assay
ChIP assays were conducted using a ChIP assay kit (Millipore, #17-295) in combination with the truChIP Chromatin Shearing kit (Covaris, #520127), following the manufacturer’s instructions. In each cell line, 1 × 107 cells were cross-linked using 1% formaldehyde and lysed and sonicated with a Covaris E220 evolution ultrasonicator (using parameters: 150 peak incident power, 5% duty factor, 200 cycles, 8 min) to shear chromatin. For immunoprecipitation, 200 µL of sheared chromatin was diluted to a final volume of 1 ml. Antibodies specific to H3K27me3 (ChIP grade) were used alongside IgG, and the mixture was incubated overnight at 4 °C. Protein A/G agarose beads were added and incubated for 30 minutes at 4 °C. Following this, DNA was purified utilizing a DNA purification kit (Omega #D6492), adhering to the manufacturer’s guidelines. Subsequently, the purified DNA was used as a template for real-time PCR analysis. Primer sequences for qPCR can be found in Supplementary tables.
RNA sequencing and statistical analysis
In the RNA sequencing experiments, total RNA was isolated from the SW620, HT29, and CT26-shDDR1 cell lines, which were subjected to stimulation with collagen I and a low dose of IFNγ (20 ng/ml). The TRIzol reagent (Invitrogen, #15596018) was utilized for RNA extraction. Subsequently, R software was used for the analysis of differentially expressed genes (DEGs) in the chemokine profile analysis, following established methods from previous studies31. Additionally, KEGG pathway enrichment analysis of DEGs and Gene Ontology analysis were conducted. A significance threshold of p < 0.05 was applied to determine statistical significance.
Results
DDR1 was identified as an ICI resistance gene by in vivo CRISPR screening
We conducted an in vivo single guide RNA (sgRNA) screening on the CT26 cell line, which is a MSS CRC cell line. These cells were transplanted into BALB/c mice using the methodology outlined in the mouse model section (Fig. 1a). Among the various pools of candidate sgRNAs, Pool A was selected due to its significant reduction in tumor volume when treated with PD-1 blockade, as compared to the control group (Fig. 1b and Figure S1A-E). Subsequently, tumor genomic DNA was extracted for PCR amplification and sequencing analysis. The human kinome tree analysis revealed the presence of overlapped kinases in both the negative and positive selection groups, based on which eight candidate kinase genes with highly enriched sgRNAs were identified, which were among the top-ranking depleted sgRNAs upon PD-1 blockade treatment (Fig. 1e, f).
a CRISPR sgRNA library screening flow chart. b Round 1: We identified downregulated sgRNAs in pool A that could sensitize the PD-1 blockade effect by the CRISPR/Cas9 method (n = 5-6/each group). c Round 2: Mouse model experiments were conducted to verify the top 8 candidate genes in pool A (n = 5-6/each group). d Tumor inhibition rate of tumor volume in Round 2 with knock out effect of 8 candidate genes on CT26 mouse models. e RNA-sequencing analysis results of pool A.Heatmap (left) showing the downregulated kinase genes with their specific sgRNAs in sample 1 and sample 2 in the PD-1 blockade group and control group. Venn diagram (right) showing the overlapping and top 8 ranked kinases downregulated in both sample 1 and sample 2. (p < 0.05;** p < 0.01;***, p < 0.001, n.s., not significant). f Kinome tree presenting protein kinases that were significantly downregulated in sample 1 and sample 2 (Pool A PD-1 blockade group). Dark blue circles represent the overlapping kinase genes that were downregulated in both sample 1 and sample 2 with Pool A sgRNA deleted and treated with PD-1 blockade (same as the following). The gray blue and light blue circles represent the kinase genes downregulated in sample 1 and sample 2, respectively. The red rectangle represents the kinase genes that were upregulated in both sample 1 and sample 2. The pink and purple rectangles represent the kinase genes upregulated in sample 1 and sample 2, respectively. Image created using KinMap, illustration reproduced courtesy of Cell Signaling Technology, Inc. (https://www.cellsignal.com).
Multiple criteria were applied to select candidate genes from the screening data, prioritizing genes with sgRNAs exclusively downregulated in the PD-1 blockade group, indicative of their potential role in inducing immunotherapy resistance. Genes were also ranked based on their fold change, and preference was given to genes targeted by two or more sgRNAs. Consequently, the top eight candidate kinases identified were Acvr1b, Axl, Csf1r, Ddr1, Blk, Btk, Cdkn1a, and Dryk3 (Fig. 1e, right). To validate these candidates, CT26 cells were transfected with sgRNAs to generate knockout cell lines for each gene, which were then implanted into mouse models for a subsequent round of experiments (Fig. 1c, d and Figure S2A-2I). After excluding genes exclusively expressed in murine cells, discoidin ___domain receptor 1(DDR1) emerged as a promising candidate for further investigation.
DDR1 was highly expressed in CRC and correlated with invasion and migration in vitro
Compared to the normal colon epithelial cell line CCD841con, both RNA and protein levels of DDR1 were significantly elevated in CRC cell lines (Fig. 2a). This pattern of increased DDR1 expression was consistently observed in cancer tissues compared to adjacent normal tissues, as demonstrated by both paired patient tissues and the GEPIA database for CRC (Fig. 2b, c).
a mRNA and protein expression levels of DDR1 in colon cancer cell lines compared to normal colon epithelial cells (detected by real-time PCR, fold change shown relative to GAPDH. Three independent experiments are shown). b Protein expression level of DDR1 in colorectal carcinoma tissue compared to normal adjacent tissue. c The gene expression profile of DDR1 across all tumor samples and paired normal tissues (bar plot, the highest bar represents the median expression of certain tumor types or normal tissues. The Y-axis represents transcripts per million (TPM) according to the Gene Expression Profiling Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn/). d Western blotting confirmed the construction of MSS and MSI CRC cell lines of both human and mouse origin with stable and transient knockdown of DDR1 induced by shRNA and siRNA transfection. Human MSS cell lines: HT29, SW620; human MSI cell lines: HCT116 and DLD1. Mouse MSS cell line: CT26; mouse MSI cell line: MC38. To achieve a better nockdown effect, we chose si 1 and si 2 for CT26 and MC38, si 1, si 2 and si 3 for HCT116 and SW620, sh1 and sh3 for CT26 and MC38, sh1 and sh2 for SW620 and DLD1 and sh1 and sh4 for HCT116 and HT29 for the following experiments. e Cell proliferation was assessed by CCK-8 assay in both human and mouse CRC cell lines with DDR1 knockdown vs. control siRNA (siNC) transfection. f Apoptosis was assessed based on the percentage of apoptotic cells detected by Annexin V/PI double staining by flow cytometry among the human and mouse CRC cell lines (cells with transient knockdown of DDR1 compared to control cells). g Invasion and migration capacity was assessed by Transwell assay in CRC cell lines with transient knockdown of DDR1 or control transfection. Scale bar, 100 μm. (p < 0.05;** p < 0.01;***, p < 0.001, n.s., not significant).
To investigate the potential relationship between DDR1 expression and immunotherapy in CRC, we conducted transient siRNA transfection and established stable DDR1 knockdown in various CRC cell lines, including both mouse and human cell lines. Specifically, we utilized the MSS mouse cell line CT26, the MSI mouse cell line MC38, the MSS human CRC cell lines HT29 and SW620, and the MSI human CRC cell lines HCT116 and DLD1 (Fig. 2d). Remarkably, knocking down DDR1 in CRC cells did not lead to significant changes in proliferation and apoptosis compared to their respective controls, regardless of whether they were mouse or human cell lines (Fig. 2e, f). However, suppressing DDR1 in CRC cells resulted in a significant reduction in invasion and migration (Fig. 2g and Fig. S3A-S3G).
Knockdown of DDR1 sensitizes MSS CRC tumors to ICIs and increases intratumoral functional CD8+ T cell infiltration
Murine CRC cell lines CT26 and MC38 with stable knockdown of DDR1 (shDDR1) or control shRNA (shNC) were generated. Subsequently, these cell lines were subcutaneously implanted into BALB/c and C57BL/6 mice, following the experimental design outlined in Fig. 3a, b. The treatment groups received four doses of RMP1-14 (a murine PD-1 blockade), while the control group received normal saline (Fig. 3b). Upon tumor harvesting, immune cells within the tumor tissue were analyzed via FACS based on the gating strategy outlined in Figure S3H. These results revealed that knockdown of DDR1 enhanced the response to PD-1 blockade therapy in MSS CRC but not in MSI CRC. Particularly, the shDDR1 treatment group exhibited the most substantial reduction in tumor volume compared to the control group (CT26-shNC) (Fig. 3c, top). Analysis of immune cell infiltration within tumors using FACS indicated that CD8+ T cells, especially functional T cells such as GZMB+ and IFNγ+ CD8+ T cells, played a crucial role in sensitizing MSS tumors to PD-1 blockade (Fig. 3d). Additionally, immunofluorescence of tumor sections from mouse models and dMMR CRC patients revealed that CD8 + T cells were more abundant in areas with lower expression of DDR1 and collagen I (Fig. 3g, top). In contrast, there was no significant reduction in tumor volumes in the shDdr1 + PD-1 blockade group when using MC38 cell lines compared to the group treated with PD-1 blockade alone in C57BL/6 mice (Fig. 3c, bottom).
a The knockdown effect of DDR1 was confirmed by western blotting, and sh1 and sh3 were chosen for the following animal experiments in CT26 and MC38 models, respectively. b Flow chart of the animal experiments for CT26 and MC38. RMP1-14 (mouse PD-1 blockade, 10 mg/kg) was given 7 days after subcutaneous inoculation of each group with tumor cells (1 × 106 cells for each mouse, right flank), and 4 doses were given in total. Mice were sacrificed 3 days after the last dose of RMP1-14, and tumors were harvested and dissociated into single cells for FACS. c Effect of Ddr1 knockdown and the combination of PD-1 blockade on CT26- and MC38-bearing mice shown by the tumor volume curve of each group. Mice bearing CT26 and MC38 tumors were transfected with DDR1 shRNA, treated with PD-1 blockade and their combination. The image on the right side shows the sizes of the tumors in each group (n = 5 in each group).Data are the mean ± SEM. Information on sample sizes, experimental number, times, biological replicates, statistical tests, and p values is available in Methods. d Flow cytometric determination of the percentage of each type of immune cell infiltrated in the tumor with the gating strategy in (sup Fig. 3B) in the Ddr1 knockdown CT26 animal experiment, which showed increased infiltration of CD8+ T cells, especially GZMB+ and IFNγ + CD8 + T cells, in the shDdr1+PD-1 blockade group compared to the other groups. Error bars show the mean ± SEM ; p values were calculated by one-way ANOVA. e Treatment schedule, tumor growth curve and representative tumor images for treating CT26 and MC38 tumors with DDR1 inhibitor 7rh and PD-1 blockade (n = 5 tumors per group. Error bars show the mean ± SEM. p values were determined by two-way ANOVA). f Flow cytometric determination of the percentage of each type of immune cell infiltrated in the tumor in the 7rh and PD-1 blockade combination in CT26 mouse models. The results showed increased CD8+ Tcell infiltration, especially functional infiltration, such as GZMB+CD8+ T cells, in the 7rh + PD-1 blockade group and shDdr1+7rh+PD-1 blockade group. n = 5 tumors per group. Error bars show the mean ± SEM. ; p values were calculated by one-way ANOVA. g Representative fluorescence confocal images of Ddr1 (red), collagen I (yellow) and CD8 (green) expression in the TME showing increased CD8+ T cell infiltration in areas with low Ddr1 and collagen I expression. Scale bar, 50 μm. h Representative fluorescence confocal images of dMMR CRC patient samples. Intratumoral CD8+ T cells were more enriched in the tumor area with low expression of DDR1 and collagen I than in that with high expression of these factors (scale bar: 50 μm).
Treatment with DDR1 kinase inhibitor 7rh sensitizes MSS CRC tumors to ICIs and increases intratumoral functional CD8+ T cell infiltration
To investigate the potential impact of inhibiting the DDR1 kinase ___domain using the DDR1 kinase inhibitor 7rh on MSS CRC tumors, we conducted additional animal experiments to assess the combined therapeutic effect of 7rh and PD-1 blockade. In the MSS CT26 mouse CRC model, we observed a significant decrease in tumor volume in the group receiving the combination of 7rh and PD-1 blockade compared to the group treated with PD-1 blockade alone (Fig. 3e, top). Analyses using FACS revealed the presence of functional CD8+ T-cell infiltration in the combined treatment groups compared to the other groups (Fig. 3f). Furthermore, IF staining of murine tumor slides demonstrated that CD8+ T cells were more abundant in regions with lower expression of both DDR1 and collagen I in the combined treatment group (Fig. 3g, bottom). The combination of 7rh and PD-1 blockade increased the number of infiltrating CD8+ T cells and enhanced their functions, as confirmed by both FACS and IF analyses of tumor slides compared to the shNC+PD-1 blockade group (Fig. 3f, g, bottom). These findings collectively suggest that depletion of the DDR1 gene and inhibition of DDR1 kinase activity can recruit more CD8 + T cells in MSS CRC tumors.
In contrast, the MC38 cell line was previously reported to be sensitive to PD-1 blockade, consistent with our result in Fig. 3c-shDdr1, which showed no significant effect on tumor shrinkage in the PD-1 blockade group. Therefore, we conducted experiments to assess whether 7rh could sensitize MC38 cells to a lower dose of PD-1 blockade (5 mg/kg). However, we did not observe any significant reduction in the group receiving both 7rh and PD-1 blockade compared to the group treated with PD-1 blockade alone, indicating that DDR1 kinase inhibition was unable to sensitize MC38 cells to a lower dose of PD-1 blockade (Fig. 3e, bottom).
Additionally,we performed IF staining for DDR1, collagen I, and CD8 markers on tumor slides collected from 10 dMMR/MSI-H CRC patients who had received PD-1 blockade treatment. Our analysis revealed that areas with lower expression of DDR1 and collagen I exhibited a higher abundance of CD8+ cells (Fig. 3h), indicating a negative correlation between DDR1, collagen I, and CD8+ markers in CRC. These findings suggest that both DDR1 depletion and DDR1 kinase inhibition have the potential to enhance the response of MSS CRC tumors to PD-1 blockade by promoting the intratumoral infiltration of CD8+ T cells, especially functional and cytotoxic CD8+ T cells.
Knockdown of DDR1 in MSS CRC cell lines enhanced CXCL10 secretion
Chemokines play a pivotal role in influencing immune cell infiltration, and our study provides compelling evidence confirming the correlation between DDR1 and the recruitment of cytotoxic CD8+ T cells in MSS CRC tumors, implying that the interaction between collagen and DDR1 may impact the recruitment of CD8+ T cells by modulating specific chemokines. To further assess this phenomenon, we conducted a chemokine profile analysis using a 12-chemokine signature which correlated with CRC. RNA-seq was performed on shDDR1 CT26, HT29 and SW620 cell lines after stimulation with collagen I and a low dose of IFNγ revealing an upregulation of CXCL10 expression in shDDR1 MSS CRC cell lines (Fig. 4a). Enrichment analysis based on DEGs suggested that the increased CXCL10 expression in shDDR1 MSS CRC cell lines could be associated with histone modification pathways and chromosome regions, as indicated by the biological process and cellular component analyses (Fig. 4c, d). Furthermore, KEGG analysis suggested a potential link between this phenomenon and proteoglycans in cancer, indicating a possible connection to the desmoplasia reaction, also known as tumor fibrosis (Fig. 4b).
a Volcano and Venn plots showing significantly elevated CXCL10 RNA expression by chemokine profile analysis in the shDdr1 mouse MSS CRC cell line CT26 and the human MSS CRC cell lines HT29 and SW620 after stimulation with collagen I and IFNγ (20 ng/ml) compared to control RNA-transfected cells. |Log2fold change | >1.2, | p adj | >0.05). b, c GO and KEGG enrichment analysis of the DEGs (DEGs, differentially expressed genes; GO,gene ontology). Dot plot and bar plot showed DEGs enriched in histone modification in the BP (biological process) category, chromosome region in the CC (cellular component) category and proteoglycans in cancer in shDDR1 CRC cell lines compared to shNC cell lines after collagen I and IFNγ (20 ng/ml) by KEGG and GO analysis. d Chord diagram showing the top 100 ranked upregulated genes by GO analysis in SW620-shDDR1 cells compared to SW620-shNC cells stimulated with collagen I and IFNγ (20 ng/ml). e Western blotting of DDR1 knockdown CRC cell lines showed decreased levels of DDR1 and phosphorylated DDR1 kinase ___domain (Tyr792) but increased supernatant CXCL10 levels in shDDR1 cell lines stimulated with collagen I and a small dose of IFNγ (20 ng/ml (WB protein levels were normalized to β-actin level). f ELISA verified elevated supernatant CXCL10 levels and increased RNA expression of CXCL10 in the shDDR1 human MSS CRC cell lines HT29 and SW620 compared to the control group stimulated with collagen I and a small dose of IFNγ (20 ng/ml) (ELISA was quantified with the same volume of supernatant of 1 × 107 cells). g, h CXCL10 RNA levels were quantified by RT-PCR and normalized to GAPDH levels (detected by real-time PCR, fold change shown relative to GAPDH). Three independent experiments are shown with stimulation by IFNγ (20 ng/ml) and stimulation by both collagen I and IFNγ (20 mg/ml).
The upregulation of CXCL10, but not CXCL9, at the RNA level, was consistently observed in the shDDR1 MSS cell lines in response to a small dose of IFNγ, whether with or without collagen I stimulation, which was then confirmed through RNA-seq and qRT-PCR analyses (Fig. 4a, g and Figure S3I). However, no similar changes in CXCL10 or CXCL9 expression were observed in the MSI CRC cell line (Figure S4A-S4B). Moreover, upon collagen I stimulation, the levels of CXCL10 increased in the supernatant of shDDR1 MSS CRC cell lines, as confirmed by ELISA and Western blotting (Fig. 4e, f and Figure S4C). Additionally, we observed the suppression of DDR1 expression and the phosphorylation of the DDR1 kinase ___domain at Tyr792 in response to collagen I stimulation.
Taken together, these findings indicate that the enhanced sensitivity of MSS CRC tumors to PD-1 blockade resulting from the depletion or inhibition of the kinase ___domain of DDR1 may be associated with the upregulation of the Th1 chemokine CXCL10 but not CXCL9.
DDR1 increased the enrichment of EZH2 and H3K27me3 at the CXCL10 promoter
We hypothesized that DDR1 kinase ___domain phosphorylation, triggered by the binding of ECM collagen, might have an impact on H3K27 methylation,and subsequently on CXCL10 transcription. This effect is likely mediated by various factors, including Polycomb Repressive Complex 2 (PRC2), Jumonji Domain-containing Protein D3 (JMJD3) and Ubiquitously Transcribed Tetratricopeptide Repeat X-Linked (UTX), which is encoded by the KDM6A gene. These factors are indicated as upregulated in shDDR1 MSS CRC cell lines in Fig. 4d.
EZH2 is upregulated in both MSI and MSS CRC cell lines compared to normal epithelial cell line CCD841con (Fig. 5a). However, EZH2 expression was lower in shDDR1 MSS CRC cells than in control cells, even with both collagen I and IFNγ stimulation (Fig. 5b), which is different from the pattern observed in shDDR1 MSI CRC cells (Figure S4D). To further explore the involvement of the PRC2 complex in CXCL10 expression, we examined the changes in the three primary components of PRC2 (EZH2, SUZ12 and EED). Specifically, EZH2 exhibited decreased levels in shDDR1 MSS cell lines following stimulation with collagen I and IFNγ, whereas SUZ12 and EED showed minimal changes (Fig. 5c). Moreover, the H3K27me3 level in shDDR1 MSS CRC cell lines was reduced compared to the control shNC cell lines upon stimulation with collagen I, which was consistent with the EZH2 change (Fig. 5c). To investigate the interaction between H3K27me3 and the CXCL10 promoter, ChIP-qPCR at the CXCL10 promoter was performed, and the results showed that the H3K27me3 occupancy at the CXCL10 promoter increased in shNC CRC cells compared to shDDR1 CRC cells (Fig. 5d).
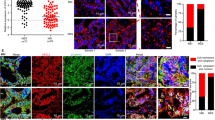
a EZH2 is highly expressed in human CRC cell lines (relative to NAPDH in both MSS and MSI CRC cell lines) compared to normal epithelial cells (CCD841con). b Downregulation of EZH2 has been observed in shDDR1 both human and mouse MSS CRC cell lines either by stimulation byIFN-γ (20 ng/ml, same as below) or by the combination of IFNγ and collagen I which were quantified and normalized to GAPDH level by qt-PCR. c With stimulation using collagen I and IFNγ, we found decreased levels of EZH2 and H3K27me3 in shDDR1 MSS CRC cell lines compared with the cell with only stimulation of IFNγ. d ChIP‒qPCR results of shDDR1 SW620 and HT29 cell lines showed increasing H3K27me3 binding at the CXCL10 promoter with stimulation by both collagen I and IFNγ. e IFNγR2was not changed in shDDR1 CRC cell lines stimulated by both collagen I and IFNγ. f Protein expression of DDR1, EZH2 and H3K27me3 in the third and fourth round of CT26 mouse models’ tumor specimens. ×400, scale bar 50 μm). g, h Protein expression of DDR1 and collagen I in tumor tissue of CRC patients.The level of DDR1 expression was classified as negative,weak,moderate and strong (×40 and ×200, scale bar 500 μm and 100 μm respectively, same as below). i Expression of DDR1 and disposition of collagen I in dMMR compared to the one in pMMR patients (×200, scale bar 100 μm). j, k Immunohistochemical score of DDR1 and collagen I in 13 dMMR and 13 pMMR CRC patients which indicated both DDR1 and collagen I expression showed higher level than the ones in pMMR group. DDR1 showed no correlation with collagen I in dMMR group but positively correlated with collagen I in pMMR group. l Schematic diagram of collagen I and DDR1 function in CXCL10 transcription in MSS CRC cells.
To remove potential confounding variables, we assessed DNMT1, as well as the demethylases JMJD3 and UTX, under identical experimental conditions. In the shDDR1 MSS CRC cell lines (CT26, HT29, SW620), we observed reduced levels of DNMT1 compared to the shNC cell lines. Furthermore, there were no significant changes detected in IFNGR2 (Fig. 5e and S4E). CRC cell lines exhibited increased UTX levels and concurrently decreased JMJD3 levels compared to normal epithelial cells (CCD841con) (Figure S4F). In MSS CRC cell lines with DDR1 knockdown, such as SW620 and CT26, JMJD3 expression was elevated. Conversely, the HT29 cell line, when subjected to DDR1 knockdown and treated with collagen I and IFNγ, displayed an upregulation of UTX expression (Figure S4G).
The expression of DDR1 in colorectal cancer tissues
In order to confirm the correlation between DDR1, EZH2 and H3K27me3, we performed IHC stain of these three markers in CT26 tumor specimens in Fig. 3c, e. Notably, we found downregulated EZH2 and H3K27me3 expression level with the knockdown of DDR1 or application of DDR1 inhibitor 7rh (Fig. 5f), which was consistent with the in vitro circumstances in Fig. 5c.
To validate the potential correlation between DDR1 and collagen deposition in CRC patients, we conducted immunohistochemical staining on surgical specimens obtained from 13 dMMR and 13 pMMR patients and their IHC scores were determined (Fig. 5g, h). In most dMMR CRC tumors, we observed reduced DDR1 expression and diminished collagen I deposition in comparison to pMMR CRC tumors (Fig. 5i). The pMMR group exhibited higher IHC scores for both DDR1 and collagen I than the dMMR group (Fig. 5j). Notably, DDR1 demonstrated no correlation with collagen I deposition in the dMMR group, but it displayed a positive correlation with collagen I deposition in the pMMR group (Fig. 5k).
Discussion
The immune response is influenced by a range of factors, encompassing both intrinsic elements (e.g., genetic mutations) and extrinsic factors (e.g. Infection and ECM fibrosis). The immune-excluded tumor phenotype10,11 is characterized by an abundance of immune cells confined to the fibrotic and collagen-rich stroma surrounding tumor nests in the ECM. The presence of fibrotic and collagen-deposited stroma acts as a barrier, preventing CD8+ T cells from accessing tumor cells and impairing their cytotoxic functions12,13,14,15. As a result, the efficacy of ICI-induced tumor cell killing is hindered. Therefore, the effectiveness of immunotherapy is strongly influenced by the interplay among tumor cells, immune cells, and ECM components.Deciphering the function of collagen deposition and it’s receptor in immunotherapy resistance may provide novel insights into improve efficacy of ICIs.
In this study, we conducted in vivo CRISPR sgRNA screening utilizing a mouse model of MSS CRC to pinpoint potential kinases associated with ICI resistance. Our screening process identified eight candidate genes, among which DDR1 was identified as the most promising. Given DDR1 is one of the key collagen receptors to exert ICI resistance effects, pinpointing the mechanism of DDR1 is critical for understanding function of collagen on tumor cells. DDR1, a member of the RTK family and a receptor for collagen, possesses RTK attributes. Functionally, DDR1 operates as a collagen receptor, with its extracellular discoidin ___domain binding to collagen, its transmembrane ___domain facilitating the recognition of external signals, and its intracellular kinase ___domain transmitting ECM signals via kinase ___domain phosphorylation32. The released ECD can bind to collagen and regulate collagen alignment and physically establishes a barrier impeding the infiltration of immune cells into the tumor parenchyma, as observed in breast cancer33, which prompts further exploration into the potential role of the kinase ___domain in modulating the immune response by affecting tumor cells.Additionally, DDR1 was reported relate to metastasis in CRC23.Through an examination of tumor cell behaviors, we discerned that DDR1 played a role in the invasion and migration of CRC cells, indicating its potential involvement in the ECM, which aligns with previous research findings23.
Our subsequent validation using in vivo murine models confirmed the significance of DDR1, particularly with knockdown of DDR1, in selectively recruiting functional CD8+ T cells within MSS CRC tumors, which led to increased sensitivity to ICIs in CRC tumor cells. Of note, ECD of DDR1 has been reported to regulate collagen alignment in ECM and subsequently the infiltration of T cells in breast cancers33. However, there is very limited knowledge on intracellular kinase ___domain of DDR1 contents afftect the recruitment of CD8+T cells.Interestingly, we found the inhibition of kinase ___domain but not the ECD of DDR1 by using its selective inhibitor 7rh also could enhanced the infiltration of functional CD8+ T cells and sensitize MSS CRC to PD-1 blokade. Reports indicated 7rh as a DDR1 inhibitor that exhibited antitumor effect in nasopharyngeal carcinoma alone or in combination with dasatinib34. Notably, our study is the first study to explore the function of kinase ___domain of DDR1 in ICIs’ resistance and potential therapeutic effect of combination therapy of 7rh and PD-1 blockade in MSS CRC.
Upon the RNA sequencing analysis of MSS CRC cells with knockdown of DDR1, we identified one of the Th1-type chemokine CXCL10 was unregulated. In addition, these cells are able to secrete more CXCL10 but not CXCL9 in supernatant.CXCL9 and CXCL10 are chemokines induced by interferon-γ (IFN-γ), primarily secreted by monocytes, endothelial cells, fibroblasts, and cancer cells, and functioned in regulating immune cell recruitment, differentiation, and activation,particularly CD8+ T cells35,36 Previous studies have reported an association between Th1-type chemokine and H3K27 methylation in ovarian cancer, which is mediated by PRC2 complex37. EZH2 is a key methyltrasferase of the PRC2 complex responsible for trimethylation of histone H3 at lysine 27 (H3K27me3), which was a repressive histone mark that promotes gene silencing by binding to gene promoters like CXCL9 and CXCL10. Therefore, we hypothesized that DDR1 might inhibit CXCL10 transcription via similar signal routes.In our study, EZH2 and H3K27me3 level was downregulated in MSS CRC cell with DDR1 knockdown no matter with or without stimulation of collagen I. Additionally, trimethylation of H3K27 was upregulated at CXCL10 promotor in theses cells. However, the other histone modification related enzymes such as DNA methylase DNMT1, histone demethylase JMJD3 and UTX were observed as subtle change. In clinical CRC specimens, we observed the positive correlation between DDR1 and collagen I deposition in MSS CRC but not MSI CRC, which were align with the result obtained in murine MSS CRC tumor specimens.
Given the emerging clinical studies have shown that collagen deposition was significantly correlated with poor response to ICIs in cancers, gaining new insights in to collagen–collagen receptors (DDR1) axis in the context of stroma-rich tumors like MSS CRC can provide therapeutic avenues. Our investigation highlights the significance of DDR1’s kinase ___domain in modulating the response to immunotherapy, proposing it as a promising target for overcoming resistance to ICIs. Given the modest response rates observed with monotherapy involving ICIs, the use of combination therapeutic approaches could be imperative to enhance their efficacy. In this regard, 7rh emerges as a promising candidate for potential synergy with ICIs, with previous clinical evaluations in cancer patients supporting its consideration. Notably, DDR1 has been previously associated with cancer cell metastasis, as has CXCL1038. Moreover, ECM components have been shown to influence the migratory and invasive properties of tumor cells. Thus, it could be plausible that immune cell infiltration into the ECM mimics some of the migratory and invasive characteristics of tumor cells. Involvment of CXCL10 is linked to metastasis and the immune response, as they possess the capacity to draw CD8 + T cells into the TME39,40,41. Interestingly, our observations indicate that in MSS CRC cell lines with DDR1 knockdown, there is a partial upregulation of EZH2 following stimulation with collagen I, indicating the presence of an alternative pathway through which collagen can influence EZH2.
Our study has certain limitations, primarily due to the lack of patient data to substantiate the role of DDR1 in immunotherapy for MSS CRC patients. Several pertinent aspects, including the intricate interplay between DDR1 kinase phosphorylation and EZH2 downregulation, warrant further elucidation. The precise reasons underlying the selective impact of EZH2 on CXCL10, as opposed to CXCL9, remain unclear. Nevertheless, it is crucial to carefully assess the potential toxicity of combination therapies as the dosage of 7rh used in our study, adapted from previous investigations for use in combination with ICIs, led to adverse outcomes, including mortality, in our mouse models.
Collectively, our findings suggest that the disruption of H3K27 tri-methylation at the CXCL10 promoter may be linked to downregulation of EZH2, which is triggered by the activation of DDR1 through ECM collagen binding and subsequent phosphorylation of its kinase ___domain. Consequently, the suppression of CXCL10 transcription and reduction in its levels leads to a decrease in the recruitment and activation of functional CD8 + T cells within the TME, ultimately resulting in resistance to ICIs in fibrotic MSS CRC. Additionally, the DDR1 kinase inhibitor 7rh was found to enhance the therapeutic efficacy of ICIs (Fig. 5n).
Data availability
All authors had access to the data published in this paper. Data have been uploaded to the Research Data online platform (https://www.researchdata.org.cn/).
Code availability
RNA-seq data processing was conducted on a Linux Ubuntu platform using R (version 4.3.1). The processed expression matrix and R scripts used in this study are available on GitHub (https://github.com/DerrickNeuro/DDR1.git). The RNA-seq expression matrix for TCGA-COAD was obtained from UCSC Xena, and single-cell RNA sequencing data from real-world CRC samples by Lee HO et al. can be accessed via the Gene Expression Omnibus (GEO) under accession number GSE13246542.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Wu, X. et al. Application of PD-1 blockade in cancer immunotherapy. Comput. Struct. Biotechnol. J. 17, 661–674 (2019).
Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 38, 255 (2019).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv324 (2016).
He, Y., Zhang, L., Zhou, R., Wang, Y. & Chen, H. The role of DNA mismatch repair in immunotherapy of human cancer. Int J. Biol. Sci. 18, 2821–2832 (2022).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Galon, J. et al. Type, density, and ___location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Platten, M., Nollen, E. A. A., Rohrig, U. F., Fallarino, F. & Opitz, C. A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18, 379–401 (2019).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Kuczek, D. E. et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 7, 68 (2019).
Renouf, D. J. et al. Survival for metastatic colorectal cancer in the bevacizumab era: a population-based analysis. Clin. Colorectal Cancer 10, 97–101 (2011).
Slack, B. E., Siniaia, M. S. & Blusztajn, J. K. Collagen type I selectively activates ectodomain shedding of the discoidin ___domain receptor 1: involvement of Src tyrosine kinase. J. Cell. Biochem. 98, 672–684 (2006).
Shariati, M. et al. Combined inhibition of DDR1 and CDK4/6 induces synergistic effects in ER-positive, HER2-negative breast cancer with PIK3CA/AKT1 mutations. Oncogene 40, 4425–4439 (2021).
Vehlow, A. et al. Interaction of discoidin ___domain receptor 1 with a 14-3-3-Beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 26, 3672–3683 e3677 (2019).
Jeitany, M. et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol. Med. 10, e7918 (2018).
Scheer, M. G., Sloots, C. E., van der Wilt, G. J. & Ruers, T. J. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann. Oncol. 19, 1829–1835 (2008).
Valencia, K. et al. Inhibition of collagen receptor discoidin ___domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin. Cancer Res. 18, 969–980 (2012).
Romayor, I. et al. Tumor DDR1 deficiency reduces liver metastasis by colon carcinoma and impairs stromal reaction. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G1002–G1013 (2021).
Romayor, I., Badiola, I. & Olaso, E. Inhibition of DDR1 reduces invasive features of human A375 melanoma, HT29 colon carcinoma and SK-HEP hepatoma cells. Cell Adh Migr. 14, 69–81 (2020).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Kanungo, J. Puromycin-resistant lentiviral control shRNA vector, pLKO.1 induces unexpected cellular differentiation of P19 embryonic stem cells. Biochem. Biophys. Res. Commun. 486, 481–485 (2017).
Cheng, T. L. & Chang, W. T. Construction of simple and efficient DNA vector-based short hairpin RNA expression systems for specific gene silencing in mammalian cells. Methods Mol. Biol. 408, 223–241 (2007).
Tokunaga, R. et al. 12-Chemokine signature, a predictor of tumor recurrence in colorectal cancer. Int J. Cancer 147, 532–541 (2020).
Vogel, W., Gish, G. D., Alves, F. & Pawson, T. The discoidin ___domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1, 13–23 (1997).
Sun, X. et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 599, 673–678 (2021).
Lu, Q. P. et al. Antitumor activity of 7RH, a discoidin ___domain receptor 1 inhibitor, alone or in combination with dasatinib exhibits antitumor effects in nasopharyngeal carcinoma cells. Oncol. Lett. 12, 3598–3608 (2016).
Tokunaga, R. et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat. Rev. 63, 40–47 (2018).
Ayers, M. et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest 127, 2930–2940 (2017).
Nagarsheth, N. et al. PRC2 epigenetically silences Th1-Type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 76, 275–282 (2016).
Zipin-Roitman, A. et al. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 67, 3396–3405 (2007).
Reschke, R. et al. Immune cell and tumor cell-derived CXCL10 is indicative of immunotherapy response in metastatic melanoma. J. Immunother. Cancer 9, e003521 (2021).
House, I. G. et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin. Cancer Res. 26, 487–504 (2020).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723.e714 (2017).
Lee, H. O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52, 594–603 (2020).
Acknowledgements
We thank Professor Ranyi Liu for the helpful support for this work. We are thankful to Dr. Xin Yue for providing the kinase library for the research. The work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82473398 and 82172812), Guangdong Basic and Applied Basic Research Foundation (Grant Nos. 2021A1515010096 and 2023A1515010243), China Postdoctoral Science Foundation (Grant No. 2019M660227), Chinese Society of Clinical Oncology Foundation (Grant Nos. Y-HR2018-319, Y-L2017-002, and Y-JS2019-009), Sun Yat-sen University Basic Research Fund (Grant No. 19ykpy180), and open research funds from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (202011-103, 202301-314).
Author information
Authors and Affiliations
Contributions
R.Z. and G.C. contributed to the study conception and design. Material preparation, data collection and analysis were performed by M.W., W.M., and G.L. Acquisition and analysis of data (e.g., provided animals, acquired and managed patients, provided facilities) were performed by M.W., G.L. and C.L. Administrative, technical, or material support were performed by X.W., X.C., X.P. Study supervision was performed by C.T., Z.P., R.L., and G.C. The first draft of the manuscript was written by M.W. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committees of Sun Yat-Sen University Cancer Center in accordance with the Declaration of Helsinki (No. B2023-023-01). All patients participated in this study voluntarily and signed written informed consent.
Consent for publication
All authors agreed to publish the article.
Patient consent for publication
Consent was obtained directly from the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, M., Ma, W., Lv, G. et al. DDR1 is identified as an immunotherapy target for microsatellite stable colon cancer by CRISPR screening. npj Precis. Onc. 8, 253 (2024). https://doi.org/10.1038/s41698-024-00743-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00743-2