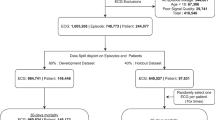
Abstract
Findings from a previous study (ClinicalTrials.gov: NCT05118035) demonstrated that an AI-enabled electrocardiogram (AI-ECG), combining AI reports and physician alerts, effectively identified hospitalized patients at high risk of mortality and reduced all-cause mortality. This study evaluates its cost-effectiveness from the health payer’s perspective in Taiwan over a 90-day post-intervention period. Cost data were obtained from electronic health records of participating hospitals, and incremental cost-effectiveness ratios (ICERs) per death averted were calculated. Non-parametric bootstrap techniques were used to address uncertainty. Among 15,965 patients, 90-day all-cause mortality was 3.6% in the intervention group versus 4.3% in controls. Medication and ICU costs were higher in the AI-ECG group, but overall medical cost was similar ($6204 vs. $5803). The ICER was $59,500 (95% CI: $-4657 to $385,950) per death averted. The cost-effectiveness acceptability curve showed that 95% of the probability mass lies below a willingness-to-pay threshold of $409,321, supporting favorable cost-effectiveness despite uncertainty.
Similar content being viewed by others
Introduction
Clinical decision support systems (CDSS) embedded in electronic health records (EHRs) enhance patient safety and guide clinicians toward evidence-based care1. By leveraging “big data” and predictive algorithms, CDSS optimize workflows, reduce medication errors, and identify patients at high risk of adverse outcomes, such as in-hospital mortality2,3. Advances in artificial intelligence (AI) have further expanded the capabilities of CDSS, resulting in innovative tools and commercial products that improve decision-making and patient outcomes. Many of these systems are now integrated into medical devices, offering new ways to visualize and interpret clinical data4,5. However, while AI-driven CDSS hold promise, there is limited evidence supporting their economic value and clinical impact, particularly in real-world settings6.
The electrocardiogram (ECG) is a widely used diagnostic tool, performed over 3 million times daily worldwide7, and is routinely utilized for admission and preoperative evaluations to assess potential cardiac risks. With the advancement of AI8, AI-enabled ECG (AI-ECG) has demonstrated the ability to detect previously undetectable conditions, such as low ejection fraction and paroxysmal atrial fibrillation9,10,11. AI-ECG has also shown strong predictive power for 1-year mortality, with area under the curve (AUC) values exceeding 0.8512,13. An AUC of 0.85 indicates excellent discrimination, meaning the AI-ECG model has a high ability to differentiate between patients who will experience an event (such as death) and those who will not, highlighting its potential for use in critical care triage.
The rapid response system (RRS) has been shown to enhance the recognition and management of high-risk patients, reducing mortality14,15. A recent randomized controlled trial (RCT) evaluated the integration of AI-ECG into a track-and-trigger system (TTS) within a RRS. This innovative approach enabled real-time identification of hospitalized patients at high mortality risk, prompting physicians to initiate timely interventions16. The trial demonstrated a significant 17% reduction in 90-day mortality (hazard ratio [HR]: 0.83; 95% confidence interval [CI]: 0.70–0.99) in the intervention group compared to controls. However, this improvement was accompanied by increased ICU admissions and additional cardiac investigations, leading to higher care cascade costs.
Despite these promising results, the cost-effectiveness of AI-ECG systems remains unclear, particularly in the context of their integration into routine clinical practice. Given that healthcare resources are limited, investments in new technologies often come at the expense of potential investments in other areas, highlighting the importance of considering opportunity costs. Therefore, it is crucial to evaluate not only clinical efficacy but also the economic impact of new technologies to support informed healthcare decision-making. This study is novel in providing the first comprehensive economic evaluation of an AI-ECG system, assessing both its clinical outcomes and incremental cost-effectiveness from a health payer’s perspective. By addressing these critical gaps, this research aims to inform the sustainable adoption of AI-driven CDSS in real-world healthcare settings.
Results
Participants and outcomes
A complete description of patients characteristics for the AI-ECG alert clinical trial is available is the main trial article16. Briefly, 15,965 patients were randomized, with 8001 assigned to the AI-ECG group and 7964 to the control group. The mean age was 60.9 ± 18.5 years in the intervention group and 61.5 ± 18.2 years in the control group, with 50.9% and 52.3% male, respectively. Baseline characteristics were well balanced between groups at admission (Supplementary Table 1). The 90-day all-cause mortality rate was 3.6% in the intervention group compared to 4.3% in the control group. Clinical and resource utilization outcomes are summarized in Table 1. On average, patients in the AI-ECG group received slightly more ECGs than those in the control group (2.41 ± 3.53 vs. 2.27 ± 2.71). The median length of hospital stay was 2 days (IQR: 7) in both groups. ICU admission within 3 days occurred in 3.6% of patients in the AI-ECG group and 3.4% in the control group. Among those admitted to the ICU, the median length of ICU stay was 8 days (IQR: 13.2) in the intervention group and 7 days (IQR: 11) in the control group.
Cost analysis
Figure 1 shows the average costs per patient for various medical cost components and their proportions in the AI-ECG and control groups over the 90 days. As presented in Fig. 1A, the average total cost was higher in the AI-ECG group ($6204) compared to the control group ($5803). The incremental cost between the two groups was $402 (95% CI, 61–735). The cost components show slight variations between the two groups, with drug ($154 difference, 38% increase) and ICU costs ($79 difference, 20% increase) being marginally higher in the AI-ECG group. Figure 1B illustrates the proportion of each cost component within the total costs. In both groups, the largest proportion of costs is attributed to drugs (21.4% vs. 20.3%), followed by examination, medical supplies and procedures. The AI-ECG group had slightly higher proportions of costs allocated to drugs and ICU, while the control group had a higher proportion of costs allocated to other cost components.
Cost-effectiveness analysis
The cost-effectiveness of the AI-ECG alert in reducing all-cause mortality is presented in Table 1. Despite the higher costs, the AI-ECG group exhibited a lower mortality rate (3.6% vs. 4.3%). The incremental cost per death averted in the AI-ECG group was $59,500, with a 95% CI of −$4657 to $385,950.
The cost-effectiveness plane presents the uncertainty around the bootstrapping estimates of ICER, as shown in Fig. 2. In most bootstrapped samples, there was a tradeoff between costs and effectiveness when comparing the AI-ECG alert system to usual care. Among the 5000 bootstrap samples, 96.8% were located in quadrant I (higher costs and more deaths averted). AI-ECG alert dominated usual care in 2.3% of cases (lower costs and more deaths averted) and was dominated in less than 0.1% of cases. The cost-effectiveness acceptability curve (Fig. 3) shows a 50% probability of cost-effectiveness at a ICER threshold of $60,628 and a 95% probability at $409,321. Furthermore, for those identified as “high risk” in the population cohort, the incremental cost per death averted in the AI-ECG group was $19,863 (95% CI: −7954 to 73,753). The distribution of bootstrap samples was shown in Supplementary Fig. 1.
The cost-effectiveness acceptability curve illustrates the probability that the AI-ECG intervention is cost-effective across a range of willingness-to-pay (WTP) thresholds. The dashed lines indicate the WTP values at which the AI-ECG alert system reaches a 50% probability ($60,628) and a 95% probability ($409,321) of being cost-effective.
Sensitivity analyses
To assess the robustness of the base-case results, we conducted sensitivity analyses by varying both the unit price of the AI-ECG alert system ($4, $8, and $32) and the override rate (0%, 40%, and 60%). We selected these three override levels to represent ideal, moderate, and high alert fatigue scenarios while maintaining interpretability. The analysis showed that although higher override rates and increased AI-ECG costs led to slightly increased ICERs, the intervention remained within a potentially acceptable cost-effectiveness range under all scenarios (Table 2).
Subgroup analyses revealed notable variation in the cost-effectiveness of AI-ECG alerts across different patient groups (Supplementary Table 2). The intervention appeared more cost-effective among inpatients, males, and individuals with hypertension. In contrast, some subgroups, such as females and those aged 65 to 74, showed less favorable or more uncertain results, highlighting the importance of targeted application and the need for further validation. Among high-risk patients, cost-effectiveness was generally more favorable and consistent (Supplementary Table 3). In this group, several subpopulations, including those with heart failure or hypertension, demonstrated dominant results. Compared to the overall study population, the intervention in high-risk patients was associated with greater mortality reduction and more stable ICERs, indicating its potential value in guiding more focused implementation strategies.
Discussion
The present study reports the direct medical costs associated with implementing AI-ECG alert system in predicting mortality risk compared to usual care in hospitalized patients from a RCT. Results of this economic evaluation revealed the implementation of AI-ECG resulted in a modest increase in short-term healthcare costs while reducing 90-day all-cause mortality. From a healthcare payer’s perspective, the intervention appears to represent a cost-effective strategy, particularly when used in a population with elevated baseline risk.
Using AI to analyze existing ECGs can provide clinicians with a wealth of additional information. Beyond indicating high mortality risk12,13, AI can also identify conditions like low ejection fraction and paroxysmal atrial fibrillation9,10,11, prompting clinicians to consider further interventions. Previous RCT have demonstrated that this type of AI-ECG can prompt clinicians to arrange echocardiograms for high-risk patients to confirm the possibility of low ejection fraction17. The increase in interventions following such alerts is not unique to AI-ECG; other studies have also shown that AI alerts can influence physician decision-making18. The present findings also indicate that the AI-ECG alert system for mortality risk may alter physician behavior, such as by prompting more diagnostic tests or increasing the provision of intensive care16. In the present study, the observed differences in total mean costs per patient were primarily associated with higher drug costs, followed by ICU stay costs and medical material costs. Therefore, the focus of the cost-effectiveness analysis is on the relationship between the costs of these additional medical interventions and the observed reduction in mortality.
In the base-case analysis, the ICER was $59,500 per death averted. The cost-effectiveness acceptability curve indicated a 95% probability that the intervention would be considered cost-effective at a WTP threshold of $409,321. This wide range of uncertainty is likely due to the relatively small differences in both costs and mortality between the intervention and control groups. When such differences are modest, even small variations in either can cause large fluctuations in the ICER estimates, especially when using resampling methods. The base-case ICER corresponds to approximately 1.8 times Taiwan’s WTP threshold for a QALY gained, while the upper bound aligns with 12.3 times that threshold. Given the average age of patients in the trial (61 years) and a life expectancy of around 80 years in Taiwan, each death averted could conservatively translate into 7 to 12 additional QALYs. Using a WTP threshold equivalent to one time Taiwan’s GDP per capita, the implied acceptable range for a death averted would be between $232,638 and $398,808. In this context, the findings suggest that the intervention is likely to be cost-effective in Taiwan’s healthcare system and potentially applicable to other middle- or high-income countries with similar or higher WTP thresholds.
In this cost-effective analysis, the cost of the AI-ECG alert system itself is the first factor to consider. However, the results of this study suggest that the costs of the AI-ECG alert system have no significant impact on healthcare team expenditures. Since ECGs are inherently very inexpensive tests, past research has emphasized that the cost-effectiveness analysis of ECGs should focus more on the care cascades triggered by ECGs. In asymptomatic adults, low-value ECGs can initiate care cascades that lead to substantial economic burdens19,20. However, the in-hospital population targeted in this study had a higher mortality rate (4.3% in the control group), making ECG both reasonable and in line with standard clinical criteria21. In the base-case analysis, each AI-ECG alert was priced at $4, equivalent to the cost of a standard ECG, and gradually increased up to eightfold. Despite this, the ICERs remained stable, suggesting that the system’s cost-effectiveness is not significantly impacted by the cost associated with AI analysis itself.
Many clinical support systems have been designed to assist physicians and improve patient safety, yet these alerts are often overridden due to alert fatigue. Previous studies have shown that override rates can reach up to three-quarters in inpatient settings and nearly two-thirds in emergency departments22,23, though most of these were related to prescribing alerts. As most costs stem from the testing and treatment following an AI-ECG alert, even if a higher override rate at 60% is seen with large-scale implementation in the future, while the mortality reduction effect may weaken, the ICER would remain relatively stable, indicating that the economic benefits of implementing AI-ECG alerts are unlikely to be diminished by alert fatigue, in part because the additional costs of the AI-enabled ECG system is relatively low.
Critical care, particularly admission to ICU, is widely recognized for significantly reducing patient mortality24. An important issue is the extremely high cost of ICU care, and numerous studies have previously focused on identifying patients who would benefit most cost-effectively from ICU interventions. Cost-effectiveness analysis has shown that ICU intervention is economically beneficial for patients with a Simplified Acute Physiology Score (SAPS) II predicted mortality greater than 5%, and it is even more cost-effective for those with predicted mortality exceeding 40%25. Another study suggests considering the withdrawal of ICU care for patients with an Acute Physiology and Chronic Health Evaluation (APACHE) III predicted mortality greater than 90%26. Two seemingly opposing pieces of evidence highlight a key point: interventions should be directed toward patients with a moderate risk of mortality. In the control group of this study, the high-risk patients had a mortality rate of 23%, while low-risk patients had a 2.4% mortality rate, aligning with the recommendations of previous studies27. A study has reported that 62% of inpatients who experienced cardiac arrest showed signs of physiologic instability for more than 6 h before the event, yet only 22% had explicit documentation28. This indicates that critical care should focus on the early identification of deterioration signs, rather than recognizing them only when the patient enters an irreversible process of dying29. By appropriately allocating medical resources to patients showing early signs of deterioration, mortality rates can be reduced. This study demonstrates that AI-ECG can substantially enhance the cost-effectiveness of critical care.
A key limitation of the study is that it was conducted in Taiwan, with cost analysis tailored to the Taiwanese healthcare system, which may restrict the generalizability of the findings to other countries. Differences in healthcare systems, along with demographic and socioeconomic disparities, could influence the cost-effectiveness. Previous studies comparing AI-ECG for asymptomatic left ventricular dysfunction screening to usual care have shown significant variations in ICERs, primarily due to differences in healthcare costs across regions30,31. Therefore, further investigation and validation across diverse healthcare settings are necessary to ensure broader applicability. Moreover, although development, staff training, maintenance, and administrative costs are important components of a comprehensive cost-effectiveness analysis, these implementation-related expenses were not included in the base-case analysis. Omitting such costs may lead to an underestimation of the ICER, as they contribute meaningfully to the real-world cost and effectiveness of the intervention32. However, these expenses are often difficult to quantify during early-stage development and are likely to become clearer during commercialization and reimbursement planning. Importantly, our sensitivity analyses showed that the ICER per death averted remained stable even when the cost of the AI-ECG system was increased by up to eightfold, suggesting that the findings are robust to variations in the cost of the intervention itself.
Additionally, the evaluation was conducted from a health payer’s perspective, excluding costs borne by patients and their families after hospitalization, which may result in an underestimation of the total economic impact. We also did not include future healthcare costs associated with extended survival (i.e., life extension costs), which are sometimes considered in lifetime models. This exclusion may further contribute to a conservative estimate of the ICER. Lastly, this study did not extrapolate deaths averted into QALYs gained or adopted a lifetime horizon, as the trial population was heterogeneous, and the follow-up period was limited to 90 days. Future research may consider these approaches to capture longer-term health outcomes.
The AI-ECG alert significantly reduced all-cause mortality among hospitalized patients. Although it incurred slightly higher costs than usual care, the incremental costs per death averted suggest favorable cost-effectiveness, even when accounting for the large uncertainty in the ICER. The AI-ECG alert system presents a promising and cost-effective approach to improving patient outcomes. However, further validation across diverse clinical settings is necessary to ensure the generalizability of these findings.
Methods
The economic evaluation was a cost-effectiveness analysis conducted alongside a pragmatic randomized clinical trial in Taiwan, assessing the impact of an AI-ECG alert system on clinical outcomes16. The study period ran from December 15, 2021, to April 30, 2022, with follow-up extending to July 31, 2022, allowing for a 90-day post-intervention analysis for all patients included up to the end of the intervention period. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist was used to demonstrate adherence to its reporting guidelines33.
Trial design
Both the RCT and the cost-effectiveness analysis were approved by the Institutional Review Board of Tri-Service General Hospital, Taipei, Taiwan (approval numbers A202105120 and A202405157). The trial included 39 eligible attending physicians from the internal medicine and emergency medicine departments, all of whom provided informed consent. As the research team did not have direct contact with patients and de-identified patient-level data were collected through EHRs, patient consent was waived by the ethics committee. The inclusion criteria encompassed any patient in the emergency department or inpatient wards who underwent at least one ECG for any clinical indication during the study period. Patients under 18 years of age and those with a time delay of more than 2 h between ECG recording and AI-ECG analysis were excluded. All eligible patients were included in the analysis.
Randomization was conducted based on the hospital’s seven-digit serial medical record numbers. This patient-level randomization ensured longer patient follow-up and minimized potential loss of follow-up that could occur with physician-level randomization. The randomization process was completed before the trial began, using simple random sampling. Half of the possible medical record numbers were allocated to the intervention group, meaning the randomization may have occurred prior to the actual creation of the medical record numbers. The trial commenced on 15 December 2021, when the AI-ECG support system was activated for patients in the intervention group, and concluded on 30 April 2022, with approximately 8000 patients assigned to each arm.
Intervention and comparator
The AI-ECG system used in this study is a convolutional neural network, with its technical details previously described in an earlier publication13. The model was trained on more than 450,000 ECGs, using all-cause mortality as the primary label and incorporating survival data with censored events. The AI-ECG system was designed to detect high-risk mortality indicators from ECGs and immediately notify attending physicians through a warning message sent to their smartphones. This notification included a high-risk assessment and provided a link to access the detailed ECG and AI-ECG prediction results.
The system aimed to prompt timely medical evaluations and interventions without altering standard procedures or treatment criteria, which remained unaffected by AI-ECG findings before the trial. While the intervention emphasized high-risk alerts, the AI-ECG system also provided risk levels for all patients in the intervention group, enabling physicians to review these assessments at their discretion through the EHR system. In contrast, patients in the control group received usual care and were not covered by the active warning message service provided by the AI-ECG system.
Clinical outcome
The endpoint of the study was all-cause mortality within 90 days post-intervention, chosen for its robustness against clinician biases. Mortality status was tracked using EHRs, with additional verification to confirm the survival status of any censored patients up to their last recorded hospital visit. While it is possible that some patients may have died outside the hospital and were not captured in the EHR, we ensured that all censored patients were confirmed alive up to the time of their most recent recorded hospital encounter.
Health resource utilization and cost
This economic evaluation was conducted from the perspective of Taiwan’s National Health Insurance (NHI). The total cost for each participant was determined by aggregating all medical resource utilization incurred during the hospital stay. Cost data were extracted directly from the EHR systems of the participating hospitals, where they were originally recorded in New Taiwan Dollars (NTD$). These data included both costs reimbursed by the NHI and out-of-pocket expenses paid by patients. The extracted data were categorized into eight groups: drugs, examinations, medical supplies, procedures, diagnoses, wards, intensive care units (ICU), and others. Procedure costs included fees for various medical treatments and interventions, while medical supplies encompassed both consumables and more advanced medical devices. Diagnosis costs covered fees for consultations and assessments by physicians and specialists. Costs were converted to USD according to the currency rate obtained from the Bank of Taiwan on November 18, 2024.
Cost-effectiveness was evaluated by calculating the incremental cost-effectiveness ratio (ICER), which represents the additional costs of one intervention compared to another, divided by the additional effects gained from one intervention compared to another. Since there is no established consensus on the willingness-to-pay (WTP) threshold for a death averted, we used the WTP for a quality-adjusted life year (QALY) gained as a proxy. Following WHO guidelines and broader health economic literature, one Gross Domestic Product (GDP) per capita was considered an appropriate threshold for assessing cost-effectiveness in Taiwan34,35. Accordingly, the threshold was set at $33,234 per QALY gained, based on Taiwan’s published 2024 GDP per capita. As this analysis focused on a 90-day post-intervention period, discounting was not applied in the cost estimation.
Statistical methods
To address uncertainty in cost and outcome data, we employed non-parametric bootstrapping with 5000 replications to generate distributions of mean costs and effects for both the AI-ECG and control groups. These bootstrap replications were used to calculate the incremental cost per death averted and to construct 95% confidence intervals (CIs) around the ICERs. To further assess uncertainty in decision-making, we constructed a cost-effectiveness acceptability curve (CEAC) by plotting the proportion of bootstrap replications that fall below varying WTP thresholds for a death averted. Notably, there were no missing data in this trial, as all relevant medical records were assumed complete and available for analysis.
We conducted sensitivity analyses by varying two key variables to estimate their impact on the ICER: (1) the cost of the AI-ECG service and (2) the override rate of the AI-ECG alerts. In the absence of clear pricing guidelines for AI-ECG, we initially set the cost at $4, equivalent to a standard ECG, and progressively increased it up to eightfold. To account for potential non-adherence to AI alerts in real-world practice, we modeled override rates of 40 and 60%. In these analyses, we assumed that patients whose AI-ECG alerts were overridden would have outcomes similar to those receiving usual care. Accordingly, we sampled cost and outcome data with replacement from the control group at proportions corresponding to the override rates.
We also performed subgroup analyses to explore potential heterogeneity in cost-effectiveness across patient characteristics (e.g., age, sex, comorbidities) and hospital settings (e.g., academic medical center vs. community hospital, emergency vs. inpatient department). For each subgroup, we calculated incremental costs, absolute reduction in mortality, and the ICER per death averted.
Data availability
Patient data cannot be made publicly available due to privacy concerns. De-identified tabular data can be obtained from the corresponding author on approval from the ethics committee of the Tri-Service General Hospital. Approval from this committee can be requested from the Tri-Service General Hospital’s Clinical Trial Management System (https://tsgh.cims.tw/wiPtms/index.html), with an expected review period of approximately 2–3 months. After approval, researchers will be granted VPN access to perform analyses, ensuring data security and confidentiality (summary data can be exported), with measures in place to prevent any breach of personal information.
References
Sutton, R. T. et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. npj Digital Med. 3, 17 (2020).
Kwan, J. L. et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ 370, m3216 (2020).
Bates, D. W., Saria, S., Ohno-Machado, L., Shah, A. & Escobar, G. Big data in health care: using analytics to identify and manage high-risk and high-cost patients. Health Aff. 33, 1123–1131 (2014).
Shortliffe, E. H. & Sepúlveda, M. J. Clinical decision support in the era of artificial intelligence. JAMA 320, 2199–2200 (2018).
Hinton, G. Deep learning—a technology with the potential to transform health care. JAMA 320, 1101–1102 (2018).
Gomez Rossi, J., Rojas-Perilla, N., Krois, J. & Schwendicke, F. Cost-effectiveness of artificial intelligence as a decision-support system applied to the detection and grading of melanoma, dental caries, and diabetic retinopathy. JAMA Netw. Open 5, e220269 (2022).
Shenasa, M. Learning and teaching electrocardiography in the 21st century: a neglected art. J. Electrocardiol. https://doi.org/10.1016/j.jelectrocard.2018.02.007 (2018).
Siontis, K. C., Noseworthy, P. A., Attia, Z. I. & Friedman, P. A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 18, 465–478 (2021).
Attia, Z. I. et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 25, 70–74 (2019).
Chen, H. Y. et al. Artificial intelligence-enabled electrocardiography predicts left ventricular dysfunction and future cardiovascular outcomes: a retrospective analysis. J. Pers. Med. 12, 455 (2022).
Attia, Z. I. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394, 861–867 (2019).
Raghunath, S. et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat. Med. 26, 886–891 (2020).
Tsai, D. J. et al. Mortality risk prediction of the electrocardiogram as an informative indicator of cardiovascular diseases. Digit. Health 9, 20552076231187247 (2023).
Maharaj, R., Raffaele, I. & Wendon, J. Rapid response systems: a systematic review and meta-analysis. Crit. Care 19, 254 (2015).
Teuma Custo, R. & Trapani, J. The impact of rapid response systems on mortality and cardiac arrests—a literature review. Intensive Crit. Care Nurs. 59, 102848 (2020).
Lin, C. S. et al. AI-enabled electrocardiography alert intervention and all-cause mortality: a pragmatic randomized clinical trial. Nat. Med. 30, 1461–1470 (2024).
Yao, X. et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat. Med. 27, 815–819 (2021).
Parikh, R. B. et al. Machine learning approaches to predict 6-month mortality among patients with cancer. JAMA Netw. Open 2, e1915997 (2019).
Bhatia, R. S. et al. Electrocardiograms in low-risk patients undergoing an annual health examination. JAMA Intern. Med.177, 1326–1333 (2017).
Ganguli, I. et al. Prevalence and cost of care cascades after low-value preoperative electrocardiogram for cataract surgery in fee-for-service medicare beneficiaries. JAMA Intern. Med. 179, 1211–1219 (2019).
Crawford, M. H. et al. ACC/AHA Guidelines for Ambulatory Electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J. Am. Coll. Cardiol. 34, 912–948 (1999).
Nanji, K. C. et al. Medication-related clinical decision support alert overrides in inpatients. J. Am. Med. Inform. Assoc. 25, 476–481 (2017).
Yoo, J. et al. Alert override patterns with a medication clinical decision support system in an academic emergency department: retrospective descriptive study. JMIR Med. Inf. 8, e23351 (2020).
Pronovost, P. J. et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA 288, 2151–2162 (2002).
Edbrooke, D. L. et al. Implications of ICU triage decisions on patient mortality: a cost-effectiveness analysis. Crit. Care 15, R56 (2011).
Glance, L. G., Osler, T. & Shinozaki, T. Intensive care unit prognostic scoring systems to predict death: a cost-effectiveness analysis. Crit. Care Med. 26, 1842–1849 (1998).
Wilcox, M. E., Vaughan, K., Chong, C., Neumann, P. J. & Bell, C. M. Cost-effectiveness studies in the ICU: a systematic review. Crit. Care Med. 47, 1011–1017 (2019).
Perkins, G. D., Temple, R. M. & George, R. Time to intervene: lessons from the NCEPOD report. Resuscitation 83, 1305–1306 (2012).
Hodgetts, T. J. et al. Incidence, ___location and reasons for avoidable in-hospital cardiac arrest in a district general hospital. Resuscitation 54, 115–123 (2002).
Tseng, A. S. et al. Cost effectiveness of an electrocardiographic deep learning algorithm to detect asymptomatic left ventricular dysfunction. Mayo Clin. Proc. 96, 1835–1844 (2021).
Liu, W. T. et al. Opportunistic screening for asymptomatic left ventricular dysfunction with the use of electrocardiographic artificial intelligence: a cost-effectiveness approach. Can. J. Cardiol. 40, 1310–1321 (2024).
Gandjour, A. Inclusion of phase III clinical trial costs in health economic evaluations. BMC Health Serv. Res. 24, 1158 (2024).
Husereau, D. et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 25, 3–9 (2022).
Marseille, E., Larson, B., Kazi, D. S., Kahn, J. G. & Rosen, S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull. World Health Organ. 93, 118–124 (2015).
Bertram, M. Y. et al. Cost-effectiveness thresholds: pros and cons. Bull. World Health Organ. 94, 925–930 (2016).
Acknowledgements
This study was supported by funding from the National Science and Technology Council, Taiwan (NSTC 113-2320-B-016-011 to P.H.H.). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
P.H.H., C.L., and C.S.T. conceptualized the study. P.H.H., C.L., T.K.L., and C.S.T. designed the methodology. C.S.L. and W.T.L. did the data collection. P.H.H., C.L., and D.J.T. did the data analysis. P.H.H. wrote the original draft of the manuscript. All authors reviewed and edited the manuscript. C.S.T. supervised the data collection and analyses. P.H.H., C.L., W.T.L., and C.S.T. did project administration. Y.J.H., Y.H.C., C.Y.L., S.H.L., and C.S.T. acquired funding. P.H.H. and C.L. verified the data. All authors have read and agreed to the final version of the manuscript and to the submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hsieh, PH., Lin, C., Lin, CS. et al. Economic analysis of an AI-enabled ECG alert system: impact on mortality outcomes from a pragmatic randomized trial. npj Digit. Med. 8, 348 (2025). https://doi.org/10.1038/s41746-025-01735-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-025-01735-7