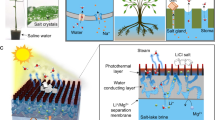
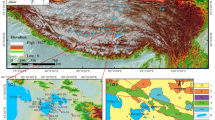
Abstract
In recent years, the demand for lithium (Li) has been on the rise as Li-ion batteries are playing an increasingly important role in powering the global transition to a low-carbon society. In contrast to the predominant production of lithium from hard rock, lithium extraction from brine sources has proven more economical and sustainable. However, substantial challenges remain, including the low efficiency of the extraction process, especially for brines of high salinity, complex composition and poor selectivity against magnesium, the major competing species. Here we show a loose nanofiltration process involving ethylenediaminetetraacetic acid (EDTA) for direct and efficient Li+ extraction as well as effective Mg2+ utilization from salt-lake brines. Taking advantage of selective binding between EDTA4− and Mg2+, our process achieves ultrahigh Mg2+ rejection of 99.85%, ultrafast Li+ flux of ~4.34 mol m−2 h−1 and unprecedented Li+/Mg2+ separation factor (~679) under industrial conditions (127.06 g l−1). More importantly, the Li+ recovery rate reaches 89.90% through a two-stage filtration process, while Mg2+ waste is converted to nanostructured Mg(OH)2 and 98.87% of EDTA4− can be regenerated. Our scalable process minimizes environmental impact while maximizing resource utilization, thereby catalysing the shift toward a more sustainable future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are provided as Source Data and in the Supplementary Information.
References
Chen, X. et al. Spatially separated crystallization for selective lithium extraction from saline water. Nat. Water 1, 808–817 (2023).
The Growing Role of Minerals and Metals for a Low Carbon Future (World Bank Group, 2017).
Haddad, A. Z. et al. How to make lithium extraction cleaner, faster and cheaper in six steps. Nature 616, 245–248 (2023).
Ding, T. et al. Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau. Geosci. Front. 14, 101485 (2023).
Xu, S. et al. Extraction of lithium from Chinese salt-lake brines by membranes: design and practice. J. Memb. Sci. 635, 119441 (2021).
Zhang, Y., Hu, Y., Wang, L. & Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. Miner. Eng. 139, 105868 (2019).
Tabelin, C. B. et al. Towards a low-carbon society: a review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 163, 106743 (2021).
Vera, M. L., Torres, W. R., Galli, C. I., Chagnes, A. & Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Environ. 4, 149–165 (2023).
Zeng, Y. et al. Electrochemically mediated lithium extraction for energy and environmental sustainability. Adv. Funct. Mater. 34, 2400416 (2024).
DuChanois, R. M. et al. Prospects of metal recovery from wastewater and brine. Nat. Water 1, 37–46 (2023).
Yang, S., Zhang, F., Ding, H., He, P. & Zhou, H. Lithium metal extraction from seawater. Joule 2, 1648–1651 (2018).
Peng, H. & Zhao, Q. A nano-heterogeneous membrane for efficient separation of lithium from high magnesium/lithium ratio brine. Adv. Funct. Mater. 31, 2009430 (2021).
Lu, J. et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal–organic frameworks. Nat. Mater. 19, 767–774 (2020).
Hafer, E. et al. Qualitative and quantitative 1H-NMR spectroscopy for determination of divalent metal cation concentration in model salt solutions, food supplements, and pharmaceutical products by using EDTA as chelating agent. Magn. Reson. Chem. 58, 653–665 (2020).
Wang, R., Alghanayem, R. & Lin, S. Multipass nanofiltration for lithium separation with high selectivity and recovery. Environ. Sci. Technol. 57, 14464–14471 (2023).
Guo, B.-B. et al. Double charge flips of polyamide membrane by ionic liquid-decoupled bulk and interfacial diffusion for on-demand nanofiltration. Nat. Commun. 15, 2282 (2024).
Peng, H., Su, Y., Liu, X., Li, J. & Zhao, Q. Designing Gemini-electrolytes for scalable Mg2+/Li+ separation membranes and modules. Adv. Funct. Mater. 33, 2305815 (2023).
Peng, H. et al. Quaternization-spiro design of chlorine-resistant and high-permeance lithium separation membranes. Nat. Commun. 14, 5483 (2023).
Zhao, G., Gao, H., Qu, Z., Fan, H. & Meng, H. Anhydrous interfacial polymerization of sub-1 Å sieving polyamide membrane. Nat. Commun. 14, 7624 (2023).
Wang, R., He, R., He, T., Elimelech, M. & Lin, S. Performance metrics for nanofiltration-based selective separation for resource extraction and recovery. Nat. Water 1, 291–300 (2023).
Wang, L. et al. Novel positively charged metal-coordinated nanofiltration membrane for lithium recovery. ACS Appl. Mater. Interfaces 13, 16906–16915 (2021).
Yuan, B. et al. Self-assembled dendrimer polyamide nanofilms with enhanced effective pore area for ion separation. Nat. Commun. 15, 471 (2024).
Hamzaoui, A. H., M’nif, A., Hammi, H. & Rokbani, R. Contribution to the lithium recovery from brine. Desalination 158, 221–224 (2003).
Swain, B. Recovery and recycling of lithium: a review. Sep. Purif. Technol. 172, 388–403 (2017).
Flexer, V., Baspineiro, C. F. & Galli, C. I. Lithium recovery from brines: a vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 639, 1188–1204 (2018).
Soyekwo, F., Wen, H., Liao, D. & Liu, C. Fouling-resistant ionic graft-polyamide nanofiltration membrane with improved permeance for lithium separation from MgCl2/LiCl mixtures. J. Memb. Sci. 659, 120773 (2022).
Lejars, M., Margaillan, A. & Bressy, C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 112, 4347–4390 (2012).
Li, Q. & Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: measurements and mechanisms. Environ. Sci. Technol. 38, 4683–4693 (2004).
Yang, X. et al. Chelation-directed interface engineering of in-place self-cleaning membranes. Proc. Natl Acad. Sci. USA 121, e2319390121 (2024).
Zhang, X. et al. Effect of chemical cleaning on nanofiltration process in treating surface water. J. Water Process Eng. 50, 103271 (2022).
Foo, Z. H., Rehman, D., Bouma, A. T., Monsalvo, S. & Lienhard, J. H. Lithium concentration from salt-lake brine by Donnan-enhanced nanofiltration. Environ. Sci. Technol. 57, 6320–6330 (2023).
Cui, R. Solubility measurement and prediction of phase equilibria in the quaternary system LiCl+NaCl+KCl+H2O and ternary subsystem LiCl+NaCl+H2O at 288.15 K. Chin. J. Chem. Eng. 28, 2137–2141 (2020).
Lian, L., Song, X., Chen, H. & Yu, J. Phase equilibrium and evaporation process simulation for the lithium recovery from NaCl-LiCl-H2O system with high presence of organic NMP. Desalination 562, 116703 (2023).
Zhong, C.-Y., Lv, Y.-P., Wen, W.-F., Chen, Q. & Zhang, W.-M. Sustainable production of lithium acetate by bipolar membrane electrodialysis metathesis. ACS Sustain. Chem. Eng. 10, 6045–6056 (2022).
Xu, R., Kang, Y., Zhang, W., Zhang, X. & Pan, B. Oriented UiO-67 metal–organic framework membrane with fast and selective lithium-ion transport. Angew. Chem. Int. Ed. 61, e202115443 (2022).
Battaglia, G. et al. Recovery of lithium carbonate from dilute Li-rich brine via homogenous and heterogeneous precipitation. Ind. Eng. Chem. Res. 61, 13589–13602 (2022).
Huang, Y. & Feng, X. Polymer-enhanced ultrafiltration: fundamentals, applications and recent developments. J. Memb. Sci. 586, 53–83 (2019).
Rivas, B. Water-soluble polymer–metal ion interactions. Prog. Polym. Sci. 28, 173–208 (2003).
Qiu, L., Xie, R., Ding, P. & Qu, B. Preparation and characterization of Mg(OH)2 nanoparticles and flame-retardant property of its nanocomposites with EVA. Compos. Struct. 62, 391–395 (2003).
Battistel, A., Palagonia, M. S., Brogioli, D., La Mantia, F. & Trócoli, R. Electrochemical methods for lithium recovery: a comprehensive and critical review. Adv. Mater. 32, 1905440 (2020).
He, L. et al. New insights into the application of lithium-ion battery materials: selective extraction of lithium from brines via a rocking-chair lithium-ion battery system. Glob. Chall. 2, 1700079 (2018).
Ma, H. et al. Dual-channel-ion conductor membrane for low-energy lithium extraction. Environ. Sci. Technol. 57, 17246–17255 (2023).
Acknowledgements
Z.L. acknowledges support from the Monash University Start-up Fund (Project No. MSRI8001003).
Author information
Authors and Affiliations
Contributions
M.Y. and M.T. designed the experiment. M.Y. conducted the experiment and analysed the results. Z.L. and X.Z. proposed and supervised the project. L.S. contributed to data analysis. E.L. and F.X. contributed to the technical and economic analysis. G.Z. and L.X. contributed to conducting SEM, 1H-NMR characterization, AFM force measurement and project discussion. X.R., K.W. and Y.C. carried out the DFT calculation. M.Y. wrote and revised the paper. Z.L., E.L. and X.Z. discussed the results, commented on and revised the paper. H.W. provided constructive suggestions for the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Jian Jin, Qiaoying Wang, Walter Torres and Amir Razmjou for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–52, Note 1 and Tables 1–9.
Source data
Source Data Fig. 2
Processed NMR patterns and statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Processed XRD pattern and statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yong, M., Tang, M., Sun, L. et al. Sustainable lithium extraction and magnesium hydroxide co-production from salt-lake brines. Nat Sustain 7, 1662–1671 (2024). https://doi.org/10.1038/s41893-024-01435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-024-01435-2