Abstract
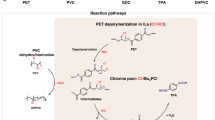
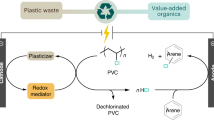
Co-upcycling of mixed plastics offers a viable approach to reusing carbon resources in plastic wastes and realizing circular economy. However, the presence of polyvinyl chloride (PVC) often complicates the co-upcycling processes, because chlorine (Cl) released from PVC can deactivate catalysts and enter final products. Moreover, existing plastic upcycling processes usually require harsh reaction conditions. Here we present a strategy enabling efficient co-upcycling of PVC and polypropylene (PP) at mild conditions. We use chlorine-resistant ionic liquids butylpyridinium chloride-aluminium chloride to dechlorinate PVC and simultaneously depolymerize the PP–PVC mixture into Cl-free liquid hydrocarbons, with the co-production of hydrogen chloride (HCl) as byproduct. This conversion approach operates at room temperature without the use of external hydrogen or noble metal catalysts. The Cl-free liquid hydrocarbon yield is up to 97.4 wt% of C and H in the feed PP–PVC mixture. This work can incentivize further technical development in plastic upcycling and improve the sustainability of plastic waste management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Xu, Z. et al. Cascade degradation and upcycling of polystyrene waste to high-value chemicals. Proc. Natl Acad. Sci. USA 119, e2203346119 (2022).
Lebreton, L. & Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5, 6 (2019).
Hahladakis, J. N., Velis, C. A., Weber, R., Iacovidou, E. & Purnell, P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 344, 179–199 (2018).
Tournier, V. et al. Enzymes’ power for plastics degradation. Chem. Rev. 123, 5612–5701 (2023).
The New Plastics Economy: Catalysing Action (Ellen MacArthur Foundation, 2017); https://ellenmacarthurfoundation.org/the-new-plastics-economy-catalysing-action
Sardon, H. & Dove, A. P. Plastics recycling with a difference. Science 360, 380–381 (2018).
Xu, Z. et al. Chemical upcycling of polyethylene, polypropylene, and mixtures to high-value surfactants. Science 381, 666–671 (2023).
Sun, J. et al. Bifunctional tandem catalytic upcycling of polyethylene to surfactant-range alkylaromatics. Chem 9, 2318–2336 (2023).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Kots, P. A. et al. Polypropylene plastic waste conversion to lubricants over Ru/TiO2 catalysts. ACS Catal. 11, 8104–8115 (2021).
Gao, Z., Ma, B., Chen, S., Tian, J. & Zhao, C. Converting waste PET plastics into automobile fuels and antifreeze components. Nat. Commun. 13, 3343 (2022).
Jia, X., Qin, C., Friedberger, T., Guan, Z. & Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2, e1501591 (2016).
Li, Y. et al. Catalytic transformation of PET and CO2 into high-value chemicals. Angew. Chem. Int. Ed. 61, e202117205 (2022).
Mi, R. et al. Solvent-free heterogeneous catalytic hydrogenation of polyesters to diols. Angew. Chem. Int. Ed. 62, e202304219 (2023).
Sun, B. et al. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustain. 6, 712–719 (2023).
Jiao, Y., Wang, M. & Ma, D. Catalytic cracking of polylactic acid to acrylic acid. Chin. J. Chem. 41, 2071–2076 (2023).
Cao, R. et al. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. 13, 4809 (2022).
Tian, S. et al. Catalytic amination of polylactic acid to alanine. J. Am. Chem. Soc. 143, 16358–16363 (2021).
Jaydev, S. D., Martín, A. J. & Pérez-Ramírez, J. Direct conversion of polypropylene into liquid hydrocarbons on carbon-supported platinum catalysts. ChemSusChem 14, 5179–5185 (2021).
Arroyave, A. et al. Catalytic chemical recycling of post-consumer polyethylene. J. Am. Chem. Soc. 144, 23280–23285 (2022).
Conk, R. J. et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561–1566 (2022).
Zhao, D., Wang, X., Miller, J. B. & Huber, G. W. The chemistry and kinetics of polyethylene pyrolysis: a process to produce fuels and chemicals. ChemSusChem 13, 1764–1774 (2020).
Li, H. et al. Aliphatic amines from waste polyolefins by tandem pyrolysis, hydroformylation, and reductive amination. Green Chem. 26, 8718–8727 (2024).
Li, H. et al. Hydroformylation of pyrolysis oils to aldehydes and alcohols from polyolefin waste. Science 381, 660–666 (2023).
Demarteau, J. et al. Circularity in mixed-plastic chemical recycling enabled by variable rates of polydiketoenamine hydrolysis. Sci. Adv. 8, eabp8823 (2022).
Yadav, G. et al. Techno-economic analysis and life cycle assessment for catalytic fast pyrolysis of mixed plastic waste. Energy Environ. Sci. 16, 3638–3653 (2023).
Zou, L. et al. Chemical recycling of polyolefins: a closed-loop cycle of waste to olefins. Natl Sci. Rev. 10, nwad207 (2023).
Ragaert, K. et al. Design from recycling: a complex mixed plastic waste case study. Resour. Conserv. Recycl. 155, 104646 (2020).
Matjašič, T. et al. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 752, 141959 (2021).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 378, 207–211 (2022).
Albertsson, A.-C. & Hakkarainen, M. Designed to degrade. Science 358, 872–873 (2017).
The Facts 2021: An Analysis of European Plastics Production, Demand and Waste Data (Plastics Europe, 2021).
Feng, B., Jing, Y., Liu, X., Guo, Y. & Wang, Y. Waste PVC upcycling: transferring unmanageable Cl species into value-added Cl-containing chemicals. Appl. Catal. B 331, 122671 (2023).
Svadlenak, S. et al. Upcycling of polyvinyl chloride to hydrocarbon waxes via dechlorination and catalytic hydrogenation. Appl. Catal., B 338, 123065 (2023).
Fagnani, D. E., Kim, D., Camarero, S. I., Alfaro, J. F. & McNeil, A. J. Using waste poly(vinyl chloride) to synthesize chloroarenes by plasticizer-mediated electro(de)chlorination. Nat. Chem. 15, 222–229 (2023).
Kots, P. A., Vance, B. C., Quinn, C. M., Wang, C. & Vlachos, D. G. A two-stage strategy for upcycling chlorine-contaminated plastic waste. Nat. Sustain. 6, 1258–1267 (2023).
Oster, K. et al. Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids. Green Chem. 22, 5132–5142 (2020).
Zhao, T. et al. A highly efficient approach for dehydrochlorinating polyvinyl chloride: catalysis by 1-butyl-3-methylimidazolium chloride. Green Chem. 12, 1062–1065 (2010).
Cao, R. et al. Co-upcycling of polyvinyl chloride and polyesters. Nat. Sustain. 6, 1685–1692 (2023).
Zhang, W. et al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 379, 807–811 (2023).
Zhang, W. et al. Chloride and hydride transfer as keys to catalytic upcycling of polyethylene into liquid alkanes. Angew. Chem. Int. Ed. 63, e202319580 (2024).
Vollmer, I. et al. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423 (2020).
Zhou, J. et al. Understanding the pyrolysis mechanism of polyvinylchloride (PVC) by characterizing the chars produced in a wire-mesh reactor. Fuel 166, 526–532 (2016).
Li, D. et al. Study on the pyrolysis behaviors of mixed waste plastics. Renewable Energy 173, 662–674 (2021).
Wu, J. et al. Kinetic study of polyvinyl chloride pyrolysis with characterization of dehydrochlorinated PVC. ACS Sustain. Chem. Eng. 12, 7402–7413 (2024).
O’Rourke, G. et al. Catalytic tandem dehydrochlorination–hydrogenation of PVC towards valorisation of chlorinated plastic waste. Chem. Sci. 14, 4401–4412 (2023).
Zhang, Y. et al. Promotion of protolytic pentane conversion on H-MFI zeolite by proximity of extra-framework aluminum oxide and Brønsted acid sites. J. Catal. 370, 424–433 (2019).
Brouwer, D. M. & Hogeveen, H. in Progress in Physical Organic Chemistry Vol. 9 (ed. Taft, R. W.) 179–240 (John Wiley & Sons, Inc., Publishing, 1972).
Rey, J., Raybaud, P., Chizallet, C. & Bučko, T. Competition of secondary versus tertiary carbenium routes for the type B isomerization of alkenes over acid zeolites quantified by ab initio molecular dynamics simulations. ACS Catal. 9, 9813–9828 (2019).
Timken, H. K. et al. Commercial Applications of Ionic Liquids (ed. Shiflett, M. B.) Ch. 2 (Springer International Publishing, 2020).
Acknowledgements
This work is financially supported by the Natural Science Foundation of Shanghai (23ZR1418800) and National Natural Science Foundation of China (22302069, 22472059). Z.G. acknowledges the Fundamental Research Funds for the Central Universities (YBNL TS2023-07). We appreciate the help of East China Normal University Multifunctional Platform for Innovation for support of SEM characterizations (004).
Author information
Authors and Affiliations
Contributions
W.Z. and Y.L. conceived the research. Z.G., Y.W., L.Y., X.S., Y.S., M.Z. and S.F. performed most of the experiments. Z.G., Y.W., W.Z. and Y.L. analysed results. J.J. collected and analysed NMR spectra. The paper was written and revised by all the authors together.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Jinwen Zhang, George Huber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24 and Tables. 1–3.
Source data
Source Data Figs. 1–6
Statistical source data of Figs. 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Z., Wang, Y., Yuan, L. et al. Room-temperature co-upcycling of polyvinyl chloride and polypropylene. Nat Sustain 7, 1691–1698 (2024). https://doi.org/10.1038/s41893-024-01468-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-024-01468-7