Abstract
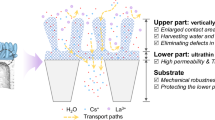
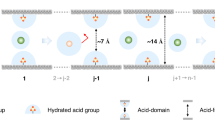
The membrane-based osmotic power generation technology can both provide sustainable energy and address environmental pollution utilizing an eco-friendly energy conversion mechanism. Covalent organic framework (COF) membranes are an attractive option for this application due to their porosity, well-defined pores and tunable surface chemistry. However, precise engineering of the porous structure for rapid ion transport remains a challenge. Here we engineer the initially randomly oriented COF nanochannels into a highly axially aligned configuration, delivering a metal ion-coordinated COF framework, through interfacial polymerization followed by coordination to different ions, including Ca2+, Mg2+, Al3+, Fe3+, Zn2+, Co2+ and Cu2+. Notably, the representative Ca-COF demonstrates a superior cation selectivity of 0.93 and ionic conductivity of 0.06 S m−1. When applied to osmotic energy harvesting, the Ca-COF membranes deliver a record output power density of 320.8 W m−2 in the presence of a mixture of natural seawater and river water. By highlighting the importance of aligning metal ion-coordinated COF nanochannels in improving ion selectivity and permeability, our strategy suggests a pathway in unlocking the potential of osmotic energy harvesting technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings in this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Wang, M. et al. Ultrafast seawater desalination with covalent organic framework membranes. Nat. Sustain. 5, 518–526 (2022).
Ying, Y. et al. Ultrathin covalent organic framework membranes via a multi-interfacial engineering strategy for gas separation. Adv. Mater. 34, 2104946 (2022).
Wang, M. et al. Electrochemical interfacial polymerization toward ultrathin COF membranes for brine desalination. Angew. Chem. Int. Ed. 62, e202219084 (2023).
Knebel, A. & Caro, J. Metal–organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 17, 911–923 (2022).
Kong, Y. et al. Manipulation of cationic group density in covalent organic framework membranes for efficient anion transport. J. Am. Chem. Soc. 145, 27984–27992 (2023).
Cao, L. et al. Giant osmotic energy conversion through vertical-aligned ion-permselective nanochannels in covalent organic framework membranes. J. Am. Chem. Soc. 144, 12400–12409 (2022).
Fan, H. et al. High-flux vertically aligned 2D covalent organic framework membrane with enhanced hydrogen separation. J. Am. Chem. Soc. 142, 6872–6877 (2020).
Cao, L. et al. Oriented two-dimensional covalent organic framework membranes with high ion flux and smart gating nanofluidic transport. Angew. Chem. Int. Ed. 61, e202113141 (2022).
Jiang, C. et al. Constructing universal ionic sieves via alignment of two-dimensional covalent organic frameworks (COFs). Angew. Chem. Int. Ed. 57, 16072–16076 (2018).
Guo, H., Jiang, J., Fang, C. & Zhu, L. Highly crystalline and robust covalent organic framework membranes for predictable solvent transport and molecular separation. J. Mater. Chem. A 11, 19374–19383 (2023).
Jing, X. et al. Gradient channel segmentation in covalent organic framework membranes with highly oriented nanochannels. J. Am. Chem. Soc. 145, 21077–21085 (2023).
Li, Y., Sui, J., Cui, L.-S. & Jiang, H.-L. Hydrogen bonding regulated flexibility and disorder in hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 145, 1359–1366 (2023).
Fan, C. et al. Scalable fabrication of crystalline COF membranes from amorphous polymeric membranes. Angew. Chem. Int. Ed. 60, 18051–18058 (2021).
Yin, C. et al. Perpendicular alignment of covalent organic framework (COF) pore channels by solvent vapor annealing. J. Am. Chem. Soc. 145, 11431–11439 (2023).
Liu, K. et al. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 11, 994–1000 (2019).
Zhang, W. et al. Reconstructed covalent organic frameworks. Nature 604, 72–79 (2022).
Yang, R. et al. Potential difference-modulated synthesis of self-standing covalent organic framework membranes at liquid/liquid interfaces. J. Am. Chem. Soc. 144, 11778–11787 (2022).
Zhu, T. et al. 3D covalent organic framework membrane with fast and selective ion transport. Nat. Commun. 14, 5926 (2023).
Yang, J. et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 17, 622–628 (2022).
Cheng, B., Zhong, Y., Qiu, Y., Vaikuntanathan, S. & Park, J. Giant gateable osmotic power generation from a goldilocks two-dimensional polymer. J. Am. Chem. Soc. 145, 5261–5269 (2023).
Hou, S. et al. Free-standing covalent organic framework membrane for high-efficiency salinity gradient energy conversion. Angew. Chem. Int. Ed. 60, 9925–9930 (2021).
Wang, K. et al. Monolayer-assisted surface-initiated Schiff-base-mediated aldol polycondensation for the synthesis of crystalline sp2 carbon-conjugated covalent organic framework thin films. J. Am. Chem. Soc. 145, 5203–5210 (2023).
Zuo, H. et al. Bioinspired gradient covalent organic framework membranes for ultrafast and asymmetric solvent transport. Adv. Mater. 36, 2305755 (2024).
Zhang, P. et al. Covalent organic framework nanofluidic membrane as a platform for highly sensitive bionic thermosensation. Nat. Commun. 12, 1844 (2021).
Lin, Z. et al. Tuning the p-orbital electron structure of s-block metal Ca enables a high-performance electrocatalyst for oxygen reduction. Adv. Mater. 33, e2107103 (2021).
Wang, Q. et al. Asymmetric coordination induces electron localization at Ca sites for robust CO2 electroreduction to CO. Adv. Mater. 35, e2300695 (2023).
Man, Z. et al. Serosa-mimetic nanoarchitecture membranes for highly efficient osmotic energy generation. J. Am. Chem. Soc. 143, 16206–16216 (2021).
Li, C. et al. One porphyrin per chain self-assembled helical ion-exchange channels for ultrahigh osmotic energy vonversion. J. Am. Chem. Soc. 144, 9472–9478 (2022).
Huang, Z. et al. Essence of the enhanced osmotic energy conversion in a covalent organic framework monolayer. ACS Nano 16, 17149–17156 (2022).
Zhou, J. et al. Maximizing ion permselectivity in MXene/MOF nanofluidic membranes for high‐efficient blue energy generation. Adv. Funct. Mater. 32, 2209767 (2022).
Hao, J. et al. A euryhaline-fish-inspired salinity self-adaptive nanofluidic diode leads to high-performance blue energy harvesters. Adv. Mater. 34, e2203109 (2022).
Chen, Y. et al. Large-scale, ultrastrong Cu2+ cross-linked sodium alginate membrane for effective salinity gradient power conversion. ACS Appl. Polym. Mater. 3, 3902–3910 (2021).
Safaei, J. et al. Vacancy engineering for high-efficiency nanofluidic osmotic energy generation. J. Am. Chem. Soc. 145, 2669–2678 (2023).
Zhang, F., Yu, J., Si, Y. & Ding, B. Meta-aerogel ion motor for nanofluid osmotic energy harvesting. Adv. Mater. 35, e2302511 (2023).
Jiang, Y. et al. Surface diffusion enhanced ion transport through two-dimensional nanochannels. Sci. Adv. 9, eadi8493 (2023).
Zhou, S. et al. Surfactant-assisted sulfonated covalent organic nanosheets: extrinsic charge for improved ion transport and salinity-gradient energy harvesting. Adv. Mater. 35, 2208640 (2023).
Liang, Q. et al. Efficient osmosis-powered production of green hydrogen. Nat. Sustain. 7, 628–639 (2024).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Humphrey, W., Dalke, A. & Schulten, K. VMD–Visual Molecular Dynamics. J. Molec. Graphics 14, 33–38 (1996).
Guo, X. et al. Effect of alloying elements on the interface of fcc-Fe/Ni3Al by first principle calculations. Comp. Mater. Sci. 214, 111673 (2022).
Mitra, S. et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 138, 2823–2828 (2016).
Lu, T. Sobtop Version 1.0 (dev3.1). http://sobereva.com/soft/Sobtop (2024).
Rakhshani, H., Dehghanian, E. & Rahati, A. Enhanced GROMACS: toward a better numerical simulation framework. J. Mol. Model 25, 355 (2019).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Acknowledgements
We gratefully acknowledge the support by the National Natural Science Foundation of China (grant numbers 52272182, 52472294 and 52073009 to Y. Zhu), the Youth Innovation Promotion Association of CAS (grant number 2021029 to Y. Zhou) and the International Partnership Program of the Chinese Academy of Sciences for Future Network Project (174GJHZ2022047FN to Y. Zhou). We are grateful to the Analysis & Testing Center of Beihang University for the facilities, and the scientific and technical assistance. We gratefully acknowledge the assistance provided by Beam Lines BL14W1 (X-ray absorption fine structure) at Shanghai Synchrotron Radiation Facility for conducting synchrotron-based measurements. We also thank G. M. Liu and M. Wang from the Institute of Chemistry Chinese Academy of Sciences for their contributions to GIWAXS characterization and analyses. In addition, we thank J. Zhu from the National Center for Nanoscience and Technology for his assistance with computation.
Author information
Authors and Affiliations
Contributions
The idea and experimental design were developed by W.J. and Y. Zhu with project supervision provided by Y. Zhu. W.J. conducted the experiments and data analysis, while Y. Zhu, W.J., X.W. and Y. Zhou contributed to the discussion of the numerical and molecular dynamics simulations, offering valuable suggestions. X.Z. contributed to structural and molecular dynamics simulations of materials, and J.H. assisted with the computational numerical simulations. In addition, J.Z., M.F. and D.Z. helped with XAFS data analysis and characterization, while H.W. and L.J. participated in discussion of the characterization results. Manuscript organization and writing were undertaken by W.J. with contributions from Y. Zhu in terms of discussion and revision. All authors participated in the manuscript discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Anthony Straub, Li-Hsien Yeh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–96, Tables 1–14, Text, and Materials and Methods.
Source data
Source Data Fig. 1
Microstructural properties of membranes, source data.
Source Data Fig. 2
Energy conversion performances of membrane, source data.
Source Data Fig. 3
Molecular dynamics simulations results, source data.
Source Data Fig. 4
Universal strategy for M-COF membrane.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, W., Zhou, J., Zhong, X. et al. Axial alignment of covalent organic framework membranes for giant osmotic energy harvesting. Nat Sustain 8, 446–455 (2025). https://doi.org/10.1038/s41893-024-01493-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-024-01493-6
This article is cited by
-
Nanopore aligned, membrane redefined
Nature Sustainability (2025)