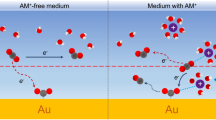
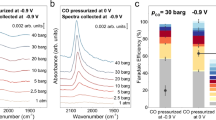
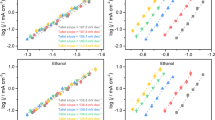
Abstract
The nature of the cations in an electrolyte has a substantial impact on the performance of the electrochemical CO2 and CO reduction reaction (CO(2)RR), however, its mechanism at the molecular level remains the subject of debate. Major gaps in our understanding include how cations affect key physicochemical variables at electrochemical interfaces and the elementary steps of the CO(2)RR. In this work, we have quantitatively determined the impact of cations on the enthalpy and entropy of CO adsorption on Cu under electrochemical conditions. CO adsorption becomes increasingly unfavourable in the sequence Li+ > Na+ > K+ > Cs+ with a substantial enthalpy–entropy compensation effect. Importantly, cations affect the stability of the initial and transition states of the CORR in opposite directions. Our results provide insights into the effect of cations on individual elementary steps in the CORR and demonstrate that the ability to stabilize the transition state in the conversion of adsorbed CO is a decisive factor.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The Python script to run the AlMD simulations is included in Supplementary Data 2.
References
Luna, P. D. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Hori, Y., KiKuchi, K., Murata, A. & Suzuki, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 15, 897–898 (1986).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Malkani, A. S. et al. Understanding the electric and nonelectric field components of the cation effect on the electrochemical CO reduction reaction. Sci. Adv. 6, eabd2569 (2020).
Li, J. et al. Hydroxide is not a promoter of C2+ product formation in the electrochemical reduction of CO on copper. Angew. Chem. Int. Ed. 59, 4464–4469 (2020).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Ovalle, V. J., Hsu, Y., Agrawal, N., Janik, M. J. & Waegele, M. M. Correlating hydration free energy and specific adsorption of alkali metal cations during CO2 electroreduction on Au. Nat. Catal. 5, 624–632 (2022).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1, 466–472 (2009).
Huang, B. et al. Cation- and pH-dependent hydrogen evolution and oxidation reaction kinetics. JACS Au 1, 1674–1687 (2021).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Shin, S. et al. A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 13, 5482 (2022).
Singh, M. R. et al. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13013 (2016).
Li, J., Li, X., Gunathunge, C. M. & Waegele, M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl Acad. Sci. USA 116, 9220–9229 (2019).
Zhang, H., Gao, J., Raciti, D. & Hall, A. S. Promoting Cu-catalyzed CO2 electroreduction to multicarbon products by tuning the activity of H2O. Nat. Catal. 6, 807–817 (2023).
Xiong, H. et al. Correlating the experimentally determined CO adsorption enthalpy with the electrochemical CO reduction performance on Cu surfaces. Angew. Chem. Int. Ed. 62, e202218447 (2023).
Schouten, K. J. P., Gallent, E. P. & Koper, M. T. M. The influence of pH on the reduction of CO and CO2 to hydrocarbons on copper electrodes. J. Electroanal. Chem. 716, 53–57 (2014).
Gunathunge, C. M., Ovalle, V. J., Li, Y., Janik, M. J. & Waegele, M. M. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 8, 7507–7516 (2018).
Haynes, W. M. (ed.) CRC Handbook of Chemistry and Physics 97th edn (CRC, 2016).
Kumeda, T., Tajiri, H., Sakata, O., Hoshi, N. & Nakamura, M. Effect of hydrophobic cations on the oxygen reduction reaction on single‒crystal platinum electrodes. Nat. Commun. 9, 4378 (2018).
Ferrari, A. M., Ugliengo, P. & Garrone, E. Ab initio study of the adducts of carbon monoxide with alkaline cations. J. Chem. Phys. 105, 4129–4139 (1996).
Malkani, A. S., Anibal, J. & Xu, B. Cation effect on interfacial CO2 concentration in the electrochemical CO2 reduction reaction. ACS Catal. 10, 14871–14876 (2020).
Li, J. et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 12, 3264 (2021).
Waegele, M. M., Gunathunge, C. M., Li, J. & Li, X. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 151, 160902 (2019).
Kim, Y., Baricuatro, J. H., Javier, A., Gregoire, J. M. & Soriaga, M. P. The evolution of the polycrystalline copper surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR potential: a study by operando EC-STM. Langmuir 30, 15053–15056 (2014).
Mähler, J. & Persson, I. A study of the hydration of the alkali metal ions in aqueous solution. Inorg. Chem. 51, 425–438 (2012).
Yang, X. et al. Cation-induced interfacial hydrophobic microenvironment promotes the C–C coupling in electrochemical CO2 reduction. J. Am. Chem. Soc. 146, 5532–5542 (2024).
Barth, J. V. Transport of adsorbates at metal surfaces: from thermal migration to hot precursors. Surf. Sci. Rep. 40, 75–149 (2000).
Nilekar, A. U., Greeley, J. & Mavrikakis, M. A simple rule of thumb for diffusion on transition-metal surfaces. Angew. Chem. Int. Ed. 45, 7046–7049 (2006).
Yildirim, H., Sankaranarayanan, S. K. R. S. & Greeley, J. P. Periodic trends in adsorption and activation energies for heterometallic diffusion on (100) transition metal surfaces. J. Phys. Chem. C. 116, 22469–22475 (2012).
Spitzer, A., Ritz, A., & Lüth, H. The adsorption of H2O on Cu(100) surfaces. Surf. Sci. 152/153, 543–549 (1985).
Schiros, T. et al. Structure of water adsorbed on the open Cu(110) surface: H-up, H-down, or both? Chem. Phys. Lett. 429, 415–419 (2006).
Lu, X., Shinagawa, T. & Takanabe, K. Product distribution control guided by a microkinetic analysis for CO reduction at high-flux electrocatalysis using gas-diffusion Cu electrodes. ACS Catal. 13, 1791–1803 (2023).
Chang, X. et al. C–C coupling is unlikely to be the rate-determining step in the formation of C2+ products in the copper-catalyzed electrochemical reduction of CO. Angew. Chem. Int. Ed. 61, e202111167 (2022).
Xu, Y., Yang, H., Chang, X. & Xu, B. Introduction to electrocatalytic kinetics. Acta Phys. Chim. Sin. 39, 2210025 (2023).
Koper, M. T. M. Theory and kinetic modeling of electrochemical cation‑coupled electron transfer reactions. J. Solid State Electrochem. 28, 1601–1606 (2024).
Bligaard, T. et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004).
Gao, W., Xu, Y., Fu, L., Chang, X. & Xu, B. Experimental evidence of distinct sites for CO2-to-CO and CO conversion on Cu in the electrochemical CO2 reduction reaction. Nat. Catal. 6, 885–894 (2023).
Mortensen, J. J., Hansen, L. B. & Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 71, 035109 (2005).
Enkovaara, J. et al. Real-space grid implementation of the projector augmented wave method. J. Phys. Condens. Matter 22, 253202 (2010).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Larsen, A. H. et al. Localized atomic basis set in the projector augmented wave method. Phys. Rev. B 80, 195112 (2009).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Lee, K. et al. Higher-accuracy van der Waals density functional. Phys. Rev. B 82, 081101(R) (2010).
Gonzalez, F. J. et al. Assessing the dynamics of CO adsorption on Cu(110) using the vdW-DF2 functional and artificial neural networks. J. Chem. Phys. 159, 224709 (2023).
Kastlunger, G., Lindgren, P. & Peterson, A. A. Controlled-potential simulation of elementary electrochemical reactions: proton discharge on metal surfaces. J. Phys. Chem. C. 122, 12771–12781 (2018).
Acknowledgements
This work was supported by the Beijing Natural Science Foundation Key Research Program (grant no. Z240026) and the Beijing National Laboratory for Molecular Sciences. We acknowledge the support of the National Natural Science Foundation of China (22122304 (H.X.) and 22303038 (Z.X.)). We are grateful to the Center for Computational Science and Engineering at SUSTech and the CHEM High Performance Supercomputer Cluster (CHEM HPC) at the Department of Chemistry at SUSTech for providing computational resources.
Author information
Authors and Affiliations
Contributions
Y.X. and B.X. conceived the idea and designed the experiments. Y.X. conducted the electrochemical tests, SEIRAS experiments and statistical thermodynamic analysis. Z.X. and H.X. performed the AIMD simulations. W.G. conducted parts of the electrochemical tests. Y.X., Z.X., H.X. and B.X. analysed the data and co-wrote the paper, with input from all of the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Chang Hyuck Choi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Table 1, Notes 1–6 and References 1–22.
Supplementary Data 1
Initial and final structures in the AIMD simulations.
Supplementary Data 2
Python script to run the AIMD simulations.
Source data
Source Data Fig. 1
Enthalpy and entropy of CO adsorption determined by SEIRAS.
Source Data Fig. 2
AIMD simulations of CO adsorption on Cu(100) with Li+ and Cs+.
Source Data Fig. 3
Correlation between the relative enthalpy and entropy of CO adsorption on Cu.
Source Data Fig. 4
Electrochemical activation parameters in MOH.
Source Data Fig. 6
Influence of cation concentration on the elementary steps of CORR.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Xia, Z., Gao, W. et al. Cation effect on the elementary steps of the electrochemical CO reduction reaction on Cu. Nat Catal 7, 1120–1129 (2024). https://doi.org/10.1038/s41929-024-01227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01227-z
This article is cited by
-
Non-isothermal CO2 electrolysis enables simultaneous enhanced electrochemical and anti-precipitation performance
Nature Communications (2025)
-
Scaled CO Electroreduction to Alcohols
Nature Communications (2025)
-
Identifying Cu reconstruction mechanism in CO2 and CO electroreduction via Cu+ detection and in situ atomic force microscopy
Science China Materials (2025)