Abstract
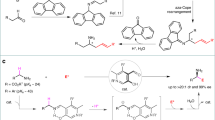
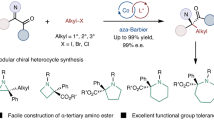
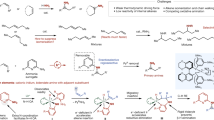
Catalytic enantioselective α-C–H functionalization of widely available achiral alkyl amines could provide an ideal synthetic approach towards chiral amines. However, the inert nature of the α-C–H of alkyl amines renders their activation as carbanionic nucleophiles for catalytic asymmetric reactions an important yet unmet challenge. Here we describe how N-arylidene-protected alkyl amines could be activated as carbanions for asymmetric conjugate addition and the Mannich reaction. These results represent an intriguing and generally useful approach to the synthesis of chiral α,α-dialkyl amines. More importantly, they highlight the enormous potential of N-arylidene-protected amines as readily available and widely applicable synthons for the asymmetric synthesis of chiral amines.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support this study are available within this article and its Supplementary Information, or from the authors upon reasonable request. Crystallographic data for compounds 8 and 5Ca were deposited in the Cambridge Structural Database under deposition nos. 2253297 (8) and 2250265 (5Ca). Copies of the data can be obtained free of charge via the Cambridge Crystallographic Data Centre at https://www.ccdc.cam.ac.uk/structures/.
References
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, 2010).
Hashimoto, T. & Maruoka, K. Recent development and application of chiral phase-transfer catalysts. Chem. Rev. 107, 5656–5682 (2007).
Wang, Q., Gu, Q. & You, S. L. Enantioselective carbonyl catalysis enabled by chiral aldehydes. Angew. Chem. Int. Ed. 58, 6818–6825 (2019).
Chen, J. et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science 360, 1438–1442 (2018).
Wen, W. et al. Chiral aldehyde catalysis for the catalytic asymmetric activation of glycine esters. J. Am. Chem. Soc. 140, 9774–9780 (2018).
Zhong, X. et al. Chiral Lewis acid-bonded picolinaldehyde enables enantiodivergent carbonyl catalysis in the Mannich/condensation reaction of glycine ester. Chem. Sci. 12, 4353–4360 (2021).
Zhang, J. et al. Enantio- and diastereodivergent construction of 1,3-nonadjacent stereocenters bearing axial and central chirality through synergistic Pd/Cu catalysis. J. Am. Chem. Soc. 143, 12622–12632 (2021).
Chen, Y. J., Seki, K., Yamashita, Y. & Kobayashi, S. Catalytic carbon−carbon bond-forming reactions of aminoalkane derivatives with imines. J. Am. Chem. Soc. 132, 3244–3245 (2010).
Gan, X. C., Zhang, C. Y., Zhong, F., Tian, P. & Yin, L. Synthesis of chiral anti-1,2-diamine derivatives through copper (I)-catalyzed asymmetric α-addition of ketimines to aldimines. Nat. Commun. 11, 4473–4480 (2020).
Wen, W. et al. Diastereodivergent chiral aldehyde catalysis for asymmetric 1,6-conjugated addition and Mannich reactions. Nat. Commun. 11, 5372–5382 (2020).
Hou, C. et al. Catalytic asymmetric α-C(sp3)–H addition of benzylamines to aldehydes. Nat. Catal. 5, 1061–1068 (2022).
Park, Y. S., Boys, M. L. & Beak, P. (−)-Sparteine-mediated α-lithiation of N-Boc-N-(p-methoxyphenyl) benzylamine:enantioselective syntheses of (S) and (R) mono- and disubstituted N-Boc-benzylamines. J. Am. Chem. Soc. 118, 3757–3758 (1996).
Wang, T. C. et al. Palladium-catalyzed enantioselective C(sp3)–H/C(sp3)–H umpolung coupling of N-allylimine and α-aryl ketones. J. Am. Chem. Soc. 143, 20454–20461 (2021).
Ji, P. et al. Direct asymmetric α-C–H addition of N-unprotected propargylic amines to trifluoromethyl ketones by carbonyl catalysis. Angew. Chem. Int. Ed. 61, e202206111 (2022).
Cheng, M. X. & Yang, S. D. Recent advances in the enantioselective oxidative α-C–H functionalization of amines. Synlett 28, 159–174 (2017).
Cordier, C. J., Lundgren, R. J. & Fu, G. C. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 135, 10946–10949 (2013).
Ahneman, D. T. & Doyle, A. G. C–H functionalization of amines with aryl halides by nickel-photoredox catalysis. Chem. Sci. 7, 7002–7006 (2016).
Chan, J. Z. et al. Direct conversion of N-alkylamines to N-propargylamines through C–H activation promoted by Lewis acid/organocopper catalysis: application to late-stage functionalization of bioactive molecules. J. Am. Chem. Soc. 142, 16493–16505 (2020).
Chen, L., Yang, Y., Liu, L., Gao, Q. & Xu, S. Iridium-catalyzed enantioselective α-C(sp3)–H borylation of azacycles. J. Am. Chem. Soc. 142, 12062–12068 (2020).
Jain, P., Verma, P., Xia, G. & Yu, J. Q. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 9, 140–144 (2017).
Rand, A. W. et al. Dual catalytic platform for enabling sp3 α-C–H arylation and alkylation of benzamides. ACS Catal. 10, 4671–4676 (2020).
Shu, X., Huan, L., Huang, Q. & Huo, H. Direct enantioselective C(sp3)-H acylation for the synthesis of α-amino ketones. J. Am. Chem. Soc. 142, 19058–19064 (2020).
Shu, X., Zhong, D., Lin, Y., Qin, X. & Huo, H. Modular access to chiral α-(hetero) aryl amines via Ni/photoredox-catalyzed enantioselective cross-coupling. J. Am. Chem. Soc. 144, 8797–8806 (2022).
Proctor, R. S., Chuentragool, P., Colgan, A. C. & Phipps, R. J. Hydrogen atom transfer-driven enantioselective minisci reaction of amides. J. Am. Chem. Soc. 143, 4928–4934 (2021).
Yan, X. B. et al. Ni-catalyzed hydroalkylation of olefins with N-sulfonyl amines. Nat. Commun. 12, 5881–5890 (2021).
Li, L., Liu, Y. C. & Shi, H. Nickel-catalyzed enantioselective α-alkenylation of N-sulfonyl amines: modular access to chiral α-branched amines. J. Am. Chem. Soc. 143, 4154–4161 (2021).
Uraguchi, D. & Ooi, T. Development of P-spiro chiral aminophosphonium salts as a new class of versatile organic molecular catalyst. J. Syn. Org. Chem. 68, 1185–1194 (2010).
Formica, M., Rozsar, D., Su, G., Farley, A. J. & Dixon, D. J. Bifunctional iminophosphorane superbase catalysis: applications in organic synthesis. Acc. Chem. Res. 53, 2235–2247 (2020).
Kondoh, A. & Terada, M. Development of molecular transformations on the basis of catalytic generation of anionic species by organosuperbase. Bull. Chem. Soc. 94, 339–356 (2021).
Wu, Y., Hu, L., Li, Z. & Deng, L. Catalytic asymmetric umpolung reactions of imines. Nature 523, 445–450 (2015).
Luo, J., Deng, Y., Deng, T. & Deng, L. Catalytic enantioconvergent conjugate addition of organosilanes via a strategy of fluorodesilylation. J. Am. Chem. Soc. 144, 23264–23270 (2022).
Fontaine, P., Chiaroni, A., Masson, G. & Zhu, J. One-pot three-component synthesis of α-iminonitriles by IBX/TBAB-mediated oxidative Strecker reaction. Org. Lett. 10, 1509–1512 (2008).
Gualtierotti, J. B., Schumacher, X., Wang, Q. & Zhu, J. Synthesis of iminonitriles by oxone/TBAB-mediated one-pot oxidative three-component Strecker reaction. Synthesis 45, 1380–1386 (2013).
Li, Z., Hu, B., Wu, Y., Fei, C. & Deng, L. Control of chemoselectivity in asymmetric tandem reactions: direct synthesis of chiral amines bearing non-adjacent stereocenters. Proc. Natl Acad. Sci. USA 115, 1730–1735 (2018).
Han, X. L. et al. Catalytic asymmetric imine cross-coupling reaction. J. Am. Chem. Soc. 145, 4400–4407 (2023).
Hu, B. & Deng, L. Catalytic asymmetric synthesis of trifluoromethylated-amino acids through the umpolung addition of trifluoromethyl imines to carboxylic acid derivatives. Angew. Chem. Int. Ed. 57, 2233–2237 (2018).
Hu, B. et al. Origin of and a solution for uneven efficiency by cinchona alkaloid-derived, pseudoenantiomeric catalysts for asymmetric reactions. J. Am. Chem. Soc. 140, 13913–13920 (2018).
Kitamura, M., Shirakawa, S. & Maruoka, K. Powerful chiral phase-transfer catalysts for the asymmetric synthesis of α-alkyl- and α,α-dialkyl-α-amino acids. Angew. Chem. Int. Ed. 44, 1549–1551 (2005).
Corey, E. J., Xu, F. & Noe, M. C. A rational approach to catalytic enantioselective enolate alkylation using a structurally rigidified and defined chiral quaternary ammonium salt under phase transfer conditions. J. Am. Chem. Soc. 119, 12414–12415 (1997).
Lucet, D., Le Gall, T. & Mioskowski, C. The chemistry of vicinal diamines. Angew. Chem. Int. Ed. 37, 2580–2627 (1998).
Kizirian, J. C. Chiral tertiary diamines in asymmetric synthesis. Chem. Rev. 108, 140–205 (2008).
Zhu, Y., Cornwall, R. G., Du, H., Zhao, B. & Shi, Y. Catalytic diamination of olefins via N–N bond activation. Acc. Chem. Res. 47, 3665–3678 (2014).
Gupta, A. K. & Hull, K. L. Synthesis of 1,2-diamines via hydroamination reactions. Synlett 26, 1779–1784 (2015).
Stephenson, M. D. & Hardie, M. J. Extended structures of transition metal complexes of 6,7-dicyanodipyridoquinoxaline: p-stacking, weak hydrogen bonding, and CN‧‧‧p interactions. Cryst. Growth Des. 6, 423–432 (2006).
Tian, Z., Ren, X., Li, Y., Song, Y. & Meng, Q. A new quasi-1D spin system with spin transition exhibiting novel CN‧‧‧p interactions. Inorg. Chem. 46, 8102–8104 (2007).
Tian, Z. F. et al. An intriguing NO2‧‧‧p and CN‧‧‧p interactions in [1-(40-nitrobenzyl)pyrazinium][Ni(mnt)2]: Crystal structure, magnetic property and DFT calculation. Inorg. Chem. Commun. 12, 65–68 (2009).
Wang, D. X. & Wang, M. X. Anion−π interactions: generality, binding strength, and structure. J. Am. Chem. Soc. 135, 892–897 (2013).
Yang, Q. et al. Holistic prediction of pKa in diverse solvents based on machine learning approach. Angew. Chem. Int. Ed. 59, 19282–19291 (2020).
Acknowledgements
We thank the Instrumentation and Service Center for Molecular Sciences and the Instrumentation and Service Center for Physical Sciences at Westlake University for assistance in measurement/data interpretation. We thank X. Lu and X. Shi at Westlake University for assistance in the NMR measurement, Y. Chen at Westlake University for assistance of the HRMS measurement, and X. Miao and F. Leng at Westlake University for assistance in the X-ray measurement. We thank the High-Performance Computing Center of Westlake University for providing computational resources. We thank J. Lu at Westlake University for providing amine 1m. We are grateful for financial support provided by the National Natural Science Foundation of China (U22A20389 to L.D, 22371232 to J.L.), Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang 2020R01004 and the Foundation of Westlake University.
Author information
Authors and Affiliations
Contributions
J.L. and L.D. conceived the project. J.L., T.D. and X.-L.H. designed and developed the reaction. C.C., X.L., Y.G., K.W. and Z.L. contributed to the catalysts and materials preparation. Y.Y. carried out theoretical calculation. J.L. and L.D. supervised the research. L.D. acquired funding. T.D., X.-L.H., Y.Y., J.L. and L.D. contributed to the writing and editing of the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Xi Lu, Gui Lui, Choon-Hong Tan and Donghui Wei for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, including materials preparation, computational methods, single X-ray crystal diffraction data, nuclear magnetic resonance spectra and SFC spectra, References, Figs. 1–332 and Tables 1–17.
Supplementary Data 1
The cif file of 5Ca.
Supplementary Data 2
The checklist of 5Ca.
Supplementary Data 3
The pdf file of structure of 5Ca.
Supplementary Data 4
The png file of structure of 5Ca.
Supplementary Data 5
The cif file of 8.
Supplementary Data 6
The checklist of 8.
Supplementary Data 7
The pdf file of structure of 8.
Supplementary Data 8
The png file of structure of 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, T., Han, XL., Yu, Y. et al. Organocatalytic asymmetric α-C–H functionalization of alkyl amines. Nat Catal 7, 1076–1085 (2024). https://doi.org/10.1038/s41929-024-01230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01230-4
This article is cited by
-
Phase-transfer-catalyst enabled enantioselective C–N coupling to access chiral boron-stereogenic BODIPYs
Nature Communications (2025)