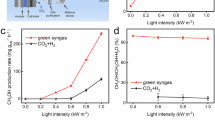
Abstract
High-purity carbon monoxide (CO), crucial for various high-tech industries, requires complex purification and further energy input. Here we show that pure fluorite ZrO2 can produce clean CO without purification by driving formic acid dehydration and completely shutting off the formic acid dehydrogenation pathway. An explosion method is developed for synthesizing pristine fluorite ZrO2 nanosheets that achieve a pure CO production rate of 55 mmol g−1 h−1 at 250 °C. Integrated with a homemade photothermal reactor, the fluorite ZrO2 nanosheets show a pure CO productivity of 83 mmol g−1 h−1 under 0.5 sun irradiation and a photochemical energy conversion efficiency of 12.3%. Moreover, this system generates over 1,538 l m−2 of pure CO per day under outdoor sunlight irradiation. This work charts a promising course for purification-free pure CO generation without secondary energy input.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are available via figshare at https://doi.org/10.6084/m9.figshare.27300834.v1 (ref. 51) or from the corresponding author upon reasonable request.
References
Wittig, C., Hassler, J. C. & Coleman, P. D. Constant-wave laser oscillation in a carbon monoxide chemical laser. Nature 226, 845–846 (1970).
Harrison, P. G. & Willett, M. J. The mechanism of operation of tin(iv) oxide carbon monoxide sensors. Nature 332, 337–339 (1988).
D’Angelo, M. et al. In-situ formation of SiC nanocrystals by high temperature annealing of SiO2/Si under CO: a photoemission study. Surf. Sci. 606, 697–701 (2012).
Ma, X. et al. Carbon monoxide separation: past, present and future. Chem. Soc. Rev. 52, 3741–3777 (2023).
Karatok, M. et al. Achieving ultra-high selectivity to hydrogen production from formic acid on Pd-Ag alloys. J. Am. Chem. Soc. 145, 5114–5124 (2023).
Matsumoto, N., Watanabe, T. & Kato, K. Impurity analyses of high-purity carbon monoxide gas using micro gas chromatography for development as a certified reference material. J. Chromatogr. A 1282, 190–193 (2013).
Xu, W., Lindbråthen, A., Janakiram, S., Ansaloni, L. & Deng, L. Enhanced CO2/H2 separation by GO and PVA-GO embedded PVAm nanocomposite membranes. J. Membr. Sci. 671, 121397 (2023).
Baker, R. W. & Low, B. T. Gas separation membrane materials: a perspective. Macromolecules 47, 6999–7013 (2014).
Kasuya, F. & Tsuji, T. High purity CO gas separation by pressure swing adsorption. Gas. Sep. Purif. 5, 242–246 (1991).
Dutta, N. & Patil, G. Developments in CO separation. Gas. Sep. Purif. 9, 277–283 (1995).
Onishi, N., Laurenczy, G., Beller, M. & Himeda, Y. Recent progress for reversible homogeneous catalytic hydrogen storage in formic acid and in methanol. Coord. Chem. Rev. 373, 317–332 (2018).
Kwon, S., Lin, T. C. & Iglesia, E. Elementary steps and site requirements in formic acid dehydration reactions on anatase and rutile TiO2 surfaces. J. Catal. 383, 60–76 (2020).
Qi, Y. et al. Photoinduced defect engineering: enhanced photothermal catalytic performance of 2D black In2O3−x nanosheets with bifunctional oxygen vacancies. Adv. Mater. 32, 1903915 (2020).
Tedsree, K. et al. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat. Nanotechnol. 6, 302–307 (2011).
Grasemann, M. & Laurenczy, G. Formic acid as a hydrogen source-recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012).
Malinowski, M., Malinowska, K. & Zatorski, L. W. A. The catalytic decomposition of formic acid into carbon monoxide. Bull. Soc. Chim. Belg. 92, 225–227 (1983).
Yu, Z. et al. Formic acid as a Bio-CO carrier: selective dehydration with γ-Mo2N catalysts at low temperatures. ACS Sustain. Chem. Eng. 8, 13956–13963 (2020).
Popova, G. Y., Andrushkevich, T. V., Chesalov, Y. A. & Stoyanov, E. S. In situ FTIR study of the adsorption of formaldehyde, formic acid, and methyl formiate at the surface of TiO2 (anatase). Kinet. Catal. 41, 805–811 (2000).
Popova, G. Y., Zakharov, I. & Andrushkevich, T. Mechanism of formic acid decomposition on P-Mo heteropolyacid. React. Kinet. Catal. Lett. 66, 251–256 (1999).
Dziembaj, R., Molenda, M. & Chmielarz, L. Synthesis and specific properties of the ceria and ceria-zirconia nanocrystals and their aggregates showing outstanding catalytic activity in redox reactions—a review. Catalysts 13, 1165 (2023).
Yang, H. et al. One-step synthesis of highly active and stable Ni-ZrO2 catalysts for the conversion of methyl laurate to alkanes. J. Catal. 413, 297–310 (2022).
Lee, H. J., Kang, D. C., Pyen, S. H., Shin, M. & Shin, C. H. Production of H2-free CO by decomposition of formic acid over ZrO2 catalysts. Appl. Catal. A 531, 13–20 (2017).
Tazuke, S. & Kitamura, N. Photofixation of carbon dioxide to formic acid in vitro using water as hydrogen source. Nature 275, 301–302 (1978).
Li, Y. et al. Selective light absorber-assisted single nickel atom catalysts for ambient sunlight-driven CO2 methanation. Nat. Commun. 10, 2359 (2019).
Gray, J. T. et al. Unravelling the reaction mechanism of gas-phase formic acid decomposition on highly dispersed Mo2C nanoparticles supported on graphene flakes. Appl. Catal. B 264, 118478 (2020).
Fisher, G., Seacrist, M. R. & Standley, R. W. Silicon crystal growth and wafer technologies. P. IEEE 100, 1454–1474 (2012).
Anthony, J. E. The larger acenes: versatile organic semiconductors. Angew. Chem. Int. Ed. 47, 452–483 (2008).
Li, Y. et al. Cu-based high-entropy two-dimensional oxide as stable and active photothermal catalyst. Nat. Commun. 14, 3171 (2023).
Li, Y. et al. General heterostructure strategy of photothermal materials for scalable solar-heating hydrogen production without the consumption of artificial energy. Nat. Commun. 13, 776 (2022).
Ricca, C., Ringuedé, A., Cassir, M., Adamo, C. & Labat, F. A comprehensive DFT investigation of bulk and low-index surfaces of ZrO2 polymorphs. J. Comput. Chem. 36, 9–21 (2015).
Barnard, A. S., Yeredla, R. R. & Xu, H. Modelling the effect of particle shape on the phase stability of ZrO2 nanoparticles. Nanotechnology 17, 3039 (2006).
Song, X. et al. Thermophysical and mechanical properties of cubic, tetragonal and monoclinic ZrO2. J. Mater. Res. Technol. 23, 648–655 (2023).
Ohtaka, O. et al. Phase relations and equations of state of ZrO2 under high temperature and high pressure. Phys. Rev. B 63, 174108 (2001).
Motomura, H., Tamao, D., Nambu, K., Masuda, H. & Yoshida, H. Athermal effect of flash event on high-temperature plastic deformation in Y2O3-stabilized tetragonal ZrO2 polycrystal. J. Eur. Ceram. Soc. 42, 5045–5052 (2022).
Jaipal, M. & Chatterjee, A. Relative occurrence of oxygen-vacancy pairs in yttrium-containing environments of Y2O3-doped ZrO2 can be crucial to ionic conductivity. J. Phys. Chem. C 121, 14534–14543 (2017).
Biçer, H. et al. Multicycle flash sintering of cubic Y2O3-stabilized ZrO2: an in situ energy dispersive synchrotron X-ray diffraction study with high temporal resolution. Mater. Today Commun. 33, 104272 (2022).
Bankar, B. D., Naikwadi, D. R. & Biradar, A. V. Hydrogenation of CO2 to formic acid over efficient heterogeneous ruthenium oxide supported on cubic phase zirconium oxide catalyst. Appl. Surf. Sci. 631, 157556 (2023).
Zhong, Y., Li, Z. & Wang, X. Seed-crystal-induced directional solidification toward Al2O3/(Y0.2Er0.2Yb0.2Ho0.2Lu0.2)3Al5O12/ZrO2 ternary eutectic ceramics. Acta Mater. 22, 119369 (2023).
Chen, Y. X., Heinen, M., Jusys, Z. & Behm, R. J. Kinetics and mechanism of the electrooxidation of formic acid-spectroelectrochemical studies in a flow cell. Angew. Chem. Int. Ed. 45, 981–985 (2006).
Huo, B., Li, B., Chen, C. & Zhang, Y. Surface etching and early age hydration mechanisms of steel slag powder with formic acid. Constr. Build. Mater. 280, 122500 (2021).
Ning, S. et al. Co0–Coδ+ interface double-site-mediated C–C coupling for the photothermal conversion of CO2 into light olefins. Angew. Chem. Int. Ed. 135, e202302253 (2023).
Belhadj, H., Hakki, A., Robertson, P. K. J. & Bahnemann, D. W. In situ ATR-FTIR study of H2O and D2O adsorption on TiO2 under UV irradiation. Phys. Chem. Chem. Phys. 17, 22940 (2015).
Li, Y. et al. Low temperature thermal and solar heating carbon-free hydrogen production from ammonia using nickel single atom catalysts. Adv. Energy Mater. 12, 2202459 (2022).
Lv, C. et al. Nanostructured materials for photothermal carbon dioxide hydrogenation: regulating solar utilization and catalytic performance. ACS Nano 17, 1725–1738 (2023).
Li, Y. et al. A Ni–O–Ag photothermal catalyst enables 103-m2 artificial photosynthesis with 17% solar-to-chemical energy conversion efficiency. Sci. Adv. 10, eadn5098 (2024).
Gazsi, A., Bánsági, T. & Solymosi, F. Decomposition and reforming of formic acid on supported Au catalysts: production of CO-free H2. J. Phys. Chem. C 115, 15459–15466 (2011).
Sadovskaya, E. M., Chesalov, Y. A., Goncharov, V. B., Sobolev, V. I. & Andrushkevich, T. V. Formic acid decomposition over V-Ti oxide catalyst: mechanism and kinetics. Mol. Catal. 430, 52–64 (2017).
Ivanez, J., Garcia-Munoz, P., Ruppert, A. M. & Keller, N. UV—a light-assisted gas-phase formic acid decomposition on photo-thermo Ru/TiO2 catalyst. Catal. Today 380, 138–146 (2021).
Bulushev, D. A., Beloshapkin, S. & Ross, J. R. H. Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal. Today 154, 7–12 (2010).
Cao, J. et al. Hydrogen production from formic acid over morphology-controllable molybdenum carbide catalysts. J. Alloys Compd. 735, 1463–1471 (2018).
Li, Y. et al. High-purity carbon monoxide production via photothermal formic acid decomposition over fluorite ZrO2. figshare https://doi.org/10.6084/m9.figshare.27300834.v1 (2024).
Acknowledgements
We thank Qingbo Meng (Institute of Physics, Chinese Academy of Sciences) for the guidance on the formic acid dehydration. We are grateful for the TEM technical support provided by the Microanalysis Center, College of Physics Science and Technology, Hebei University. We thank Jianmin Lv (Dalian Institute of Chemical Physics, Chinese Academy of Sciences), Wei Zhou (Tianjin University) and Ruqian Lian (Hebei University) for assistance with the theoretical calculations. This work is supported by the National Natural Science Foundation of China (52371220, Y.Li; U23A20139, J.Y.), Natural Science Foundation of Hebei Province (B2023204034, Y.Li; B2023201107, D.Y.; B2022201090, Y.Li; B2021201074, Y.Li; B2024201096, J.Y.; 2023HBQZYCXY001, J.Y.), Hebei Education Department (QN2022059, D.Y.), Interdisciplinary Research Program of Natural Science of Hebei University (521100311, J.Y.; DXK202109, Y.Li), the Advanced Talents Incubation Program of Hebei University (grant no. 050001-521100223213, J.Y.) and the Scientific Research Foundation of Hebei Agricultural University (YJ201939, D.Y.).
Author information
Authors and Affiliations
Contributions
Y. Li and J.Y. conceived the project and contributed to the design of the experiments and analysis of the data. Y. Luo, B.L. and D.Y. performed the preparation and characterizations of the catalysts. D.Y., H.W., Q.W. and J.W. performed the photothermal reactors’ characterizations. X.S. and Y.W. conducted the SEM and TEM examinations. Y. Li and J.Y. wrote the paper. All the authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Hermenegildo Garcia and Andrew Logsdail for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–28 and References.
Supplementary Video 1
The fast-burning video of the mixture of zirconium salt and explosive.
Supplementary Video 2
The water droplets produced from thermal formic acid decomposition over F-ZrO2.
Supplementary Video 3
The working video of photothermal formic acid dehydration.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, B., Yuan, D. et al. High-purity carbon monoxide production via photothermal formic acid decomposition over fluorite ZrO2. Nat Catal 7, 1350–1358 (2024). https://doi.org/10.1038/s41929-024-01249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01249-7