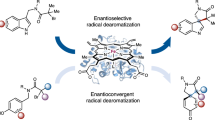
Abstract
The scope of nature’s catalytic abilities has been expanded by recent advancements in biocatalysis to include synthetic transformations with no biological equivalent. However, these newly introduced catalytic functions represent only a small fraction of reactions utilized in synthetic catalysis. Here we present a biocatalytic platform that combines photoredox and metalloenzymatic catalysis for enantioselective radical transformations. Under green light irradiation, the eosin Y photocatalyst enables 4-hydroxyphenylpyruvate dioxygenases to catalyse enantioselective decarboxylative azidation and thiocyanation of N-hydroxyphthalimide esters. The final optimized variant obtained through directed evolution can afford diverse chiral organic azide and thiocyanate compounds with up to 77% yield, 385 total turnovers and 94% enantiomeric excess. Mechanistic studies show that the eosin Y catalyst mediates the generation of both C(sp3) radical and Fe(III)‒N3/Fe(III)‒NCS intermediate, leading to efficient enantioselective C‒N3 and C‒SCN bond formation in the enzyme active site. These findings establish an adaptable biocatalytic platform for introducing abiological metallophotoredox catalysis into biology.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data required to assess the conclusions of this study are included in the Article and its Supplementary Information or can be obtained from the authors upon reasonable request.
References
Lu, Y., Yeung, N., Sieracki, N. & Marshall, N. M. Design of functional metalloproteins. Nature 460, 855–862 (2009).
Bertini, G. et al. Biological Inorganic Chemistry: Structure and Reactivity (University Science Books, 2007).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Miller, D. C., Athavale, S. V. & Arnold, F. H. Combining chemistry and protein engineering for new-to-nature biocatalysis. Nat. Synth. 1, 18–23 (2022).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Ren, X. & Fasan, R. Engineered and artificial metalloenzymes for selective C–H functionalization. Curr. Opin. Green. Sustain. Chem. 31, 100494 (2021).
Mondal, D., Snodgrass, H. M., Gomez, C. A. & Lewis, J. C. Non-native site-selective enzyme catalysis. Chem. Rev. 123, 10381–10431 (2023).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Huang, X. & Groves, J. T. Oxygen activation and radical transformations in heme proteins and metalloporphyrins. Chem. Rev. 118, 2491–2553 (2018).
Broderick, J. B., Duffus, B. R., Duschene, K. S. & Shepard, E. M. Radical S-adenosylmethionine enzymes. Chem. Rev. 114, 4229–4317 (2014).
Krebs, C., Galonić Fujimori, D., Walsh, C. T. & Bollinger, J. M. Jr. Non-heme Fe(IV)–oxo intermediates. Acc. Chem. Res. 40, 484–492 (2007).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)-H azidation. Science 376, 869–874 (2022).
Fu, W. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat. Catal. 6, 628–636 (2023).
Zhao, Q. et al. Engineering non-haem iron enzymes for enantioselective C(sp3)–F bond formation via radical fluorine transfer. Nat. Synth. 3, 958–966 (2024).
Zhao, L.-P. et al. Biocatalytic enantioselective C(sp3)–H fluorination enabled by directed evolution of non-haem iron enzymes. Nat. Synth. https://doi.org/10.1038/s44160-024-00536-2 (2024).
Lee, J. & Song, W. J. Photocatalytic C-O coupling enzymes that operate via intramolecular electron transfer. J. Am. Chem. Soc. 145, 5211–5221 (2023).
Hu, W.-Y. et al. Light-driven oxidative demethylation reaction catalyzed by a Rieske-type non-heme iron enzyme Stc2. ACS Catal. 12, 14559–14570 (2022).
Fu, Y. et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase. J. Am. Chem. Soc. 143, 617–622 (2021).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Fu, H. & Hyster, T. K. From ground-state to excited-state activation modes: flavin-dependent ‘ene’-reductases catalyzed non-natural radical reactions. Acc. Chem. Res. 57, 1446–1457 (2024).
Ouyang, Y., Page, C. G., Bilodeau, C. & Hyster, T. K. Synergistic photoenzymatic catalysis enables synthesis of a-tertiary amino acids using threonine aldolases. J. Am. Chem. Soc. 146, 13754–13759 (2024).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox–pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Wang, T.-C. et al. Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling. Nature 629, 98–104 (2024).
Xu, Y. et al. A light-driven enzymatic enantioselective radical acylation. Nature 625, 74–78 (2024).
Trimble, J. S. et al. A designed photoenzyme for enantioselective [2+2] cycloadditions. Nature 611, 709–714 (2022).
Sun, N. et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes. Nature 611, 715–720 (2022).
Chen, H., Liu, Y. A. & Liao, X. Recent progress in radical decarboxylative functionalizations enabled by transition-metal (Ni, Cu, Fe, Co or Cr) catalysis. Synthesis 53, 1–29 (2020).
Sivaguru, P., Ning, Y. & Bi, X. New strategies for the synthesis of aliphatic azides. Chem. Rev. 121, 4253–4307 (2021).
Schwarz, J. & König, B. Decarboxylative reactions with and without light—a comparison. Green. Chem. 20, 323–361 (2018).
Kao, S.-C. et al. Photochemical iron-catalyzed decarboxylative azidation via the merger of ligand-to-metal charge transfer and radical ligand transfer catalysis. Chem. Catal. 3, 100603 (2023).
Zhu, Y. et al. Silver-catalyzed decarboxylative azidation of aliphatic carboxylic acids. Org. Lett. 17, 4702–4705 (2015).
Liu, C., Wang, X., Li, Z., Cui, L. & Li, C. Silver-catalyzed decarboxylative radical azidation of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 137, 9820–9823 (2015).
Masterson, D. S. & Porter, N. A. Diastereoselective free radical halogenation, azidation, and rearrangement of β-silyl barton esters. Org. Lett. 4, 4253–4256 (2002).
Wu, J., Shu, C., Li, Z., Noble, A. & Aggarwal, V. K. Photoredox-catalyzed decarboxylative bromination, chlorination and thiocyanation using inorganic salts. Angew. Chem. Int. Ed. 62, e202309684 (2023).
Marcote, D. C. et al. Photoinduced decarboxylative azidation of cyclic amino acids. Org. Biomol. Chem. 17, 1839–1842 (2019).
Wang, Y., Li, L. & Fu, N. Electrophotochemical decarboxylative azidation of aliphatic carboxylic acids. ACS Catal. 12, 10661–10667 (2022).
Nyfeler, E. & Renaud, P. Decarboxylative radical azidation using MPDOC and MMDOC esters. Org. Lett. 10, 985–988 (2008).
Wang, K., Li, Y., Li, X., Li, D. & Bao, H. Iron-catalyzed asymmetric decarboxylative azidation. Org. Lett. 23, 8847–8851 (2021).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Winkler, J. R. & Gray, H. B. Electron transfer in ruthenium-modified proteins. Chem. Rev. 92, 369–379 (1992).
Waheed, A. A., Rao, K. S. & Gupta, P. D. Mechanism of dye binding in the protein assay using eosin dyes. Anal. Biochem. 287, 73–79 (2000).
Biegasiewicz, K. F., Cooper, S. J., Emmanuel, M. A., Miller, D. C. & Hyster, T. K. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases. Nat. Chem. 10, 770–775 (2018).
Gray, H. B. & Winkler, J. R. Long-range electron transfer. Proc. Natl Acad. Sci. USA 102, 3534–3539 (2005).
Hari, D. P. & König, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 50, 6688–6699 (2014).
Hagedoorn, P.-L. et al. The effect of substrate, dihydrobiopterin, and dopamine on the EPR spectroscopic properties and the midpoint potential of the catalytic iron in recombinant human phenylalanine hydroxylase. J. Biol. Chem. 276, 22850–22856 (2001).
Frantom, P. A., Seravalli, J., Ragsdale, S. W. & Fitzpatrick, P. F. Reduction and oxidation of the active site iron in tyrosine hydroxylase: kinetics and specificity. Biochemistry 45, 2372–2379 (2006).
Barrette, W. C. Jr., Sawyer, D. T., Fee, J. A. & Asada, K. Potentiometric titrations and oxidation-reduction potentials of several iron superoxide dismutases. Biochemistry 22, 624–627 (1983).
Schindler, C. E. M., Hollenbach, E., Mietzner, T., Schleifer, K.-J. & Zacharias, M. Free energy calculations elucidate substrate binding, gating mechanism, and tolerance-promoting mutations in herbicide target 4-hydroxyphenylpyruvate dioxygenase. Prot. Sci. 28, 1048–1058 (2019).
Fritze, I. M. et al. The crystal structures of Zea mays and Arabidopsis 4-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 134, 1388–1400 (2004).
Brownlee, J. M., Johnson-Winters, K., Harrison, D. H. T. & Moran, G. R. Structure of the ferrous form of (4-hydroxyphenyl)pyruvate dioxygenase from Streptomyces avermitilis in complex with the therapeutic herbicide, NTBC. Biochemistry 43, 6370–6377 (2004).
Blasiak, L. C. & Drennan, C. L. Structural perspective on enzymatic halogenation. Acc. Chem. Res. 42, 147–155 (2009).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Kille, S. et al. Reducing codon redundancy and screening effort of combinatorial protein libraries created by saturation mutagenesis. ACS Synth. Biol. 2, 83–92 (2013).
Tomohiko, F., Nobuki, K., Izumi, I., Yoshiyuki, M. & Minoru, U. Synthesis of Staudinger-type molecular probe for catch-and-release purification of the binding protein for potassium isolespedezate, a leaf-closing substance of leguminous plant. Chem. Lett. 37, 52–53 (2008).
Xu, J. et al. Synthesis and reactivity of 6,7-dihydrogeranylazides: reagents for primary azide incorporation into peptides and subsequent Staudinger ligation. Chem. Biol. Drug Des. 68, 85–96 (2006).
Acknowledgements
Financial support was provided by the Johns Hopkins University (to X.H.) and by National Institutes of Health (NIH) GM R01-125924 (to Y.G.). This work was also supported by the Generalitat de Catalunya AGAUR 2021SGR00623 project (to M.G.-B.), the Spanish MICINN (Ministerio de Ciencia e Innovación) PID2022-141676NB-I00 and TED2021-130173B-C42 projects, RYC 2020-028628-I grant (to M.G.-B) and the Spanish MIU (Ministerio de Universidades) pre-doctoral fellowship FPU18/02380 (to J.S.). Part of the computational resources used were funded by the European Research Council (ERC) under the European Union’s ERC-StG-2015 (grant agreement no. 679001), as well as by FEDER (Fondo Europeo de Desarrollo Regional) and Spanish MINECO (Ministerio de Economía, Comercio y Empresa) through projects CTQ2014-54306-P, CTQ2015-69363-P and MPCUdG2016/096.
Author information
Authors and Affiliations
Contributions
X.H. and J.R. conceived the project and designed the experiments. J.R. performed initial screening and directed evolution experiments and results analysis. X.M. and J.R. performed substrate scope study. Y.G. and J.C.P. conducted the electron paramagnetic resonance studies. M.G.-B. directed the computational modelling studies. J.S. performed density functional theory calculations and MD simulation studies under the guidance of M.G.-B. X.H. and J.R. wrote the manuscript with input from all other authors, M.G.-B. wrote the computational section.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent application covering radical C–N3 and C‒SCN bond formation has been filed through the Johns Hopkins University with X.H. and J.R. as inventors (PCT/US2023/022431). The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Qi Wu, Wei Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–6, Figs. 1–11 and Notes 1–13.
Supplementary Data 1
Coordinates from MD simulations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rui, J., Mu, X., Soler, J. et al. Merging photoredox with metalloenzymatic catalysis for enantioselective decarboxylative C(sp3)‒N3 and C(sp3)‒SCN bond formation. Nat Catal 7, 1394–1403 (2024). https://doi.org/10.1038/s41929-024-01257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01257-7