Abstract
The distinctive colour of brown adipose tissue (BAT) is attributed to its high content of haem-rich mitochondria. However, the mechanisms by which BAT regulates intracellular haem levels remain largely unexplored. Here we demonstrate that haem biosynthesis is the primary source of haem in brown adipocytes. Inhibiting haem biosynthesis results in an accumulation of the branched-chain amino acids (BCAAs) valine and isoleucine, owing to a haem-associated metabolon that channels BCAA-derived carbons into haem biosynthesis. Haem synthesis-deficient brown adipocytes display reduced mitochondrial respiration and lower UCP1 levels than wild-type cells. Although exogenous haem supplementation can restore intracellular haem levels and mitochondrial function, UCP1 downregulation persists. This sustained UCP1 suppression is linked to epigenetic regulation induced by the accumulation of propionyl-CoA, a byproduct of disrupted haem synthesis. Finally, disruption of haem biosynthesis in BAT impairs thermogenic response and, in female but not male mice, hinders the cold-induced clearance of circulating BCAAs in a sex-hormone-dependent manner. These findings establish adipose haem biosynthesis as a key regulator of thermogenesis and sex-dependent BCAA homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data and uncropped scans for all western blots are included in the Source Data files. RNA-seq data have been deposited in GEO under accession number GSE289295. Proteomics data have been deposited in PRIDE under accession number PXD060812. Primers and gRNA sequences are provided in the Supplementary Information. Source data are provided with this paper.
Code availability
All coding packages used in this study are described in the Methods and are available from the corresponding author upon request.
References
Pontzer, H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021).
Cypess, A. M. et al. Identification and Importance of brown adipose tissue in adult humans. New Engl. J. Med. 360, 1509–1517 (2009).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Li, X. et al. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. Geroscience 2495–2510 (2023).
Miller, R. A., Li, X. & Garcia, G. Aging rate indicators: speedometers for aging research in mice. Aging Biol. https://doi.org/10.59368/agingbio.20230003 (2023).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Yuko, O. O. & Saito, M. Brown fat as a regulator of systemic metabolism beyond thermogenesis. Diabetes Metab. J. 45, 840–852 (2021).
Newgard, C. B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Lotta, L. A. et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 13, e1002179 (2016).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
Green, C. L. et al. Dietary restriction of isoleucine increases healthspan and lifespan of genetically heterogeneous mice. Cell Metab. 35, 1976–1995.e6 (2023).
Yu, D. et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 33, 905–922.e6 (2021).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Zhou, M. et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68, 1730–1746 (2019).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Bornstein, M. R. et al. Comprehensive quantification of metabolic flux during acute cold stress in mice. Cell Metab. 35, 2077–2092.e6 (2023).
Verkerke, A. R. P. et al. BCAA-nitrogen flux in brown fat controls metabolic health independent of thermogenesis. Cell 187, 2359–2374.e18 (2024).
Fiorito, V. et al. The heme synthesis-export system regulates the tricarboxylic acid cycle flux and oxidative phosphorylation. Cell Rep. 35, 109252 (2021).
Mistretta, M. et al. Flvcr1a deficiency promotes heme-based energy metabolism dysfunction in skeletal muscle. Cell Rep. 43, 113854 (2024).
Yin, L. et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789 (2007).
Hira, S., Tomita, T., Matsui, T., Igarashi, K. & Ikeda‐Saito, M. Bach1, a heme‐dependent transcription factor, reveals presence of multiple heme binding sites with distinct coordination structure. IUBMB Life 59, 542–551 (2007).
Galmozzi, A. et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 576, 138–142 (2019).
Moreno-Navarrete, J. M. et al. Heme biosynthetic pathway is functionally linked to adipogenesis via mitochondrial respiratory activity. Obesity (Silver Spring) 25, 1723–1733 (2017).
Dailey, H. A. & Medlock, A. E. A primer on heme biosynthesis. Biol. Chem. 403, 985–1003 (2022).
Bishop, D. F., Henderson, A. S. & Astrin, K. H. Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome. Genomics 7, 207–214 (1990).
Rajagopal, A. et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453, 1127–1131 (2008).
Duffy, S. P. et al. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol. Cell. Biol. 30, 5318–5324 (2010).
Shayeghi, M. et al. Identification of an intestinal heme transporter. Cell 122, 789–801 (2005).
Kumar, N. et al. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology 151, 3015–3025 (2010).
Rahbani, J. F. et al. Parallel control of cold-triggered adipocyte thermogenesis by UCP1 and CKB. Cell Metab. 36, 526–540.e7 (2024).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
White, C. et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 17, 261–270 (2013).
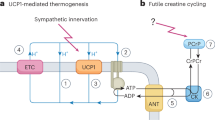
Roesler, A. & Kazak, L. UCP1-independent thermogenesis. Biochem. J. 477, 709–725 (2020).
Bertholet, A. M. et al. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab. 25, 811–822.e4 (2017).
Yin, L. et al. Rev-Erb⍺, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789 (2007).
Gerhart-Hines, Z. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 (2013).
Trefely, S., Lovell, C. D., Snyder, N. W. & Wellen, K. E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 38, 100941 (2020).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Sun, W., Dong, H. & Wolfrum, C. Local acetate inhibits brown adipose tissue function. Proc. Natl Acad. Sci. USA 118, e2116125118 (2021).
Sun, W. et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Trefely, S. et al. Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation. Mol. Cell 82, 447–462.e6 (2022).
Reszko, A. E. et al. Assessing the reversibility of the anaplerotic reactions of the propionyl-CoA pathway in heart and liver. J. Biol. Chem. 278, 34959–34965 (2003).
Barja de Quiroga, G. et al. Effect of cold acclimation on GSH, antioxidant enzymes and lipid peroxidation in brown adipose tissue. Biochem. J. 277, 289–292 (1991).
Sustarsic, E. G. et al. Cardiolipin synthesis in brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metab. 28, 159–174.e11 (2018).
Okano, S. et al. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells 15, 77–89 (2010).
Kazak, L. et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl Acad. Sci. USA 114, 7981–7986 (2017).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Davis, K. E. et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2, 227–242 (2013).
Dakin, R. S., Walker, B. R., Seckl, J. R., Hadoke, P. W. & Drake, A. J. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int. J. Obes. 39, 1539–1547 (2015).
Nookaew, I. et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J. Clin. Endocrinol. Metab. 98, E370–E378 (2013).
Manfredi, L. H., Zanon, N. M., Garófalo, M. A., Navegantes, L. C. C. & Kettelhut, I. C. Effect of short-term cold exposure on skeletal muscle protein breakdown in rats. J. Appl. Physiol. 115, 1496–1505 (2013).
Imai, D. et al. Association of elevated serum branched-chain amino acid levels with longitudinal skeletal muscle loss. J. Endocr. Soc. 8, bvad178 (2024).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Tai, E. S. et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian–Indian men. Diabetologia 53, 757–767 (2010).
Huffman, K. M. et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32, 1678–1683 (2009).
Lian, K. et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64, 49–59 (2015).
McCormack, S. E. et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 8, 52–61 (2013).
Tirosh, A. et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 11, eaav0120 (2019).
Rogers, N. H. et al. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168 (2009).
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016).
Lettieri-Barbato, D. Redox control of non-shivering thermogenesis. Mol. Metab. 25, 11–19 (2019).
Obi, C. D., Dailey, H. A., Jami-Alahmadi, Y., Wohlschlegel, J. A. & Medlock, A. E. Proteomic analysis of ferrochelatase interactome in erythroid and non-erythroid cells. Life https://doi.org/10.3390/life13020577 (2023).
Galmozzi, A. et al. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep. 9, 1584–1593 (2014).
Acknowledgements
We thank V. Lo Sardo, C. Alexander, D. Harris, D. Lamming, M. Merrins, E. Saez and L. Kazak for critical input and discussion; J. Simcox at the University of Wisconsin–Madison for sharing immortalized brown preadipocytes; V. Lo Sardo at the University of Wisconsin–Madison School of Medicine and Public Health for sharing reagents and resources; the University of Wisconsin–Madison Genome Editing and Animal Model core for assistance with the generation of the Alas1-floxed mouse model; N. Rosenthal at The Jackson Laboratory for sharing the RNA-seq data of the Diversity Outbred mice; and A. Konopka at the University of Wisconsin–Madison School of Medicine and Public Health for support with Oroboros instrumentation. This work was supported by the National Institutes of Health (NIH) grants 1R35GM150899 (A.G.), P41GM108538 and R35MG118110 (J.J.C.), R35GM147014 (J.F.), the Wisconsin Partnership Program at the University of Wisconsin School of Medicine and Public Health grant WPP5451 (A.G.) and the DRC at Washington University P30 DK020579 (A.G.). The University of Utah Metabolomics Core is supported by U54 DK110858 (J.E.C.). D.J.D. is supported by the National Institute On Aging training grant T32AG000213; H.B. is supported by NIH NCATS awards to UW-ICTR TL1TR002375 and UL1TR002373. M.F. is supported by a postdoctoral fellowship (PF-23-1070297-01-TBE; https://doi.org/10.53354/ACS.PF-23-1070297-01-TBE.pc.gr.175370) from the American Cancer Society. Y.B. is supported by the American Heart Association predoctoral fellowship 25PRE1374479. The model in Fig. 7j was created using NIAID NIH BIOART (https://bioart.niaid.nih.gov/bioart/77) (NIAID Visual and Medical Arts, 10/7/2024, Chromatin).
Author information
Authors and Affiliations
Contributions
D.J.D. and A.G. conceived the project, designed research and analysed data. D.J.D., J.K.H. and M.F. performed in vivo experiments. D.J.D., J.K.H., N.D.C. and Y.B. carried out cell-based assays. D.J.D. and H.B. performed RNA-seq analyses. D.J.D. and S.V.J. performed metabolomic analyses. D.J.D., A.J., L.Y.C., K.A.O. and E.S. prepared samples and carried out proteomic studies. M.P.K. and A.G. performed correlation expression analysis. D.J.D., J.L.C., Q.P., A.G. and J.E.C. designed and performed isotope tracing experiments. R.A.A., V.L.C., A.D.A., J.J.C. and J.F. provided advice and reagents. D.J.D. and A.G. wrote the paper and integrated comments from the other authors.
Corresponding author
Ethics declarations
Competing interests
J.J.C. is a consultant for Thermo Fisher Scientific, 908 Devices and Seer. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Expression analysis of heme acquisition pathways in differentiating adipocytes and tissues.
a) Schematic of the eight-step mammalian heme biosynthesis pathway. ALAS1 serves as the rate limiting enzyme. Succinylacetone (SA) is a competitive inhibitor of ALAD/PBGS. Substrates (black), enzymes (red) and subcellular ___location (gray). Eight molecules each of succinyl-CoA and glycine are required for the synthesis of one molecule of heme. b) mRNA relative expression of Alas1 in various mouse tissues collected from 18-week-old wild type male (n = 3) and female (n = 3) mice. c) Log2 normalized counts of mRNA of genes within the heme biosynthesis pathway in differentiating primary white (yellow), brown (black), or immortalized brown (blue) adipocytes (n = 3 biological replicates per cell type per time point). d) Expression of Alas2 was not detected throughout differentiation in any adipocyte line (n = 3 biological replicates per cell type per time point). e) mRNA counts of putative heme transporters in differentiating adipocytes as described in c (n = 3 biological replicates per cell type per time point). Data shown as mean ± SD. * p < 0.05 vs day 0 of differentiation of pBAT (black stars), pWAT (orange stars), or immBAT (blue stars), respectively. # p < 0.05 for pBAT (black hashes) and immBAT (blue hashes) vs same time point of pWAT; one-way ANOVA with multiple comparisons and a Tukey’s post-test.
Extended Data Fig. 2 Chemical disruption of heme acquisition represses the thermogenic profile without impairing adipocyte differentiation.
a) mRNA relative expression of adipogenic genes and Alas1 in primary brown adipocytes differentiated in the presence of vehicle, succinylacetone (SA), heme-depleted serum (HDS), or SA + HDS (n = 3 biological replicates per condition). b) Primary brown adipocytes treated as described in a have comparable capacity for adipogenic differentiation. Nile red (red) and Hoechst (blue) used to stain lipid droplets and nuclei, respectively. Scale bar = 100 μm. c) Volcano plot of global protein abundance in primary brown adipocytes treated with SA relative to vehicle (n = 4 biological replicates per condition). d) Bubble plot of biological pathways enriched among significantly changed genes with a log2-fold change less than -1. Data shown as mean ± SD. p values vs. FBS, one-way ANOVA with multiple comparisons and a Tukey’s post-test.
Extended Data Fig. 3 Generation of Alas1-/- immortalized preadipocytes using CRISPR-Cas9.
a) mRNA levels of Alas1 in three representative Alas1 KO clones generated using three distinct gRNAs (n = 3 biological replicates per clone). b) Pparγ expression is not impacted by Alas1 deletion. c) Ucp1 levels are significantly reduced in all Alas1 KO clones (n = 3 biological replicates per clone for a-c). d) Protein levels of Alas1 and Ucp1 confirm loss of expression in screened clones. e) The relative abundance of numerous electron transport chain proteins is significantly reduced in Alas1 KO brown adipocytes compared to WT (n = 4 biological replicates per genotype). Data shown as mean ± SD. p values vs. WT; one-way ANOVA with multiple comparisons and a Tukey’s post-test.
Extended Data Fig. 4 Exogenous heme supplementation does not compensate for loss of heme synthesis.
a) Quantification of heme in isolated nuclei and mitochondria fractions derived from WT and Alas1 KO brown adipocytes following treatment with vehicle or 10 μM HA (n = 3 biological replicates per condition). b) Changes to the global proteome are mainly explained by genotype and active heme biosynthesis (WT vs. Alas1 KO) rather than HA supplementation and differences in the intracellular heme pool (n = 4 biological replicates per condition). c, d) Quantification of intracellular heme levels and mRNA relative expression (n = 4 biological replicates per condition) of Pparγ, Alas1, and Ucp1 in WT primary brown adipocytes treated with SA, HA, or both throughout differentiation. e) Alas1 and Ucp1 protein levels in primary brown adipocytes treated with SA, HA (1 or 10 μM), or both throughout differentiation. f) In contrast to inhibition of heme synthesis (SA), exogenous heme supplementation has limited impact on gene expression in primary brown adipocytes (n = 4 biological replicates per condition). g) ATP turnover is partially restored by 10 μM HA supplementation in Alas1 KO brown adipocytes (n = 8 biological replicates per condition). h) The contribution of the futile creatine cycle to maximal respiration is higher in Alas1 KO adipocytes treated with HA compared to WT (n = 4 biological replicates per condition). i, j) Pharmacological modulation of Rev-Erbα activity does not rescue Ucp1 expression in Alas1 KO adipocytes (n = 3 biological replicates). Data are shown as mean ± SD. p values vs. WT; one-way ANOVA with multiple comparisons and a Tukey’s post-test (a, c, d, g, h), two-tailed FDR-adjusted t-test (b), Wald test (f), and two-way ANOVA with multiple comparisons and a Tukey’s post-test (j).
Extended Data Fig. 5 Alas1 substrates and their precursors accumulate upon heme biosynthesis blockade.
a, b) Relative abundance of Alas1 substrates and their metabolic precursors in differentiated WT and Alas1 KO brown adipocytes (a; n = 4 biological replicates per genotype) and primary brown adipocytes treated with vehicle or SA throughout differentiation (b; n = 3 biological replicates). Data shown as mean ± SD. p values vs. WT or vehicle; multiple two-tailed Student’s t-test.
Extended Data Fig. 6 BCAAs fuel heme synthesis in brown adipocytes.
a) Adipose, liver, and skeletal muscle correlation of Alas1 expression with genes involved in BCAA catabolism. b) Enrichment of propionate-derived carbon labeling of TCA metabolites (n = 3 biological replicates). c) Representative images and quantification of proximity ligation assay (PLA) foci for ALAS1 and PCCA in Alas1 KO brown adipocytes (n = 30 individual cells per genotype). Scale bar = 5 μm. Data are shown as mean ± SD. p values vs. WT, one-tailed Student’s t-test.
Extended Data Fig. 7 Alas2 does not compensate for the loss of Alas1 in BAKO BAT.
a) mRNA relative expression of Alas2 in red blood cells (RBC) relative to BAT collected from female WT (n = 4) and BAKO (n = 4) mice housed at thermoneutrality. b) Protein levels of ALAS1 and ALAS2 in protein lysates from red blood cells (RBC) and PBS-perfused BAT collected from female WT and BAKO mice as described in a. Data are shown as mean ± SEM. p values vs. WT; one-way ANOVA with multiple comparisons and a Dunnett’s post-test.
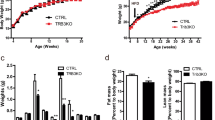
Extended Data Fig. 8 BAKO mice have normal development and metabolic physiology at thermoneutrality.
a) Female (n = 16 WT, 18 BAKO) and male (n = 7 WT, 8 BAKO) BAKO mice show no significant differences in body weight compared to WT when fed standard chow and housed at thermoneutrality. b) Tissue mass of brown (BAT), inguinal (iWAT), epididymal (eWAT) white adipose, and liver are similar in WT and BAKO females (n = 7 per genotype) and males (n = 5 WT, 7 BAKO). c) Oral glucose tolerance test (OGTT) reveals no differences between WT and BAKO females (n = 6 WT, 11 BAKO) and males (n = 7 WT, 8 BAKO). d) Insulin tolerance test (ITT) shows no significant differences between WT and BAKO females (n = 20 WT, 24 BAKO) or males (n = 6 WT, 8 BAKO). e, f) Female and male indirect calorimetry measurements of O2 consumption (VO2), CO2 production (VCO2), respiratory exchange ratio (RER), and energy expenditure (EE) in WT and BAKO males and females (n = 6 per group) housed at thermoneutrality. Data are shown as mean ± SEM. Mixed effects analysis with Šídák’s multiple comparisons test (a), unpaired two-tailed t-test for tissue weights and reverse AUC, two-way ANOVA followed by Šídák’s multiple comparisons test for OGTT and IGTT.
Extended Data Fig. 9 Altered molecular signatures of BAKO BAT at thermoneutrality.
a) Volcano plot of differentially expressed genes in BAT collected from chow-fed female WT and BAKO (n = 4 per group) mice housed at thermoneutrality. b) Volcano plot of differentially abundant proteins in BAT collected from female WT and BAKO (n = 7 per group) mice. c) Heatmap of differentially expressed genes (p < 0.05; |log2-FC | > 0.5) in BAT from WT and BAKO mice (n = 4 per group) as described in a.
Extended Data Fig. 10 Diet-induced thermogenesis is not impaired in BAKO mice housed at thermoneutrality.
a, b) Indirect calorimetry in female (n = 10 WT, 10 KO) and male (n = 7 WT, 8 BAKO) mice reveals no significant differences in diet-induced thermogenesis between WT and BAKO mice. Data are shown as mean ± SEM or Min to Max values (RER).
Supplementary information
Supplementary Information
Supplementary Fig. 1
Supplementary Tables 1–4
Table 1: Reagent/chemical information. Table 2: Antibody-based assay information including dilution factors used for western blots. Table 3: Oligo sequences used for cloning into PX459 plasmid backbone for CRISPR targeting of Alas1 gene. Table 4: PCR and qPCR primer sequences.
Source data
Source Data Fig. 1
Excel file contains statistical source data.
Source Data Fig. 1
PDF contains blot and image source data.
Source Data Fig. 2
Excel file contains statistical source data.
Source Data Fig. 2
PDF contains blot and image source data.
Source Data Fig. 3
Excel file contains statistical source data.
Source Data Fig. 3
PDF contains blot source data.
Source Data Fig. 4
Excel file contains statistical source data.
Source Data Fig. 4
Excel file contains statistical source data.
Source Data Fig. 5
PDF contains image source data.
Source Data Fig. 6
Excel file contains statistical source data.
Source Data Fig. 6
PDF contains image source data.
Source Data Fig. 7
Excel file contains statistical source data.
Source Data Fig. 7
PDF contains blot and image source data.
Source Data Extended Data Fig./Table 1
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 2
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 2
PDF contains image source data.
Source Data Extended Data Fig./Table 3
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 3
PDF contains blot source data.
Source Data Extended Data Fig./Table 4
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 4
PDF contains blot source data.
Source Data Extended Data Fig./Table 5
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 6
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 6
PDF contains image source data.
Source Data Extended Data Fig./Table 7
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 7
PDF contains blot source data.
Source Data Extended Data Fig./Table 8
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 9
Excel file contains statistical source data.
Source Data Extended Data Fig./Table 10
Excel file contains statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duerre, D.J., Hansen, J.K., John, S.V. et al. Haem biosynthesis regulates BCAA catabolism and thermogenesis in brown adipose tissue. Nat Metab 7, 1018–1033 (2025). https://doi.org/10.1038/s42255-025-01253-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-025-01253-6