Abstract
Forest-based carbon sequestration projects incentivize reforestation and restoration activities while offering opportunities to realize co-benefits such as biodiversity conservation. While conservation aspects are increasingly emphasized in these projects, the rigor of biodiversity co-benefit verification has been highly variable. Recent advances in biodiversity monitoring based on shed DNA in the environment (eDNA) offer promise for improving effectiveness, standardization, and transparency. Here we analyze 129 forest carbon projects and 396 peer-reviewed studies to identify how biodiversity co-benefits are currently verified within forest carbon markets, and to evaluate the potential of eDNA for tracking biodiversity change. Our analysis revealed that eDNA studies focused more on smaller organisms (microbes and invertebrates) and on temperate ecosystems compared with biodiversity-focused forest carbon projects. Efforts to align these two worlds via investments into broadening the geographic and taxonomic scope could allow greater adoption and increased accountability in biodiversity monitoring within forest carbon markets (i.e. standardized, auditable biodiversity data trails). Adapting advancements in eDNA technology to the biodiversity monitoring needs of nature-based initiatives will aid countries and organizations striving to meet global conservation commitments.
Similar content being viewed by others
Introduction
Forest-based carbon (FC) sequestration projects are the primary nature-based solution (NbS) for climate change mitigation1,2 and potentially offer multiple co-benefits, including supporting biodiversity conservation3. However, FC projects have inconsistently documented and certified biodiversity co-benefits4,5,6, resulting in calls for greater standardization and effectiveness7,8,9,10. Technological innovation offers promising new tools for scalable, standardized, and auditable biodiversity monitoring within these projects11,12,13. Techniques that identify the presence of organisms based on shed genetic material—notably environmental DNA or eDNA—are especially promising14,15, yet their use within FC markets remains largely unexplored.
Land-use changes, particularly deforestation, are the primary drivers of terrestrial biodiversity loss16. In response, forest restoration, management, and conservation (i.e., avoided conversion) are often counted upon to help minimize these losses7,17. Recently, privately funded projects, relying on similar approaches but focused on sequestering or conserving forest carbon stocks, have emerged5,18. While the synergies between FC projects and biodiversity conservation are readily apparent19, there is an increased focus on validating and verifying the purported co-benefits4,5,10,20. The recent emphasis placed on high-quality carbon credits18,21, and on monitoring outcomes within nature-based climate solutions7,9, suggests that expectations around quantifying biodiversity co-benefits will continue to increase.
While efforts to track and certify biodiversity benefits within FC projects have improved over time, they can still lack rigor4,5,10. To achieve biodiversity certification within an FC scheme, a project typically must demonstrate a net positive impact on biodiversity (Table 1). However, the associated criteria to support these certifications vary across programs20,22,23 and the indicators and associated monitoring programs are often viewed as inadequate4,6,9,22,24. Improving standardization, transparency, and monitoring rigor helps ensure positive biodiversity outcomes, and supports the long-term viability of biodiversity certification schemes4,5,21.
Emerging technology for biodiversity monitoring—including advancements in the utilization of aerial imagery, LiDAR, acoustic recordings, and eDNA—appear well-suited to support biodiversity-based certification within FC markets11,13,24,25. eDNA-based methods, which detect traces of genetic material left in the environment, are especially promising; they offer broad taxonomic reach, straightforward field sampling, and efficiencies at scale14,15,26. A wide variety of taxa, from microbes to vertebrates, can be simultaneously identified from a single field sample (e.g., water, soil, air, or surface swabs), permitting holistic insights into biodiversity without the bespoke monitoring typically needed for any individual taxa6,14. Such high-throughput species determination—often achieved using metabarcoding, the mass sequencing of short DNA fragments found within samples—can also lead to cost efficiencies by supplanting labor-intensive morphological approaches to species identification27,28. Both metabarcoding and single species eDNA approaches (e.g., qPCR, ddPCR) have already revolutionized biodiversity monitoring and management in aquatic habitats14,15,29 and are increasingly being applied in terrestrial habitats to enhance detectability of rare species30 and to monitor conservation outcomes31. In the context of FC markets, eDNA-based methods produce an auditable data trail that serves as a permanent record of species detected at a surveyed site32,33,34,35. Because of these benefits, there is growing interest for incorporating eDNA-based methods into national and global biodiversity monitoring schemes, many of which leverage local capacity for implementation25,26,36,37.
The breadth of eDNA-based methods available to monitor terrestrial above-ground biodiversity is now extensive28,30,35,38,39. As a result, there is value in considering how eDNA-based monitoring can be integrated into FC projects. In forested ecosystems, eDNA-based biodiversity surveys have been used to monitor specific species30,39,40 and taxonomic assemblages such as mammals28,40,41,42, birds34, amphibians27, arthropods32,35,43,44, fungi33, and plants45,46. In parallel, there is also a growing body of literature, highly relevant to FC projects, on the application of eDNA to track forest restoration progress. These studies chart the recovery of plants, fungi, bacteria, arthropods, and vertebrates post-restoration32,33,34,35,47,48.
Despite the potential benefits, implementation of eDNA within FC projects has been limited49. Best practices are emerging for eDNA-based biodiversity monitoring within forests and other ecosystems25,30,31. At the same time, guidelines for biodiversity monitoring within forest carbon projects have matured, with recommendations on sampling strategies, taxonomic focus, and field methods24,50,51. However, there is a notable absence of recommended best practices for marrying eDNA and biodiversity monitoring within FC projects. Here we conduct a systematic analysis to ascertain how biodiversity is currently monitored within FC projects, focusing on those that explicitly certify biodiversity co-benefits. We conduct a parallel analysis of the literature to identify how and where eDNA-based methods are used to monitor impacts of management or restoration on biodiversity. We use a broad definition of eDNA that includes mixed samples of trap-collected specimens as these studies involve similar molecular methods and often serve similar environmental monitoring purposes. For each pool of literature, we document which taxonomic groups are monitored, their ___location, and which methods were used. Through data synthesis techniques, we highlight the opportunities and challenges for applying eDNA as a tool to track biodiversity within FC projects. Our analysis revealed that the use of eDNA is currently rare within FC projects; and that peer-reviewed eDNA studies occur more within temperate ecosystems and focus more on smaller organisms compared with biodiversity-focused FC projects. We provide recommendations that could allow greater adoption of eDNA and increased accountability in biodiversity monitoring within FC markets.
Results
We located 72 peer-reviewed studies pertaining to FC markets and biodiversity that met our review criteria (Table 2, Supplementary Fig. 1). Of those, 7 (10%) had an explicit focus on biodiversity monitoring practices and reported either the empirical findings of biodiversity monitoring (arthropods49,52, mammals49, or plants53,54), the relative merits of different monitoring methodologies6,24, or reviewed bigger-picture approaches4. Most studies covered the effectiveness of FC project activities towards conservation (74%), while some discussed optimizing the geographic distribution of FC projects for biodiversity conservation (19%) or addressed potential negative biodiversity consequences of FC projects (17%).
We tallied 1323 FC projects across the five dominant registries on the voluntary market (Verra, American Carbon Registry, Climate Action Reserve, PlanVivo, and Gold Standard) totaling ~76.3 million ha (Supplementary Note 1). Of these, 451 projects (33.0 million ha) were identified as explicitly verifying biodiversity co-benefits under one of four standards (listed in Table 1). Of these 451 FC projects, 129 (totaling 7.0 million ha) met our review inclusion criteria (Tables 1 and 2).
Of the 324 peer-reviewed eDNA studies that met our review inclusion criteria (Table 2, Supplementary Fig. 2), 95% involved eDNA metabarcoding; 5% involved single species assays (e.g., qPCR); and 4% involved eRNA metabarcoding. Forty-one percent involved terrestrial habitats, such as forests (21%), grasslands (10%), and others such as caves or disturbed lands (10%). The most common biodiversity impacts assessed using eDNA in forested habitats were changes in habitat structure (78%), species interactions (10%), pollutants (6%), and other abiotic factors (e.g., salinity, temperature; 6%). Below, we compare the current state of eDNA-based biodiversity monitoring with the monitoring activities occurring within FC projects that verify biodiversity benefits.
Taxonomic scope
Of the 129 biodiversity-certifying FC projects analyzed (Table 1), 75% reported some on-the-ground monitoring of animal populations; the others relied on forest cover measurements, community education, or surveillance against illegal activities to support claims of animal biodiversity enhancement. Among the 97 projects that monitored animal populations, most covered birds (84%), mammals (70%), reptiles (23%), amphibians (18%), invertebrates (18%), and fish (7%) (Figs. 1 and 2). One FC project monitored fungi, while none monitored protists or bacteria. While all FC projects assessed trees for carbon, 74% of projects discussed native forest communities (beyond carbon value), including only a few that discussed understory species (e.g., shrubs, herbs). The number of taxonomic groups studied per project ranged from 1 to 6 (median: 3; Fig. 3).
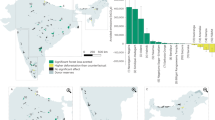
Comparison of taxonomic scope, geographic distribution, and methodological rigor of biodiversity monitoring within forest carbon (FC) projects (n = 129) and terrestrial eDNA studies (n = 134). In the top row, icons represent taxonomic groups monitored: plants, mammals, birds, amphibians, reptiles, fish, invertebrates, fungi, prokaryotes, and protists (icon size is proportional to the % of FC projects or eDNA studies). Fish were detected incidentally in some terrestrial eDNA studies. The maps in the middle row show locations of FC projects (left) and eDNA studies (right) included in this analysis. In the bottom row, the % of FC projects or eDNA studies meeting four different criteria of methodological rigor and transparency are shown. The icons in the bottom row represent the different biodiversity survey methods used, shown in decreasing order of commonness from top to bottom: formal visual observation surveys, incidental observations, camera traps, conventional traps, bioacoustics, and eDNA-based methods. Organism images from www.phylopic.org are used under Creative Commons licenses (see section “Acknowledgements”).
Taxonomic focus of biodiversity monitoring within forest carbon (FC) projects and peer-reviewed studies employing eDNA-based methods to study ecological impact in terrestrial environments. The bars show the percent of FC projects that verify biodiversity benefits (n = 129; left plot) or peer-reviewed eDNA studies (n = 134; right plot) that reported monitoring each taxonomic group listed on the y-axis.
The number of taxonomic groups monitored by environmental DNA (eDNA) studies (red symbols) or forest carbon (FC) projects (green symbols) in relation to the number of genomic regions used (eDNA studies only; left plot) or the number of field methods employed (right plot). For eDNA studies, different eDNA collection substrates were considered different field methods. Field methods for FC projects and taxonomic categories are listed in Fig. 1. Six FC projects that did not specify which field methods were used are excluded from the plot (three each from PlanVivo and Gold Standard). Individual data points are shown as open circles; boxes show the median, upper, and lower quartiles; whiskers represent the range of data not considered to include outliers (i.e., within 1.5× the interquartile range).
In contrast to taxa monitored within FC projects, eDNA studies in terrestrial habitats focused on fungi (51% of 134 studies), prokaryotes (40%), invertebrates (30%), protists (9%), and plants (7%), with fewer studies involving mammals (4%), reptiles (2%), and birds (1%; Figs. 1 and 2). Most studies monitored one taxonomic group (median: 1), while the maximum number monitored was 7 (Fig. 3). The fraction of eDNA studies that monitored vertebrates increased from ~5% in 2013 to over 20% in 2023 (binomial GLM; p = 0.03; Fig. 4).
The x-axis in both graphs represents the year published, while the y-axis is the percent of eDNA studies analyzed. The left panel shows the empirical % of eDNA studies involving vertebrates in different year intervals (sample size shown in gray at the base of each bar). The right panel shows the model-predicted values along with 95% confidence intervals (error bars) from a binomial (logistic) GLM model describing the increase.
Geographic distribution
Most (86%) of the 129 biodiversity-certifying FC projects (Table 1) were in tropical or sub-tropical regions, particularly Central and South America (34%), Asia (31%), and Africa (17%; Fig. 1). The remainder were in temperate areas of Asia (11%), North America (2%), Europe (1%), or Oceania (1%; Fig. 1). Most of the peer-reviewed studies on biodiversity monitoring within FC projects (83% of 41) were also in tropical or sub-tropical locations (Africa, the Americas, Asia, and Oceania); the remainder were in temperate Europe, Oceania, and North America.
In contrast, only 32% of peer-reviewed eDNA biodiversity monitoring studies we analyzed were conducted in tropical and sub-tropical areas, mainly in Asia (14%), Oceania (7%), or Central and South America (6%; Fig. 1). The majority (68%) were in temperate or arctic areas, notably in Europe (42%), North America (12%), Oceania (6%), and Asia (5%; Fig. 1).
Methodological rigor and transparency
FC project reporting varied in methodological rigor and transparency across the biodiversity certification programs we included in our analysis (Table 1, Fig. 5). Of the 97 FC projects that monitored animal populations, 72% employed a replicated sample-based survey design, 22% relied only on unstandardized observations (mostly community-derived), while 6% did not provide sufficient information for us to assess their methodology (Fig. 5). The most common forms of standardized animal monitoring were visual plot or transect surveys (49%), followed by camera traps (15%), physical traps or mist nets (7%), bioacoustics (2%), or eDNA-based mark-recapture (two studies that focused on elephants). Most projects used two survey methods (median: 2), but the maximum number of survey methods employed in a single FC project was six (Fig. 3).
Comparison of methodological and reporting aspects of animal biodiversity monitoring within forest carbon (FC) projects from three biodiversity co-benefit verification standards: the Climate, Community, and Biodiversity (CCB) Standard, PlanVivo, and Gold Standard. A fourth, the Sustainable Development Verified Impact Standard (SD VISta), is not shown as it was represented by only three projects, and none reported performing animal monitoring. The bars show the % of projects meeting the methodological or reporting criteria listed on the y-axis. Sample size for all bars is the total number of projects: CCB, n = 80; PlanVivo, n = 26; Gold Standard, n = 20.
Of the 70 FC projects that performed standardized animal surveys, only 69% reported sample sizes (37% of all projects; Figs. 1 and 5). Mean sample size was 21 for visual observations, 27 for camera traps, and 34 for conventional traps; no sample sizes were provided for the few acoustic or DNA-based monitoring efforts. Only one project reported estimates of uncertainty (e.g., error bars) around a measure of population abundance, while none reported uncertainty surrounding community metrics (e.g., species richness or diversity). Information noted as missing may exist in unpublished reports, which were mentioned in the documentation reports for several projects (n = 28), but that we found were not accessible online. Aside from aggregate species lists, only five projects made raw abundance or diversity data available (one for animals, four for plants; Figs. 1 and 5).
Nearly all the eDNA studies that we analyzed (323 of 324) employed a replicated survey design, with a median sample size of 50 (mean: 97; range: 2–1724). The most common environmental substrate sampled in terrestrial eDNA studies was soil (62% of 134 studies), followed by vegetation surfaces (3%), water (1%), and air (1%). Metabarcoding of trap-collected arthropods was also common (18%), as were studies of organisms’ diet (10%) or microbiome (10%). Thirty percent of studies targeted more than one genomic region, which generally increased the taxonomic diversity of organisms documented (Fig. 3). Most eDNA studies (72%) made raw or processed data available via public repositories, mainly those hosted by the National Center for Biotechnology Information, European Bioinformatics Institute, or Dryad digital repository (Fig. 1).
Discussion
Our analysis illuminates the current state and a potential future for eDNA in biodiversity monitoring within the voluntary forest carbon (FC) market. We confirmed that biodiversity monitoring and reporting are highly variable across projects and certifying programs, and often lack rigor and transparency. The detailed picture we provide complements previous efforts, which were mainly higher-level overviews20,22 or limited to single species and certifying programs6; no other studies have considered the potential role of eDNA within FC markets. Our analysis revealed stark taxonomic and geographic differences between biodiversity monitoring within peer-reviewed eDNA studies and FC projects. While the use of eDNA is currently rare within FC projects, the literature compiled for our analysis collectively suggests considerable potential for its inclusion, notably the many examples we found of successful forest biodiversity monitoring using eDNA. Below, we synthesize this potential, highlighting gaps that must be addressed to facilitate broad-scale use of eDNA monitoring in FC projects.
The ability to monitor a wide range of taxonomic groups simultaneously using a single field method is one of the most attractive features of eDNA-based monitoring14,15. Our analysis revealed that numerous studies already employ eDNA metabarcoding approaches to document success of forest restoration32,33,34,35,47,48. These studies could directly inform the approaches taken in FC projects in terms of documenting biodiversity changes in response to reforestation or carbon-focused forest management. Below we explore this in greater detail for major taxonomic groups.
Native plant diversity provides insights into forest health and is a common indicator of restoration success55,56. While FC projects do include plant surveys, most are focused on carbon accounting and do not quantify diversity of non-tree species. Taking a more holistic approach to plant community monitoring could be beneficial for biodiversity certification within FC projects. eDNA-based monitoring of plant diversity is still relatively uncommon45 (Fig. 2), however, the broad-spectrum monitoring it facilitates (e.g., via herbivore scat, pollen, water, or soil samples) shows great promise35,45,46. Incorporating eDNA-based monitoring into vegetation surveys may prove particularly useful within species-rich tropical forests where morphological identification is difficult. eDNA-based monitoring could also be beneficial within temperate forests if time or cost savings can be realized relative to visual vegetation sampling45. However, seed and pollen dispersal strategies could result in DNA transport from distant locations, potentially confounding datasets and interpretation; and the development of DNA reference databases for plants has lagged that of other groups46. These aspects limit the broader utility and adoption of eDNA. Thus, fundamental research is still needed to improve robustness and build out reference databases to realize the full potential of eDNA to monitor trends in plant biodiversity.
Most forest-based eDNA studies focused on fungi, protists, and bacteria (Figs. 1 and 2). This focus is likely because eDNA overcomes the challenges of conventional sampling for these groups47,48. While microbial monitoring is rare within FC projects, they may be beneficial to monitor as these taxa are sensitive to environmental changes and provide essential ecosystem services47. As the use of microbial ecological indicators continues to develop57, biodiversity reporting within FC projects could be adapted to include these aspects, resulting in a more nuanced understanding of ecosystem functioning currently absent from FC biodiversity co-benefit schemes (e.g., nutrient cycling, forest pathogens).
Terrestrial invertebrates are common monitoring targets within FC projects49,52 (Fig. 2), as they are useful bioindicators58. However, monitoring using morphology-based identification methods can be challenging due to the need for diverse trapping methods, laborious specimen sorting, and considerable taxonomic expertise35,44,52. eDNA-based approaches for arthropod monitoring that span soil44,49, water59, and plant surfaces43,60 may efficiently overcome these limitations. These approaches reduce field effort as they require no trap set-up or checking, specimen sorting, or morphological identification. At least one FC project has adopted such an approach (TerraBio initiative49), demonstrating via soil eDNA metabarcoding that invertebrate communities of carbon-rich agroforestry areas resemble those of native forest. This study joins others in demonstrating the potentially practical application of non-lethal invertebrate eDNA sampling for biodiversity monitoring44.
Vertebrates are by far the dominant group monitored within existing FC projects that verify biodiversity co-benefits (Fig. 1). While terrestrial vertebrates are underrepresented within eDNA biodiversity monitoring studies, field and lab eDNA methods for these groups are developing rapidly34,40,42,61,62. For example, eDNA sampling performed similarly to camera traps and visual transect surveys for some mammals, and showed superior performance for small and nocturnal species41,63,64. A suite of eDNA collection substrates has been successfully used to monitor vertebrates, including soil41, water27,63,64, plant surfaces40,42,61, coverboards39, air38, and invertebrate parasites62. Despite the growing interest in new eDNA-based vertebrate monitoring tools, and the increasing prevalence of vertebrates within eDNA studies (Fig. 4), we identified only three FC projects employing eDNA-based methods. The TerraBio forest carbon project49 used eDNA metabarcoding to detect Amazonian mammals, using the same soil samples to also detect arthropods. Two FC projects in Cambodia used elephant scat eDNA to estimate population sizes via mark-recapture. As with aquatic systems, more research into the dynamics of DNA deposition, persistence, and transport within terrestrial systems65 is needed to facilitate more widespread use for biodiversity change detection and other applications. Nevertheless, the existence of early adopters, and the wave of recent terrestrial vertebrate eDNA research, suggests these approaches to vertebrate biodiversity monitoring should become more commonplace within FC markets.
We found that most FC projects certified as providing biodiversity benefits occurred in tropical or sub-tropical regions (86%). This pattern likely reflects recognition that these regions contain high-biodiversity value, and that FC projects can be mechanisms to support biodiversity conservation objectives19,66. The pattern may also result from regional preference for project developers to certify via certain registries (e.g., ACR and CAR projects are prevalent in North America and do not verify biodiversity co-benefits).
In contrast to the FC projects, only 32% of eDNA studies were conducted in tropical or sub-tropical biomes. While the high species richness found in the tropics complicates morphological taxonomic identification, making eDNA approaches attractive, the genetic libraries (i.e., reference libraries) used to generate species lists from eDNA are still sparse45,67. Nevertheless, there has been rapid growth in the number of published studies that have implemented eDNA-based tools to survey in tropical rainforests27,28,49,63,67. We suggest that this growth in using eDNA surveys in the tropics would accelerate with the adoption of eDNA methods for certifying biodiversity co-benefits within FC markets. Temperate FC projects also offer a pathway to demonstrate the value of eDNA to support certification of biodiversity benefits, as eDNA application is more established in these systems and biodiversity certification for temperate FC projects is relatively rare. The decreasing costs of sequencing68 and the proliferation of eDNA sample processing companies28,36 also promise to make eDNA applications more globally accessible.
The methodological rigor and transparency of biodiversity monitoring efforts varied widely among FC projects. Only 54% of projects conducted structured, replicated animal monitoring, and only 69% of those reported sample sizes. Only one project reported uncertainty (i.e., confidence intervals) while only three corrected for detection biases (e.g., distance sampling, occupancy modeling69). These design and reporting characteristics are crucial for assessing progress in biodiversity indicators69,70 as they help distinguish true change in species richness or diversity from statistical artifact (e.g., change from observer skill level or device sensitivity). Indeed, such change detection is a key motivation for the biodiversity monitoring plans required by the four FC programs analyzed23,50 (Table 1). Historically, eDNA studies cannot report on changes in species abundance14, however, advanced sampling design (e.g., occupancy modeling) may be able to overcome these limitations70. Occupancy models can integrate eDNA-based presence–absence data to generate estimates of the extent of an organism within an area, along with uncertainty in those estimates40,70. This information can be correlated with abundance, tracked over time, used to generate robust data on species’ populations and community diversity metrics40,69,70, and can be integrated with other data commonly collected for FC projects (e.g., camera traps and bioacoustics40,69,70,71). Regardless of whether eDNA-based methods are used, biodiversity monitoring within FC projects would benefit from more careful consideration of study design—including replication, uncertainty, and detection bias—to support more robust long-term assessment of biodiversity change.
Like other remote monitoring approaches (e.g., camera traps, bioacoustics) eDNA methods leave an auditable data trail13. This outcome allows for transparent accounting, a core principle of carbon offsets, but which has not been applied as rigorously to biodiversity monitoring and reporting within FC projects4,9,10. Such accessibility would allow the data to be repurposed for improved monitoring over time, and could be leveraged more broadly to support research, biodiversity conservation, and progress towards national biodiversity targets36,37. Very few FC projects openly shared biodiversity data (only 4% of projects analyzed). In contrast, the data from eDNA studies are commonly deposited in public repositories (72% of studies analyzed). Further, standardized formats for archiving and reporting eDNA-derived biodiversity data are rapidly emerging at the local, national25, and international36,37 levels, including in relation to FC projects49. Given that the biodiversity benefits of FC projects may be realized more gradually, the ability to store, re-use, and interpret data over long time periods—ideally, via openly accessible repositories—is key to increasing the integrity of FC projects50.
Conclusions
The world is increasingly looking to NbS for sustainable climate change mitigation strategies2,7, and the large-scale implementation of NbS is likely to be necessary to achieve global climate goals1. The rapid emergence of these projects, particularly those involving FC sequestration, has been associated with increased scrutiny20. Despite the continued technical challenges72, there have been improvements in the measuring, reporting, and verification of carbon in natural systems5,20,21. At the same time, the biodiversity co-benefits of FC projects are increasingly recognized and valued8,10. With less than half of FC projects we discovered (451 of 1323 projects) currently implementing formal biodiversity co-benefit verification, there is room for much wider adoption. While the magnitude of co-benefits will vary from project to project, the need to accurately assess reference biodiversity levels and change is critical to their ultimate viability. This need highlights an opportunity to integrate emerging technologies that improve transparency and accountability in measuring these co-benefits5,10,13. As eDNA-based technology matures, there is an opportunity to develop operationalized sampling protocols and provide robust, standardized, and auditable biodiversity co-benefit data, and to contribute to broader biodiversity objectives across geographic scales4,7,11,13,25. In FC markets and other NbS, a powerful approach could include joining eDNA sampling with robust modeling frameworks such as occupancy modeling, allowing cost-effective, scientifically-sound monitoring of virtually any taxa14,27,28,70. This approach would leverage the strengths of eDNA technology to provide an auditable data trail while also facilitating the involvement of local and indigenous community members, for example, in study design and data collection23,26. Regardless of the exact approach, achieving standardized and robust biodiversity monitoring at scale within forest carbon markets will require creative solutions. The flexible and powerful suite of eDNA-based methods appears well-suited to meaningfully contribute to this effort.
Methods
We explored the potential role of eDNA for biodiversity monitoring within voluntary FC projects. To do so, we conducted a three-phase systematic review of the peer-reviewed and gray literature. Following standard practices for systematic reviews73, we first used Web of Science to search the peer-reviewed literature for studies about biodiversity within FC markets (Table 2, Supplementary Fig. 1).
Second, we obtained project reports and documentation from registries associated with the four main sustainability and biodiversity co-benefit certifying programs on the voluntary FC market5,20 (Tables 1 and 2). As part of this effort, we also assessed the total size of the FC market, including projects that do not currently verify biodiversity co-benefits. We did so by accessing three other project databases (see Supplementary Note 1): Verified Carbon Standard, including only projects not co-certified by the standards in Table 1; American Carbon Registry; and Climate Action Reserve (see Data Availability section). Together, the FC projects we considered represent >97% of the carbon credits issued on the voluntary FC market20.
Finally, we used Web of Science to systematically search for peer-reviewed literature on eDNA-based biodiversity monitoring programs. This search was limited to studies of ecological impacts, including those resulting from interventions such as restoration efforts, or from anthropogenic or natural drivers such as pollution or land-use gradients (Table 2, Supplementary Fig. 2).
This three-phase process resulted in three pools of literature. The first captured the scholarly discussion surrounding biodiversity monitoring within FC projects. The second highlighted voluntary FC projects with on-the-ground biodiversity monitoring in place. The third characterized the current state of eDNA-based monitoring of biodiversity change. To contextualize the potential role for eDNA-based biodiversity monitoring within FC markets, we collected data on each study or project report in the three final literature pools. Data on empirical studies and projects included taxa, monitoring method, question addressed, site characteristics, and geographic ___location (Table 2). Data collected on peer-reviewed literature on biodiversity monitoring within FC markets also included thematic focus and whether biodiversity monitoring practices were explicitly discussed. Additional methodological details, including review inclusion and exclusion criteria, are outlined in Table 2 and Supplementary Material (Supplementary Figs. 1 and 2, Supplementary Note 1).
Data availability
The annotated literature and forest carbon project databases are publicly available as tab-separated text files at: https://doi.org/10.5281/zenodo.13830752. Data and reports for forest carbon projects were retrieved from the respective registry websites (see Supplementary Note 1 for links).
Code availability
R code and data to reproduce the analyses and figures are publicly available via GitHub (https://github.com/mikeallen-eco/eDNA_forest_carbon) and Zenodo (https://doi.org/10.5281/zenodo.13830752).
References
Roe, S. et al. Contribution of the land sector to a 1.5 °C world. Nat. Clim. Change 9, 817–828 (2019).
Seddon, N. et al. Understanding the value and limits of nature-based solutions to climate change and other global challenges. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190120 (2020).
Sarira, T. V., Zeng, Y., Neugarten, R., Chaplin-Kramer, R. & Koh, L. P. Co-benefits of forest carbon projects in Southeast Asia. Nat. Sustain. 5, 393–396 (2022).
Phelps, J., Webb, E. L. & Adams, W. M. Biodiversity co-benefits of policies to reduce forest-carbon emissions. Nat. Clim. Change 2, 497–503 (2012).
Donofrio, S.-M., Patrick–Daley, C.-C. & Ciro–Lin, K. The Art of Integrity: State of the Voluntary Carbon Markets 2022 Q3 (Forest Trends’ Ecosystem Marketplace, 2022).
Hyde, M. et al. Refining carbon credits to contribute to large carnivore conservation: the jaguar as a case study. Conserv. Lett. 15, e12880 (2022).
International Union for Conservation of Nature. IUCN Global Standard for Nature-Based Solutions: A User-Friendly Framework for the Verification, Design and Scaling up of NbS 1st edn (International Union for Conservation of Nature, 2020).
Soto-Navarro, C. et al. Mapping co-benefits for carbon storage and biodiversity to inform conservation policy and action. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190128 (2020).
Key, I. B. et al. Biodiversity outcomes of nature-based solutions for climate change adaptation: characterising the evidence base. Front. Environ. Sci. 10, 905767 (2022).
Lou, J., Hultman, N., Patwardhan, A. & Qiu, Y. L. Integrating sustainability into climate finance by quantifying the co-benefits and market impact of carbon projects. Commun. Earth Environ. 3, 1–11 (2022).
Gonzalez, A. & Londoño, M. C. Monitor biodiversity for action. Science 378, 1147–1147 (2022).
Oliver, R. Y. et al. Camera trapping expands the view into global biodiversity and its change. Philos. Trans. R. Soc. B Biol. Sci. 378, 20220232 (2023).
Pimm, S. L. et al. Emerging technologies to conserve biodiversity. Trends Ecol. Evol. 30, 685–696 (2015).
Deiner, K. et al. Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Mol. Ecol. 26, 5872–5895 (2017).
Kelly, R. P. et al. Harnessing DNA to improve environmental management. Science 344, 1455–1456 (2014).
Jaureguiberry, P. et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 8, eabm9982 (2022).
Stanturf, J. A., Palik, B. J. & Dumroese, R. K. Contemporary forest restoration: a review emphasizing function. For. Ecol. Manag. 331, 292–323 (2014).
Carbon Direct. State of the voluntary carbon market. https://www.carbon-direct.com/research-and-reports/state-of-the-voluntary-carbon-market (2023).
Ebeling, J. & Yasué, M. Generating carbon finance through avoided deforestation and its potential to create climatic, conservation and human development benefits. Philos. Trans. R. Soc. B Biol. Sci. 363, 1917–1924 (2008).
Pan, C. et al. Key challenges and approaches to addressing barriers in forest carbon offset projects. J. For. Res. 33, 1109–1122 (2022).
The Integrity Council for the Voluntary Carbon Market. Core carbon principles, assessment framework and assessment procedure. https://policycommons.net/artifacts/4433491/ccp-foreword-final-28mar23/5230721/ (2023).
Merger, E., Dutschke, M. & Verchot, L. Options for REDD+ voluntary certification to ensure net GHG benefits, poverty alleviation, sustainable management of forests and biodiversity conservation. Forests 2, 550–577 (2011).
Richards, M. & Panfil, S. Social and Biodiversity Impact Assessment (SBIA) Manual for REDD+ Projects: Part 1—Core Guidance for Project Proponents (Climate, Community & Biodiversity Alliance, Forest Trends, Fauna & Flora International, and Rainforest Alliance, 2011).
Waldon, J., Miller, B. W. & Miller, C. M. A model biodiversity monitoring protocol for REDD projects. Trop. Conserv. Sci. 4, 254–260 (2011).
Kelly, R. P. et al. Toward a national eDNA strategy for the United States. Environ. DNA 6, e432 (2024).
UNESCO. Environmental DNA expeditions in UNESCO world heritage marine sites. https://www.unesco.org/en/edna-expeditions (2024).
Bálint, M. et al. Accuracy, limitations and cost efficiency of eDNA‐based community survey in tropical frogs. Mol. Ecol. Resour. 18, 1415–1426 (2018).
Mena, J. L. et al. Environmental DNA metabarcoding as a useful tool for evaluating terrestrial mammal diversity in tropical forests. Ecol. Appl. 31, e02335 (2021).
Beng, K. C. & Corlett, R. T. Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodivers. Conserv. 29, 2089–2121 (2020).
Valentin, R. E. et al. Moving eDNA surveys onto land: strategies for active eDNA aggregation to detect invasive forest insects. Mol. Ecol. Resour. 20, 746–755 (2020).
van der Heyde, M., Bunce, M. & Nevill, P. Key factors to consider in the use of environmental DNA metabarcoding to monitor terrestrial ecological restoration. Sci. Total Environ. 848, 157617 (2022).
Lynggaard, C. et al. DNA-based arthropod diversity assessment in Amazonian iron mine lands show ecological succession towards undisturbed reference sites. Front. Ecol. Evol. 8, 590976 (2020).
Van Der Heyde, M. et al. Changes in soil microbial communities in post mine ecological restoration: Implications for monitoring using high throughput DNA sequencing. Sci. Total Environ. 749, 142262 (2020).
Van Der Heyde, M. et al. Scat DNA provides important data for effective monitoring of mammal and bird biodiversity. Biodivers. Conserv. 30, 3585–3602 (2021).
Van Der Heyde, M. et al. Evaluating restoration trajectories using DNA metabarcoding of ground‐dwelling and airborne invertebrates and associated plant communities. Mol. Ecol. 31, 2172–2188 (2022).
International Union for Conservation of Nature. eBioAtlas: Using the Power of eDNA to Fill Global Biodiversity Knowledge Gaps and Deliver Impact in Conservation (International Union for Conservation of Nature, 2023).
Abarenkov, K. et al. Publishing DNA-derived data through biodiversity data platforms, version 1.3.0, 7 June 2023 (Global Biodiversity Information Facility, 2023).
Lynggaard, C. et al. Airborne environmental DNA for terrestrial vertebrate community monitoring. Curr. Biol. 32, 701–707 (2022).
Kyle, K. E. et al. Combining surface and soil environmental DNA with artificial cover objects to improve terrestrial reptile survey detection. Conserv. Biol. 36, e13939 (2022).
Allen, M. C. et al. Sampling environmental DNA from trees and soil to detect cryptic arboreal mammals. Sci. Rep. 13, 180 (2023).
Leempoel, K., Hebert, T. & Hadly, E. A. A comparison of eDNA to camera trapping for assessment of terrestrial mammal diversity. Proc. R. Soc. B 287, 20192353 (2020).
Newton, J. P., Bateman, P. W., Heydenrych, M. J., Mousavi-Derazmahalleh, M. & Nevill, P. Home is where the hollow is: revealing vertebrate tree hollow user biodiversity with eDNA metabarcoding. Environ. DNA 4, 1078–1091 (2022).
Allen, M. C. et al. Using surface environmental DNA to assess arthropod biodiversity within a forested ecosystem. Environ. DNA 5, 1652–1666 (2023).
Marquina, D., Esparza‐Salas, R., Roslin, T. & Ronquist, F. Establishing arthropod community composition using metabarcoding: surprising inconsistencies between soil samples and preservative ethanol and homogenate from Malaise trap catches. Mol. Ecol. Resour. 19, 1516–1530 (2019).
Banerjee, P. et al. Environmental DNA analysis as an emerging non-destructive method for plant biodiversity monitoring: a review. AoB Plants 14, plac031 (2022).
Johnson, M. D. et al. Environmental DNA as an emerging tool in botanical research. Am. J. Bot. 110, e16120 (2023).
Watson, C. D. et al. Global meta-analysis shows progress towards recovery of soil microbiota following revegetation. Biol. Conserv. 272, 109592 (2022).
Eaton, W. D., Shokralla, S., McGee, K. M. & Hajibabaei, M. Using metagenomics to show the efficacy of forest restoration in the New Jersey Pine Barrens. Genome 60, 825–836 (2017).
Dyson, K. et al. Coupling remote sensing and eDNA to monitor environmental impact: A pilot to quantify the environmental benefits of sustainable agriculture in the Brazilian Amazon. PLoS ONE 19, e0289437 (2024).
Pitman, N. Social and Biodiversity Impact Assessment Manual for REDD+ Projects: Part 3 – Biodiversity Impact Assessment Toolbox (Forest Trends, Climate, Community & Biodiversity Alliance, Rainforest Alliance and Fauna & Flora International, 2011).
Tedersoo, L. et al. Towards a co-crediting system for carbon and biodiversity. Plants People Planet 6, 18–28 (2024).
Forbes, R. J., Watson, S. J., O’Connor, E., Wescott, W. & Steinbauer, M. J. Diversity and abundance of Lepidoptera and Coleoptera in multiple-species reforestation plantings to offset emissions of carbon dioxide. Aust. For. 82, 89–106 (2019).
Nakakaawa, C., Aune, J. & Vedeld, P. Changes in carbon stocks and tree diversity in agro-ecosystems in south western Uganda: what role for carbon sequestration payments? New For. 40, 19–44 (2010).
Mekuria, W. et al. Restoring aboveground carbon and biodiversity: a case study from the Nile basin, Ethiopia. For. Sci. Technol. 11, 86–96 (2015).
Bartels, S. F. & Macdonald, S. E. Dynamics and recovery of forest understory biodiversity over 17 years following varying levels of retention harvesting. J. Appl. Ecol. 60, 725–736 (2023).
Haq, S. M. et al. Biodiversity and carbon stocks of the understory vegetation as indicators for forest health in the Zabarwan Mountain Range, Indian Western Himalaya. Ecol. Indic. 159, 111685 (2024).
Karimi, B. et al. Microbial diversity and ecological networks as indicators of environmental quality. Environ. Chem. Lett. 15, 265–281 (2017).
Borges, F. L. G., da Rosa Oliveira, M., de Almeida, T. C., Majer, J. D. & Garcia, L. C. Terrestrial invertebrates as bioindicators in restoration ecology: a global bibliometric survey. Ecol. Indic. 125, 107458 (2021).
Deiner, K., Fronhofer, E. A., Mächler, E., Walser, J.-C. & Altermatt, F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat. Commun. 7, 12544 (2016).
Macher, T., Schütz, R., Hörren, T., Beermann, A. J. & Leese, F. It’s raining species: rainwash eDNA metabarcoding as a minimally invasive method to assess tree canopy invertebrate diversity. Environ. DNA 5, 3–11 (2023).
Lynggaard, C. et al. Vertebrate environmental DNA from leaf swabs. Curr. Biol. 33, R853–R854 (2023).
Massey, A. L. et al. Invertebrates for vertebrate biodiversity monitoring: comparisons using three insect taxa as iDNA samplers. Mol. Ecol. Resour. 22, 962–977 (2022).
Coutant, O. et al. Amazonian mammal monitoring using aquatic environmental DNA. Mol. Ecol. Resour. 21, 1875–1888 (2021).
Lyet, A. et al. eDNA sampled from stream networks correlates with camera trap detection rates of terrestrial mammals. Sci. Rep. 11, 1–14 (2021).
Valentin, R. E., Kyle, K. E., Allen, M. C., Welbourne, D. J. & Lockwood, J. L. The state, transport, and fate of aboveground terrestrial arthropod eDNA. Environ. DNA 3, 1081–1092 (2021).
Corlett, R. T. & Primack, R. B. Tropical rainforest conservation: a global perspective. Trop. For. Community Ecol. 442, 457 (2008).
Jackman, J. M. et al. eDNA in a bottleneck: obstacles to fish metabarcoding studies in megadiverse freshwater systems. Environ. DNA 3, 837–849 (2021).
van der Reis, A. L., Beckley, L. E., Olivar, M. P. & Jeffs, A. G. Nanopore short-read sequencing: a quick, cost-effective and accurate method for DNA metabarcoding. Environ. DNA 5, 282–296 (2023).
Kéry, M. & Royle, J. A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS: Volume 1: Prelude and Static Models (Academic Press, 2016).
Bush, A. et al. Replicate DNA metabarcoding can discriminate seasonal and spatial abundance shifts in river macroinvertebrate assemblages. Mol. Ecol. Resour. 23, 1275–1287 (2023).
Nichols, J. D. et al. Multi-scale occupancy estimation and modelling using multiple detection methods. J. Appl. Ecol. 45, 1321–1329 (2008).
West, T. A. P. et al. Action needed to make carbon offsets from forest conservation work for climate change mitigation. Science 381, 873–877 (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906 (2021).
Acknowledgements
We thank R. Almeida, S. Dickey, C. Eddy, K. Fitz, A. Kisurin, K. Kyle, J. Ramirez-Garofalo, L. Tkacenko, A. Vastano, and E. Waltman for helpful feedback and J. Vastano for assistance with data collection. Icons in Fig. 1 were created by M. Michaud, Ghedo, T. M. Keesey, C. De Rito, F. Sayol, L. Simons, M. Tan, G. Palomo Munoz, and E. J. McTavish. Funding for this study was provided by ExxonMobil.
Author information
Authors and Affiliations
Contributions
B.D.J., J.C.A., J.D.B., J.L.L., and M.C.A. collectively conceived the idea. M.C.A. and R.I. assembled the literature and collected data. M.C.A. assembled the final databases and wrote the code to create figures and analyze the data. M.C.A. led the writing of the manuscript. All authors contributed meaningfully to shaping the manuscript during revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Martina Grecequet. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allen, M.C., Lockwood, J.L., Ibanez, R. et al. eDNA offers opportunities for improved biodiversity monitoring within forest carbon markets. Commun Earth Environ 5, 801 (2024). https://doi.org/10.1038/s43247-024-01970-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-024-01970-y