Abstract
Peatlands on the Qinghai-Tibetan Plateau (QTP) represent one of the world’s largest reservoirs of soil organic carbon (C) and nitrogen (N), but their future stability is uncertain. This study utilizes high-resolution multi-core records of C and N contents, stable isotopes, and infrared spectroscopy to reconstruct water table depths and their impacts on C and N accumulation and decomposition over the past 2.7 kyr in a slope peatland from the central QTP. Our paleo records reveal that increased decomposition in the surface oxic layer has led to decreased C and N accumulation rates over the last millennium, primarily due to water-table drawdown driven by climate warming and drying. Supporting evidence from nearby records suggests that this trend of surface drying may be widespread across QTP peatlands. Despite this, QTP peatlands retain some of the highest 1-m C and N densities globally, with values of 54.1 ± 18.9 kg C m-2 and 3.2 ± 1.5 kg N m-2. These findings highlight the vulnerability of substantial C and N reservoirs and sequestration capabilities in QTP peatlands to continued water table declines in a warming and drying climate.
Similar content being viewed by others
Introduction
Northern peatlands (north of 30°N) store 500 ± 100 gigatons (Gt) of organic carbon (C) and 10 ± 7 Gt of nitrogen (N) with only occupying 3% of the land surface area1,2. Owing to their long-term waterlogging condition, northern peatlands contain disproportionately higher amounts of C underground (up to c. 150 kg C m-2) than other terrestrial ecosystems, representing the largest soil organic C pools in terrestrial ecosystems3. Such C sequestration ability makes northern peatlands a persistent C sink since the last deglaciation4. But how northern peatlands will respond to the future climate change remain in debate, especially about whether their C and N pools would become a large source of greenhouse gases.
The strengths of C and N sequestration in peatlands are mainly determined by the balance between productivity and aerobic decomposition in the acrotelm (upper oxic layer) as the rate of decomposition is extremely low in the catotelm (anoxic layer). The water table depth (WTD) is the primary determinant modulating this balance5. Due to the inherent ecohydrological complexity in peatland ecosystems, future climate predictions cannot be directly used to infer the peatland hydrological conditions, resulting in high uncertainties in predicting C and N sequestration abilities6. Many studies have revealed that climate warming and increased atmospheric CO2 will lead to an increase in peat C sequestration in northern peatlands as increases in vegetation productivity exceed increases in decomposition7,8. But some studies also implied that the C sink in northern peatlands may be vulnerable to the multi disturbances associated with climate change, including permafrost thaw and drought9,10,11. It is widely accepted that maintaining a proper WTD is critical for sustaining the C and N sequestration in peatlands12,13. However, on longer timescales, our knowledge of how the peatland ecohydrology and C and N cycles respond to climate change remains poor, particularly in high-altitude regions where large amounts of peatland resources that face highly-variable land-atmosphere interactions.
Since the 1970s, the Qinghai–Tibetan plateau (QTP) has experienced rapid warming at a rate of c. 0.4 °C per decade, which is almost twice the global average14. As one of the regions that are intensely affected by global warming, the ecosystems in the QTP have been affected by changes in climatic and terrestrial processes, which may compromise the stability of C and N cycles in the future15,16. The QTP is the largest alpine permafrost-affected region with the largest alpine wetlands in the world17. Wetlands from the QTP store c. 2.1 Gt of C (ref. 15), with c. 1.5 Gt of C being stored in peatlands, making the QTP a region with the largest peatland C pool in China18. Wetlands from the QTP contain c. 0.06 Gt of N, which is also an important N pool in China15. Such large C and N pools are the consequences of persistent C and N sequestration over thousands of years19. However, the C and N densities and storage capacities of peatlands on the QTP remain insufficiently understood. Most existing estimates of peatland C and N densities focus on the Zoige Plateau in the eastern QTP, while core-based measurements are limited to the Three-River-Source Region (TRSR) in the central QTP, another key area of alpine wetland distribution on the QTP20,21. Furthermore, recent remote sensing and machine learning-derived estimates of wetland C density and storage differ significantly from survey-based data22, highlighting substantial uncertainties and the need of ground-based studies. These gaps impede a comprehensive understanding of the role QTP peatlands play in the global C cycle.
Currently, eddy-covariance flux observations indicate that wetlands on the QTP, including peatlands, function as strong C sinks, with net ecosystem production reaching 104.7 ± 59 g C m−2 yr-1 (ref. 23). This contemporary C sink strength is largely influenced by growing-season temperatures. Experimental warming studies further demonstrate that elevated temperatures enhance the C sequestration capacity of these high-elevation wetlands. However, contemporary C flux measurements may not reflect long-term C sink strength in peatlands, as the latter is influenced by multiple long-term ecological drivers beyond temperature before the organic matter entering the catotelm. These include ecohydrological feedbacks, variations in litter quality, and permafrost dynamics24,25,26. This discrepancy complicates accurate predictions of the future stability of C and N pools and the sequestration potential of QTP wetlands. To better project their future trajectories, it is essential to investigate how past climate changes have influenced the C and N sequestration capacities of peatlands on the QTP.
Studies about the relationships between C and N cycles and climate changes in peatlands from the QTP are mainly conducted in the Zoige Plateau18,27,28, while remain scarce for the TRSR. To address this gap, the objectives of this study are: (1) to identify the primary drivers of long-term C and N sequestration in peatlands from the TRSR on the central QTP, and (2) to evaluate the role of QTP peatlands on global C and N cycles. We investigated the responses of C and N accumulation and decomposition to environmental changes over the past 2.7 kyr in the Chadam (CD) peatland, located in the TRSR (Fig. 1a). Recognizing the hydrological and nutrient heterogeneity within the peatland induced by local topography, we collected multiple peat cores along a hillslope at approximately 4800 m a.s.l. (Fig. 1b, c). Bulk peat C isotope (δ13Cpeat) values were measured as a proxy for WTD. High-resolution measurements of physicochemical properties and infrared spectroscopy were employed to quantitatively reconstruct peat C and N accumulation and decomposition. Furthermore, a dataset of C and N densities at depth of 1 m (1-m C and N densities; kg m−2) in northern peatlands was used to contextualize the significance of the CD peatland and other QTP peatlands within the broad context of northern peatlands. Collectively, this study underscores the hydroclimatic vulnerability of peat C and N on the QTP, highlighting their critical role in understanding broader C and N dynamics.
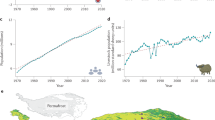
a Map of China highlighting peatland distributions67 with the ___domain of the QTP outlined by red lines (the areas with elevation >3000 m a.s.l.)68. b Aerial view showing the locations of study cores C1 to C6 (Google Earth image). c Schematic plot of the coring sites with the slope transect indicated by the yellow line in (b). Elevations are derived from a digital elevation model based on UAV aerial imagery36. The UAV aerial image is shown in the bottom left. d Photograph of the hummock-to-pool transect next to the coring ___location of core C5. Black triangles in (a) denote other sites discussed in the text: 1 – Maduo Xiang wetland37, 2 – Koucha lake32, 3 – ZigetangCo lake31, 4 – Dangxiong wetland33.
Results
Characteristics of surface samples
The hummock-to-pool transect next to the position of core C5 represents an apparent moisture gradient from a dry habitat at the top of the hummock to a wet habitat near the pool. From hummock to pool, the WTD decreases from 85 cm to 5 cm and the soil water content (SWC) increases from 129.4% to 442.8% (Supplementary Fig. 1e). The molar ratios of C to N (C:N) show no obvious trend along this gradient but significantly higher values in plant samples (29 ± 16) compared to peat samples (17 ± 3; p < 0.05; one-way analysis of variance (ANOVA)) (Supplementary Fig. 1a; Supplementary Table 3). The ratios of integrated area of C-H bands to C=O bands (C-Ha:C=Oa) derived from Fourier transform infrared spectroscopy (FTIR) show similar patterns along the gradient and between plant (0.48 ± 0.12) and peat (0.26 ± 0.05) samples (p < 0.05; one-way ANOVA) (Supplementary Fig. 1b; Supplementary Table 3). The values of N isotope (δ15N) also show similar patterns along the gradient and between plant (2.59 ± 2.33) and peat (1.46 ± 1.15) samples (p < 0.05; one-way ANOVA) (Supplementary Fig. 1d; Supplementary Table 3). However, the values of C isotope (δ13C) of plant and peat show an obvious trend along the moisture gradient, reaching about −24‰ at the top and decreasing to about −28‰ near the pool (Supplementary Fig. 1c). Significant correlations are found between δ13Cpeat and WTD (p < 0.05; Pearson) and SWC (p < 0.05; Pearson) (Supplementary Figs. 4 & 6).
Characteristics of peat core samples
The peat core samples from CD peatland are characterized by the high dry bulk density (DBD) and moderate levels of total organic C (TC) and total N (TN). The DBD range from 0.07 to 1.79 g cm−3, while the TC is from 0.08 to 41% and the TN from 0.08 to 2.46% (Supplementary Figs. 2a–c; Supplementary Table 3). These values lead to the high mean C density (73.3 ± 20.6 kg C m−3) and N density (5.34 ± 1.68 kg N m−3) in the peat core samples from CD (Supplementary Fig. 2d & e; Supplementary Table 3). The C and N densities at CD are higher than the peatlands on the QTP as a whole, where the limited data in the literature range from 27 kg C m-3 to 96 kg C m−3 and 1.5 kg N m−3 to 4.9 kg N m−3, respectively27,29,30. The C and N densities at CD are also higher than most regional estimations for northern peatlands (Fig. 4d).
C and N accumulation rates
The radiocarbon dating measurements show that the peats were accumulated since c. 2200 cal. yr B.P. for core C1, c. 2700 cal. yr B.P. for core C4, and c. 1700 cal. yr B.P. for core C6 (Supplementary Tables 1 and 2). Over the millennial timescale, the time-weighted mean rates of C accmulation and N accumulation are estimated to be 29.3 ± 2 g C m-2 yr-1 and 2.1 ± 0.3 g N m−2 yr-1, respectively (Supplementary Table 3). Over the decadal to centennial timescale, the C accmulation rates (CAR) and N accmulation rates (NAR) are highly correlated in all three cores (Fig. 3c, d). The CAR and NAR reach high values (more than 40 g C m-2 yr-1 and 2 g N m-2 yr-1) from c. 1100 to c. 700 cal. yr B.P. in C1, from c. 2100 to c. 1700 cal. yr B.P. in C4 and from c. 1700 to c. 1400 cal. yr B.P. in C6. Then, the CAR and NAR decrease to lowest values (less than 30 g C m-2 yr-1 and 2 g N m-2 yr-1) from c. 750 to c. 100 cal. yr B.P. in all three cores. Both CAR and NAR reach their highest values since c. 100 cal. yr B.P. of all three cores.
Generally, the CAR and NAR showed high values from c. 2000 to c. 1500 cal. yr B.P. but decreasing trends since c. 1000 cal. yr B.P. in all three cores (Fig. 3c, d). Thus, the dynamics of peat accumulation in CD can be devided into two major periods, namely centennial accumulation optimum (CAO; from 2000 to 1500 cal. yr B.P.) and centennial accumulation nadir (CAN; from 750 to 100 cal. yr B.P.) (Fig. 3).
Peat decomposition history
In all three cores, the C:N ratios range from 7.3 to 34.9, showing slight variations in the catotelm and a rapid increasing trend in the acrotelm (Supplementary Fig. 2i). The C:N ratios show high values from c. 2000 to c. 1400 cal. yr B.P. and low values from c. 750 to c. 100 cal. yr B.P. in C4 and C6. But the C:N show high values at c. 2200 and c. 1200 cal. yr B.P. and low values at c. 1100 and c. 750 cal. yr B.P. in C1. The C-Ha:C=Oa ratios range from 0.07 to 0.47, showing a similar trend but with greater variability compared to C:N ratios (Supplementary Fig. 2j). The percentage of original C loss (C loss %), calculated from the C-Ha:C=Oa ratios, is estimated to be up to c. 60% under the current WTD and ranges from c. 20% to c. 80% in the catotelm (Supplementary Fig. 2k). Besides, the C loss % is significantly higher in C1 than C4 and C6 (p < 0.05; one-way ANOVA; Supplementary Table 3). Across the records, the C loss % are relatively low from c. 2000 to c. 1500 cal. yr B.P. particularly in C4 and C6, followed by higher values from 1000 to 100 cal. yr B.P. and a subsequent decline since c. 100 cal. yr B.P.
Paleo ecohydrology
The values of δ13Cpeat, after accounting for the Suess effect, range from −26.6‰ to −23.8‰ (Supplementary Fig. 2l). The δ13Cpeat show low values from c. 2100 to c. 1700 cal. yr B.P. in C4 and from c. 1700 to c. 1400 cal. yr B.P. in C6. Both C4 and C6 show lower values of δ13Cpeat from c. 2000 to c. 1500 cal. yr B.P. and then increase to the highest values at the top. But the δ13Cpeat in C1 exhibits an inverse trend, with high values observed from c. 2100 to c. 1500 cal. yr B.P., lower values from c. 1000 to c. 400 cal. yr B.P., and a subsequent increase to the highest values near the surface. Notably, the δ13Cpeat in C1 is significantly higher than in C4 and C6 (p < 0.05; one-way ANOVA; Supplementary Table 3). The values of bulk peat N isotope (δ15Npeat) range from −2.27‰ to 6.26‰, showing higher values (>0‰) in the catotelm and a decreasing trend to the lowest values in the acrotelm (Supplementary Fig. 2m). Based on the relationships between δ15Npeat and C:N, possible permafrost perturbations can be identified during two 200-year periods in C1, which started at c. 2200 cal. yr B.P. and at c.1500 cal. yr B.P. (Supplementary Fig. 2n). The δ15Npeat in C4 and C6 show no signs of permafrost perturbation and indicates that these cores have predominantly experienced a state of N gain (Supplementary Fig. 2n).
C and N densities at 1-m depth of northern peatlands
We collected and calculated a total of 488 datapoints for 1-m C densities and 76 datapoints for 1-m N densities in northern peatlands. Most of the 1-m C densities in northern peatlands range from 40 to 60 kg C m-2 and most of the 1-m N densities are less than 3 kg N m-2 (Fig. 4a, b). The 1-m C and N densities at CD are estimated to be 73.5 ± 7.8 kg C m-2 and 5.6 ± 0.9 kg N m-2, respectively. Thus, the 1-m C and N densities at CD are higher than those in most of the records from northern peatlands. Despite the scarcity of reported data, the high 1-m C and N densities were also found in other peatlands on the QTP (Fig. 4a, b). The 1-m C and N densities in QTP is estimated to be 54.07 ± 18.86 kg C m-2 and 3.24 ± 1.46 kg N m-2, respectively, which are higher than most regional estimations for northern peatlands (Fig. 4c; Supplementary Table 4).
Discussion
Paleo-hydroclimate variations in the central QTP
The modern process investigation has validated the relationship between the δ13Cpeat and WTD, allowing us to interpret that the δ13Cpeat in peat cores reflects the two major periods of paleo-hydrological variations at CD peatland (Supplementary discussion). The δ13Cpeat showed lower values (indicating low WTD and wetter surface) from 2000 to 1500 cal. yr B.P., while it shifted to higher values (indicating increasing WTD and drier surface) since c. 1000 cal. yr B.P. (Fig. 2a). This trend of surface drying is pronounced in C4 and C6 (p < 0.05; one-way ANOVA; Fig. 3e), but not in C1 (p > 0.05; one-way ANOVA; Fig. 3e). Despite varying oppositely, the δ13Cpeat of C1 are significantly lower than those in other cores (p < 0.05; one-way ANOVA; Supplementary Table 3), indicating that the C1 has experienced prolonged drier condition than other cores.
The calendar age is relative to 1950 CE. a Suess-effect-corrected bulk peat δ13C (δ13Cpeat; ‰) from CD peatland. The bone lines represent 5-ponits moving average curves. Depth scale and radiocarbon dating points are shown above; b Pollen-based quantitative reconstruction of mean annual precipitation from ZigetangCo lake31; c Pollen-based quantitative reconstruction of mean annual precipitation from Koucha lake32; d Pollen-based quantitative reconstruction of summer temperature from Koucha lake32; e Biomarker-based quantitative reconstruction of warm-season temperature (from May to September) from Dangxiong wetland33.
The calendar age is relative to 1950 CE. a Suess-effect-corrected bulk peat δ13C (δ13Cpeat; ‰); b Percentages of C loss in peat samples (C loss %); c Carbon accumulation rates (CAR; g C m−2 yr-1); d Nitrogen accumulation rates (NAR; g N m−2 yr-1). For a to d, the bone lines represent 5-ponits moving average curves. The gray band denotes centennial accumulation nadir (CAN; from 750 to 100 cal. yr B.P.) and green band denotes centennial accumulation optimum (CAO; from 2000 to 1500 cal. yr B.P.). Depth scale and radiocarbon dating points are shown above. The right panel from (e) to (h) shows distributions and mean values of these data during CAN and CAO. The different letters denote statistically significant differences in post hoc Tukey’s HSD test for multiple comparisons.
The variation in surface wetness at CD peatland over the past 2 kyr could be attributed to reduced precipitation and increased temperature. A pollen-based quantitative reconstruction from ZigetangCo lake, located at c. 300 km southwest of CD, indicates a decline in mean annual precipitation (MAP) from around 2000 cal. yr B.P. to the present in the central QTP31 (Figs. 1a, 2b). This drying trend is corroborated by another pollen-based reconstruction from Koucha lake, about 250 km northeast of CD, which also shows a reduction in precipitation over the same period32 (Figs. 1a, 2c). Furthermore, the reconstruction from Koucha lake suggests that summer temperature have risen since approximately 2000 cal. yr B.P.32. (Figs. 1a, 2d). A biomarker-based reconstruction from the Dangxiong wetland, near the ZigetangCo lake, similarly indicates an increase in warm-season temperature during this time33 (Figs. 1a, 2e). It is important to note that these paleo-climate records exhibit greater variability compared to our findings and are geographically distant from CD. While paleo-climate reconstructions remain scarce for the Damqu River Basin, the trend of warming and drying in the central QTP over the past two millennia is well-supported by numerous quantitative and qualitative studies34,35.
The hydrology of the CD peatland is also influenced by local topography, particularly in terms of water recharge, which primarily comes from rainfall and snowmelt from the catchment area on the upper slopes. The hydrological dynamics vary across different elevations and slope gradients: at the higher elevation of C1 (around 4815 m) with a steeper slope (about 5.6°), water flows much faster compared to C4 and C6. In contrast, the position at C4 and C6 is characterized by a more developed pattern of pools and ridges, which helps reduce water loss36. As a result, the interaction between local topography and hydrology creates long-term dry habitat and distinct paleo hydrological variation in C1 at the upslope.
The trend of surface drying is also observed in other wetlands on central QTP, although paleo-hydrology records during the last two millennia remain scarce in this region. A reconstruction of humidity index derived from Maduo Xiang wetland, located c. 300 km northeast of CD (Fig. 1a; Supplementary Fig. 5b), indicates that regional moisture has been decreasing since c. 4000 cal. yr. B.P. with higher values from c. 2000 to c. 1000 cal. yr B.P., which aligns with our WTD record37. Similarly, a paleo-hydrology record based on multi proxies from an alpine meadow wetland at Dangxiong county, Xizang Autonomous Region (Tibet) (Fig. 1a; Supplementary Fig. 5c), reported that aquatic plants dominated the wetland from c. 2000 to c. 1000 cal. yr B.P., while terrestrial plants became more dominant after c. 1000 cal. yr B.P.33. Although data are limited, it seems to be a common feature across central QTP wetlands that they have been undergoing a shift toward drier conditions over the past millennium.
Drying-reduced peat C and N accumulation over the last millennium
Our paleo records indicate that WTD plays a dominant role in modulating the C and N sequestration abilities in CD peatland over millennial timescales. During the CAO, the low temperature and high precipitation led to a lower WTD (wetter surface), resulting in a lower degree of decomposition and consequently higher CAR and NAR, especially in C4 and C6. But during the CAN, significantly decreased precipitation and increased temperature elevated the degree of decomposition by increasing WTD (drier surface), leading to lower CAR and NAR in three cores. Although the significant difference of CAR and NAR between the two periods only exists in C4 (Fig. 3g, h), the decrease of CAR and NAR also observed in C1 and C6 (Fig. 3c, d). Overall, the water-table drawdown induced by climate warming and drying has hindered the C and N sequestration abilities in CD over the last millennium.
The observed variations of CAR and NAR in peat cores can be influenced by many factors, including chronology, peat characteristics and environmental drivers38. It has been shown that peat accumulation rates are highly sensitive to chronology38,39,40. However, in the case of CD peatland, the low CAR and NAR during CAN were not solely due to reduced accmulation rates but also due to increased C and N densities. This aligns with the effects of enhanced decomposition driven by increased WTD (drier surface) (Supplementary Fig. 2d–f).
The characteristics of peat, largely influenced by the plant components, also significantly affect CAR and NAR. Numerous studies have shown that a shift of vegetation communities from sedge to Sphagnum, the so-called fen-bog transition, can significantly promote the CAR in northern peatlands41,42,43,44. However, in the CD peatland, no noticeable changes in plant functional types were observed throughout the entire peat stratigraphy across all three cores45.
Various climatic and environmental factors, such as temperature, growing degree days, and water level, have been considered potential drivers of peat C accumulation. In permafrost-affected peatlands, permafrost aggradation and thawing are particularly important, often indicated by changes in dominant vegetation communities46,47. However, in CD peatland, the effects of permafrost activities on C and N accumulations were difficult to detect, as evidenced by the lack of noticeable changes in plant macrofossils throughout the peat stratigraphy45. The variations in δ15Npeat also suggest that permafrost perturbation was rare in CD48. Therefore, permafrost aggradation and degradation are considered secondary drivers of long-term C and N sequestration in CD.
Numerous core-based observations and Earth system models have confirmed that climate warming can enhance C accumulations in northern peatlands by extending growing degree days and/or altering vegetation communities7,8,42. However, when climate warming is combined with drying, it may lead to water-table drawdown, which can offset the positive effects of warming on peat accumulation by increasing decomposition rates26,49,50. Despite this, the CAR and NAR exhibit a significant increasing trend over the past century in CD peatland, corresponding to the marked water table drawdown and anthropogenic warming since the 20th century. This pattern may result from insufficient decomposition of peat before its transition into the catotelm, rather than direct stimulation by climate warming51. Besides, though the trend of surface drying is not pronounced in C1, the long-term dry conditions result in the lower time-weight mean rates of C accumulation and N accumulation in C1 compared to C4 and C6, supporting the inference that water table drawdown reduces C and N sequestration abilities in CD. Therefore, we argue that oxygen exposure time, which is linked to WTD, plays a crucial role in determining the C and N sequestration abilities of CD in response to climate change.
Large peatland C and N pools on the QTP and their future
Although CAR and NAR have decreased over the last millennium, the CD peatland has still stored a significant amount of C and N over the past c. 2000 years. Such high C and N densities are also observed in other peatlands on the QTP. The 1-m C and N densities in QTP are estimated at 54.1 ± 18.9 kg C m-2 and 3.2 ± 1.5 kg N m−2, respectively, which are higher than most regional estimates in northern peatlands (Fig. 4c). Our data synthesis indicates that the peatlands on the QTP serve as one of the greatest 1-m C and N pools in the terrestrial ecosystems.
a Map and histogram of 1-m C density (kg C m−2) (n = 488). b Map and histogram of 1-m N density (kg N m−2) (n = 76). c Comparison of regional 1-m C and N densities (kg m-2). d Comparison of C and N densities (g cm−3). The regional C and N densities are calculated based on the dataset of northern peatlands66.
The high 1-m C and N densities in peatlands on the QTP can be attributed to several factors. One key reason is the unique peat-forming vegetation. Dominant species, such as Kobresia spp. and Carex spp. are prevalent in QTP peatlands52. Kobresia spp., also dominant in the alpine steppe and meadow ecosystems, is characterized by significantly high below-ground biomass, leading to both high annual net primary productivity and strong N fixation capabilities53,54. This is supported by our δ15Npeat records, which indicate a long-term state of N gain in cores C4 and C6 (Supplementary Fig. 2n). Additionally, the alpine climate of the QTP promotes peat accumulation through reduced decomposition rates, which result from lower mean annual temperatures (MAT) and permafrost aggradation. This is consistent with the pattern observed in other high-latitude and high-altitude areas, such as Northern Canada and West Siberia, where high 1-m C and N densities are also prevalent (Fig. 4c). Finally, the land surface process will also help the peat accumulation. The extensive pool and ridge patterns on the land surface of CD help to reduce water loss by hindering surface runoff and decreasing hydraulic conductivity, which is beneficial for peatland formation36.
Traditionally, the widespread wetlands on the central QTP are classified as “wet meadow” or “peat pasture,” dominated by Carex and Kobresia20. These wetlands are characterized by high mineral content and low C content (typically <30%), which has led to the assumption that their C and N densities are lower than those of typical peatlands. So far, a remote sensing and machine learning-based estimate of 1-m C density for wetlands across China is 26.57 ± 3.73 kg C m−2, with values for QTP wetlands often falling below 30 kg C m−2 (ref. 22). Similarly, core-based estimates for 1-m N density on the QTP wetlands are 2.6 ± 3 kg N m-2 (ref. 55). However, our findings indicate that the 1-m C and N densities of peatlands on the QTP are significantly higher than these values. Given that C and N stored within the top meter of peatlands are highly susceptible to atmospheric exchange56, the underestimation of these densities could hinder effective land-use management and accurate C budget assessments. This is critical for informing climate mitigation strategies that rely on natural solutions57.
As mentioned above, millennial-scale surface drying has been observed in other peatlands on the central QTP33,37. This drying trend may have led to reduced C and N sequestration abilities in many peatlands on the QTP through unlocking the enzyme latch on microbial activities9. Additionally, climate warming and drying may compromise the large C and N pools in peatlands on the QTP though inducing areal loss, active layer deepening or thermokarst expansion1,11,58,59. Therefore, the substantial C and N pools and sequestration capacities of QTP peatlands may be at risk if the current trends of warming and drying continue.
In summary, our study, utilizing modern process investigations and multi-proxy reconstructions, elucidates how eco-hydroclimatic changes have influenced long-term C and N sequestration in a Kobresia-dominated slope peatland from the TRSR on the central QTP. Over the last millennium, CAR and NAR have decreased, primarily due to water-table drawdown caused by climate warming and drying. This trend of surface drying may also be affecting other peatlands on the central QTP. Despite this, peatlands on the QTP retain some of the highest C and N densities globally. Our findings suggest that the significant C and N stores and sequestration capabilities of peatlands on the QTP are at risk due to ongoing and future water-table declines in a warming and drying climate.
Methods
Study site
Chadam peatland (32.59°N, 94.25°E) is located ~10 km east of Chadam village, Qinghai province, China (Fig. 1a). The CD peatland is one of the typical Kobresia-dominated peatlands from the basin of Damqu River, and this basin represents one of the major wetland areas in the TRSR60. The MAT is 0.84 °C, and the MAP is 554 mm, based on instrumental record from 1980 to 2014 obtained from the nearest Zadoi meteorological station36. The vegetation is predominantly composed of Kobresia pygmaea, K. humilis, and Carex atrata, with a small presence of moss species.
The CD peatland features a distinctive morphology with widespread water pools and ridges, where the pools typically range from 0.25 to 3.4 m2 in size. Our sampling sites were located on a hillslope (Fig. 1b), with slope angles ranging from 0° to 14.8° and a mean of 5.6° ± 2.5° (ref. 36). The ground surface of CD was perennially inundated, with WTD varying between 7 cm and 23 cm during the sampling period. CD is also a permafrost-affected peatland, with active layer depths ranging from 0.5 m to 1.1 m in late August, 2021. The pH values range from 6.83 to 8.5. More details can be found in ref. 36.
Field sample collection
Sampling expedition was conducted in August, 2021. Six peat cores were collected along an elevational gradient on a slope peatland, ranging from low to high elevations (from C6 to C1) (Fig. 1b, c). WTD and active layer depth were measured on site at each core ___location36,45. Three cores from different slope positions—downslope (C6), midslope (C4), and upslope (C1)—were selected for detailed analyzes. To interpret the peat core records, peat-forming plants, and surface peat were collected from a hummock-to-pool transect adjacent to the position of core C5 (Fig. 1d). A total of 11 samples were gathered at 20 cm intervals, covering a moisture gradient from a hummock (with WTD at 80 cm) to a ___location near a water pool (with WTD at 5 cm). WTD was measured in the field once the water table had returned to its normal state after sampling.
Physio-chemical analyses
Moist bulk peat samples were dried in oven at 65 °C for 24 h, then the SWC was calculated as [mass of moist soil (g)—mass of oven-dried soil (g)/mass of oven-dried soil (g)] × 100 (ref. 61). TC, TN, δ13C, and δ15N was measured using an elemental analyzer (vario EL cube, Elementar, Germany) coupled with an isotope ratio mass spectrometer (Isoprime 100, Elementar, Germany). To remove inorganic C, samples were pretreated with hydrochloric acid fumigation62. TC, TN, δ13Cpeat, and δ15N peat were measured at 1 cm resolution for peat core samples. The δ13C and δ15N are expressed as ratios to their respective standards—Pee Dee Belemnite for δ13C and air N2 for δ15N. The δ13C of peat core samples were corrected by subtracting variations in atmospheric δ13C since A.D. 1000 to remove the interferences of the Suess effect63.
The apparent CAR (g C m−2 yr−1) and NAR (g N m−2 yr−1) of each 1-cm interval of peat samples were calculated by the following formulas:
where DBD is the dry bulk density (g cm−3), TC are total organic C (%), TN are total N (%), and AR are peat accumulation rates (cm yr−1).
FTIR spectra were obtained using an FTIR spectrometer (Vetex70, BRUKER, Germany) with absorption mode. Baselined correction, area normalization and peak area calculation were processed using the R packages ir64 and irpeat65. The C-Ha:C=Oa was calculated as the peak area ratio of C-H (2860 cm−1 and 2920 cm−1) bands to C=O (1610 cm−1 and 1700 cm−1) bands.
Calculation of the proportion of C loss
Our modern process investigations revealed that the C-Ha:C=Oa of peat samples varied within the range between fresh plant (0.48 ± 0.12) and ash (0.02 ± 0.51) (Supplementary Table 3). Thus, a conservative estimate of C loss from the peat can be calculated using the difference in the C-Ha: C=Oa ratio between fresh plant material and peat, based on the following equation:
where C loss % represents the percentage of the original C that has been removed, and C-Ha:C=Oa peat and C-Ha:C=Oa plant are C-Ha:C=Oa of peat and fresh peat-forming plants, respectively. This estimate of C loss is considered conservative because the mean value for the plant material is assumed to represent the original condition of the pristine organic matter.
Data compilation of C and N densities at 1-m depth in northern peatland
We compiled data on C and N densities at 1-m depth from published literatures. For cores where only the loss on ignition was measured, we calculated the C density by assuming the C content as 50% (ref. 66).
Statistical analyses
Difference between groups was assessed for significance using one-way analysis of variance (ANOVA) with the Tukey HSD test.
Data availability
The data supporting the findings of this study were deposited on figshare (https://doi.org/10.6084/m9.figshare.26954650.v3).
Code availability
The R packages utilized in this study are publicly available, and no custom code was used.
References
Hugelius, G. et al. Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw. Proc. Natl. Acad. Sci. USA. 117, 20438–20446 (2020).
Yu, Z. et al. No support for carbon storage of >1000 GtC in northern peatlands. Nat. Geosci. 14, 465–467 (2021).
Temmink, R. J. M. et al. Recovering wetland biogeomorphic feedbacks to restore the world’s biotic carbon hotspots. Science 376, eabn1479 (2022).
Brovkin, V. et al. Comparative carbon cycle dynamics of the present and last interglacial. Quat. Sci. Rev. 137, 15–32 (2016).
Limpens, J. et al. Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5, 1475–1491 (2008).
Zhang, H. et al. Decreased carbon accumulation feedback driven by climate-induced drying of two southern boreal bogs over recent centuries. Glob. Chang. Biol. 26, 2435–2448 (2020).
Charman, D. J. et al. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosciences 10, 929–944 (2013).
Gallego-Sala, A. V. et al. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Chang. 8, 907–913 (2018).
Fenner, N. & Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 4, 895–900 (2011).
Loisel, J. et al. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Chang. 11, 70–77 (2021).
Schuur, E. A. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
Evans, C. D. et al. Overriding water table control on managed peatland greenhouse gas emissions. Nature 593, 548–552 (2021).
Huang, Y. et al. Tradeoff of CO2 and CH4 emissions from global peatlands under water-table drawdown. Nat. Clim. Chang. 11, 618–622 (2021).
Kuang, X. & Jiao, J. J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res. Atmos. 121, 3979–4007 (2016).
Chen, H. et al. Carbon and nitrogen cycling on the Qinghai–Tibetan Plateau. Nat. Rev. Earth Environ. 3, 701–716 (2022).
Mu, C. et al. The status and stability of permafrost carbon on the Tibetan Plateau. Earth Sci. Rev. 211, 103433 (2020).
Wei, D. et al. Revisiting the role of CH4 emissions from alpine wetlands on the Tibetan Plateau: Evidence from two in situ measurements at 4758 and 4320 m above sea level. J. Geophys. Res. Biogeosci. 120, 1741–1750 (2015).
Wang, M. et al. Carbon dynamics of peatlands in China during the Holocene. Quat. Sci. Rev. 99, 34–41 (2014).
Yu, Z., Beilman, D. W. & Jones, M. C. Sensitivity of northern peatland carbon dynamics to Holocene climate change. in Carbon cycling in northern peatlands (eds Baird, A.J. et al.) 55–69 (American Geophysical Union, 2009).
Wei, D., Zhao, H., Huang, L., Qi, Y. & Wang, X. Feedbacks of alpine wetlands on the Tibetan Plateau to the atmosphere. Wetlands 40, 787–797 (2020).
Xue, Z. et al. Spatial and temporal changes of wetlands on the Qinghai-Tibetan Plateau from the 1970s to 2010s. Chin. Geogr. Sci. 28, 935–945 (2018).
Ren, Y. et al. China’s wetland soil organic carbon pool: new estimation on pool size, change, and trajectory. Glob. Chang. Biol. 29, 6139–6156 (2023).
Wei, D. et al. Plant uptake of CO2 outpaces losses from permafrost and plant respiration on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA. 118, e2015283118 (2021).
Flanagan, L. B. & Syed, K. H. Stimulation of both photosynthesis and respiration in response to warmer and drier conditions in a boreal peatland ecosystem. Glob. Chang. Biol. 17, 2271–2287 (2011).
Holmes, M. E. et al. Carbon accumulation, flux, and fate in Stordalen Mire, a permafrost peatland in transition. Glob. Biogeochem. Cycles 36, e2021GB007113 (2022).
Ratcliffe, J. et al. Contemporary carbon fluxes do not reflect the long-term carbon balance for an Atlantic blanket bog. Holocene 28, 140–149 (2018).
Chen, H. et al. The carbon stock of alpine peatlands on the Qinghai–Tibetan Plateau during the Holocene and their future fate. Quat. Sci. Rev. 95, 151–158 (2014).
Wang, M. et al. Higher recent peat C accumulation than that during the Holocene on the Zoige Plateau. Quat. Sci. Rev. 114, 116–125 (2015).
Large, D. J. et al. The influence of climate, hydrology and permafrost on Holocene peat accumulation at 3500 m on the eastern Qinghai–Tibetan Plateau. Quat. Sci. Rev. 28, 3303–3314 (2009).
Zeng, M., Zhu, C., Song, Y., Ma, C. & Yang, Z. Paleoenvironment change and its impact on carbon and nitrogen accumulation in the Zoige wetland, northeastern Qinghai-Tibetan Plateau over the past 14,000 years. Geochem. Geophys. Geosyst. 18, 1775–1792 (2017).
Herzschuh, U., Winter, K., Wünnemann, B. & Li, S. A general cooling trend on the central Tibetan Plateau throughout the Holocene recorded by the Lake Zigetang pollen spectra. Quat. Int. 154-155, 113–121 (2006).
Herzschuh, U., Kramer, A., Mischke, S. & Zhang, C. Quantitative climate and vegetation trends since the late glacial on the northeastern Tibetan Plateau deduced from Koucha Lake pollen spectra. Quat. Res. 71, 162–171 (2009).
Cheung, M.-C., Zong, Y., Zheng, Z., Liu, Z. & Aitchison, J. C. Holocene temperature and precipitation variability on the central Tibetan Plateau revealed by multiple palaeo-climatic proxy records from an alpine wetland sequence. Holocene 27, 1669–1681 (2017).
Chen, F. et al. Climate change, vegetation history, and landscape responses on the Tibetan Plateau during the Holocene: A comprehensive review. Quat. Sci. Rev. 243, 106444 (2020).
Zhang, C. et al. Seasonal imprint of Holocene temperature reconstruction on the Tibetan Plateau. Earth Sci. Rev. 226, 103927 (2022).
Li, Y. et al. Control of local topography and surface patterning on the formation and stability of a slope permafrost peatland at 4800-m elevation on the central Qinghai-Tibetan Plateau. Ecol. Indic. 158, 111475 (2024).
Wang, Q.-F. et al. Non-climate environmental factors matter to Holocene dynamics of soil organic carbon and nitrogen in an alpine permafrost wetland, Qinghai‒Tibet Plateau. Adv. Clim. Chang. Res. 14, 213–225 (2023).
Zhang, H. & Väliranta, M. To better detect drivers of peatland carbon accumulation rates and patterns. Environ. Res. Lett. 19, 041004 (2024).
Loisel, J. et al. Insights and issues with estimating northern peatland carbon stocks and fluxes since the Last Glacial Maximum. Earth Sci. Rev. 165, 59–80 (2017).
Xia, Z., Oppedal, L. T., Van der Putten, N., Bakke, J. & Yu, Z. Ecological response of a glacier-fed peatland to late Holocene climate and glacier changes on subantarctic South Georgia. Quat. Sci. Rev. 250, 106679 (2020).
Cleary, K. G., Xia, Z. & Yu, Z. The growth and carbon sink of tundra peat patches in Arctic Alaska. J. Geophys. Res. Biogeosci. 129, e2023JG007890 (2024).
Magnan, G. et al. Widespread recent ecosystem state shifts in high-latitude peatlands of northeastern Canada and implications for carbon sequestration. Glob. Chang. Biol. 28, 1919–1934 (2022).
Xia, Y., Yang, Z., Sun, J., Xia, Z. & Yu, Z. Late-Holocene ecosystem dynamics and climate sensitivity of a permafrost peatland in Northeast China. Quat. Sci. Rev. 324, 108466 (2024).
Yang, Q., Liu, Z. & Bai, E. Comparison of carbon and nitrogen accumulation rate between bog and fen phases in a pristine peatland with the fen-bog transition. Glob. Chang. Biol. 29, 6350–6366 (2023).
Sun, J. Climatic and Topographic Effect on the Distribution and Dynamics of Peatlands on the Tibetan Plateau: Past and Future. Ph. D. Dissertation (Northeast Normal University, 2024).
Gao, Y. & Couwenberg, J. Carbon accumulation in a permafrost polygon peatland: steady long-term rates in spite of shifts between dry and wet conditions. Glob. Chang. Biol. 21, 803–815 (2015).
Jones, M. C. et al. Rapid carbon loss and slow recovery following permafrost thaw in boreal peatlands. Glob. Chang. Biol. 23, 1109–1127 (2017).
Krüger, J. P., Conen, F., Leifeld, J. & Alewell, C. Palsa uplift identified by stable isotope depth profiles and relation of δ15N to C/N ratio. Permafrost Periglacial Process. 28, 485–492 (2017).
Barral, U. M. et al. Can anthropization govern the water and carbon dynamics? A case study of peatlands in Serra do Espinhaço Meridional, Brazil. Wetl. Ecol. Manag. 31, 479–497 (2023).
Philben, M., Kaiser, K. & Benner, R. Does oxygen exposure time control the extent of organic matter decomposition in peatlands? J. Geophys. Res. Biogeosci. 119, 897–909 (2014).
Young, D. M., Baird, A. J., Gallego-Sala, A. V. & Loisel, J. A cautionary tale about using the apparent carbon accumulation rate (aCAR) obtained from peat cores. Sci. Rep. 11, 9547 (2021).
Miehe, G. et al. The Kobresia pygmaea ecosystem of the Tibetan highlands—origin, functioning and degradation of the world’s largest pastoral alpine ecosystem: Kobresia pastures of Tibet. Sci. Total Environ. 648, 754–771 (2019).
Wang, C. et al. Changes in plant biomass and species composition of alpine Kobresia meadows along altitudinal gradient on the Qinghai-Tibetan Plateau. Sci. China Ser. C-Life Sci. 51, 86–94 (2008).
Schleuss, P.-M. et al. Nitrogen uptake in an alpine Kobresia pasture on the Tibetan Plateau: localization by 15N labeling and implications for a vulnerable ecosystem. Ecosystems 18, 946–957 (2015).
Tian, H. et al. Patterns of soil nitrogen storage in China. Glob. Biogeochem. Cycles 20, GB1001 (2006).
Jackson, R. B. et al. The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 48, 419–445 (2017).
Leifeld, J. & Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 9, 1071 (2018).
Hodgkins, S. B. et al. Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production. Proc. Natl. Acad. Sci. USA. 111, 5819 (2014).
Sun, J., Gallego-Sala, A. & Yu, Z. Topographic and climatic controls of peatland distribution on the Tibetan Plateau. Sci. Rep. 13, 14811 (2023).
Zhao, Z. et al. Dynamic changes of plateau wetlands in the Damqu River Basin, Yangtze River Source Region, China, 1988–2015. Wetlands 40, 1409–1424 (2020).
Topp, G. C., Parkin, G. W., Ferré, T. P. A., Carter, M. R. & Gregorich, E. G. Soil water content. in Soil Sampling and Methods of Analysis Vol. 2 (eds. Carter, M. R. & Gregorich, E. G.) 939–962 (CRC Press, 2008).
Harris, D., Horwáth, W. R. & van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or CARBON-13 isotopic analysis. Soil Sci. Soc. Am. J. 65, 1853–1856 (2001).
Leuenberger, M. To what extent can ice core data contribute to the understanding of plant ecological developments of the past? in Terrestrial Ecology Vol. 1 (eds. Dawson, T. E. & Siegwolf, R. T. W.) 211−233 (Elsevier, 2007).
Teickner, H. ir: Functions to handle and preprocess infrared spectra. https://zenodo.org/record/5747170 (2022).
Teickner, H. & Hodgkins, S. B. irpeat: Functions to analyze mid infrared spectra of peat samples. https://github.com/henningte/irpeat (2022).
Loisel, J. et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 24, 1028–1042 (2014).
Yu, Z., Loisel, J., Brosseau, D. P., Beilman, D. W. & Hunt, S. J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 37, L13402 (2010).
Zhang, Y. Integration dataset of Tibet Plateau boundary. A Big Earth Data Platform for Three Poles. https://doi.org/10.11888/Geogra.tpdc.270099 (2019).
Acknowledgements
We thank the Three-Rivers-Source National Park Administration for granting permission to conduct this project and for approving the sampling activities; and Zucheng Wang, Yuwen Fu, Yingfan Xia, Qiulei Yan, Shuai Zhang, Jiahong Sun, Qiao Yang and others for discussion. This work was supported by the National Natural Science Foundation of China (42494822, 42330509).
Author information
Authors and Affiliations
Contributions
Z.Y. and T.Y. conceptualized the idea and designed this research; Z.Y., J.S., Y.L., H.L., M.W., and S.Z. conducted field works and sample collections; T.Y., J.S. and Y.L. completed the analyses; T.Y., J.S., Y.L., M.W., H.L., S.W., Z.X., and Z.Y. wrote and discussed the results of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Kunshan Bao, Alexandre Christófaro Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Annie Bourbonnais, Somaparna Ghosh. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, T., Sun, J., Li, Y. et al. Impact of climate-induced water-table drawdown on carbon and nitrogen sequestration in a Kobresia-dominated peatland on the central Qinghai-Tibetan Plateau. Commun Earth Environ 6, 188 (2025). https://doi.org/10.1038/s43247-025-02168-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02168-6