Abstract
Despite the global ban on leaded gasoline, environmental lead pollution remains a concern, especially in developing countries. Asia, and particularly China, has emerged as a major emission center, yet long-term records of atmospheric lead emission are scarce and often inconsistent. Here we reconstruct a 60-year history of lead concentration (lead/calcium) and isotopic composition in a coral from Dongsha Atoll in the northern South China Sea. The record shows a fourfold increase in lead level from 1953 to the mid-2000s, followed by a ~60% decline over the next decade. Shifts in lead isotopic composition suggest a transition from local automotive emissions to more distant industrial emissions, primarily from coal combustion in mainland China after 2000. This industrial source has declined significantly after the mid-2000s in response to stricter air pollution controls. Our findings highlight both the lasting legacy of leaded gasoline and the effectiveness of recent environmental policies in reducing atmospheric lead exposure.
Similar content being viewed by others
Introduction
Lead (Pb) is one of the most toxic elements with highly detrimental effects on human health, especially on brain and nerve system development in young children1,2. The continuous release of Pb into the environment through human activities has led to one of the most severe instances of global chemical exposure3,4. Recognizing this serious public health risk, many countries took steps to phase out the primary source of Pb pollution, namely leaded gasoline, towards the end of the 20th century5,6,7. However, despite the elimination of leaded gasoline, elevated blood Pb concentration (>5 µg dL-1) in children8 has continuously been reported, predominantly in Asian, African, and South American countries. This indicates the persistence of contemporary Pb sources, even after the global phase-out of leaded gasoline.
Previous studies, drawing upon emission inventories9,10,11 and aerosol measurements12, have revealed the presence of contemporary Pb sources. These findings emphasize the dominant contribution of Asian countries, particularly China, as major Pb emitters after phasing out leaded gasoline9,10,11. Notably, Chinese-origin Pb has been identified in various locations, extending as far as the western United States13. However, investigations on suspended particulate matter and sediment records in China revealed contradictory trends in atmospheric Pb emissions during the recent decade14,15. For example, several studies present an upward trajectory in atmospheric Pb emissions16,17,18, while other studies indicate a substantial reduction or no changes in atmospheric Pb emissions during recent decades, and their quoted amounts are also substantially different14,15,19,20. These divergent findings may, in part, arise from the studies anchored in specific site analyses, potentially limited to capture the regional dynamics of atmospheric Pb depositions. Additionally, studies on sediments may lack robust age control and/or resolution, impeding their ability to accurately portray the temporal changes in Pb emissions. To address the discrepancies, it is crucial to develop records that can preserve regional signals of atmospheric Pb pollution and also bear robust chronologies. Such records can serve as indispensable tools to reconstruct Pb emission patterns, offering potential validation for emission inventories and enabling a comprehensive evaluation on the impact of policy changes on Pb emissions21,22,23,24,25.
Corals, particularly those living in shallow water of tropical oceans, are unique geochemical archives to record the history of environmental changes with annual or higher resolution. The calcium carbonate skeletons of corals incorporate and preserve a wide range of chemical constituents, including trace elements (such as Pb) and their isotopes, from the surrounding seawater26,27. In offshore surface oceans, Pb in seawater is primarily sourced (>95%) from atmospheric deposition28, and has a residence time of a few years29,30,31,32. Corals growing in surface oceans can then be used to quantitatively track the temporal variability of atmospheric Pb deposited to the oceans. Such reconstructions have been successfully demonstrated in the North Atlantic33, the Indian Ocean22,24, and the Pacific Ocean21,25.
The South China Sea (SCS) is a marginal sea in the Western Pacific. As the areas surrounding the SCS, such as Taiwan, Hong Kong, and eastern China, have undergone rapid industrialization and urbanization in recent decades, this region has observed a notable increase in anthropogenic Pb emissions23,34,35. Though previous studies on corals have addressed Pb pollution in the SCS, most study sites are located in coastal areas near major cities23,25,36,37. The obtained records are vulnerable to various localized human activities, such as mining, metal smelting, industrial wastewater discharge, and fossil fuel combustion, which may introduce both atmospheric and non-atmospheric Pb inputs into coastal waters. Moreover, the isotopic composition of Pb in coastal seawater can be substantially modified by exchanging with natural fluvial particles24,38. Therefore, these coastal Pb records (refs. 22,23) may not be suitable for regional representation. In contrast, coral records from open ocean sites, located distant away from coastal areas and local sources, have the potential to represent regional-scale emissions and can offer crucial field validation for emission inventories. To date, records from open ocean sites reflecting recent East Asian Pb emissions are rare.
In this study, we investigate the extent and impacts of Pb pollution in East Asia, particularly China, using a coral core from the outer rim of Dongsha Atoll (20.58-20.78°N, 116.68-116.92°E), northern SCS (Fig. 1). By analysing Pb concentrations and isotope ratios in the coral, we obtained an annually resolved Pb pollution record spanning a period of 60 years, from 1953 to 2016 (see Materials and Methods). We then compared our coral-based record with historical Pb emissions in the region to assess the relationship between the Pb record and anthropogenic activities. Lastly, we conducted a comparison of regional to global-scale variations in atmospheric Pb emissions, which leads to a discussion of environmental policies.
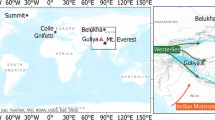
This map shows that eastern China, due to its soaring economic development and urbanization, has been dominating the regional SO2 emissions in the past decades. The emitted SO2 and its accompanying trace metals can influence the atmospheric depositions at downwind sites, including Dongsha Atoll, South China Sea. The circle marks our study ___location, the Dongsha Atoll. Other study sites of published coral records in the region are also shown: Nha Trang23 (triangle) and Phuket22 (square).
Results
Temporal variability in Dongsha coral Pb/Ca and Pb isotopes
Figure 2 presents Pb concentrations (as Pb/Ca) as well as variations in 206Pb/207Pb and 208Pb/206Pb in Dongsha coral DS86. The record can be separated into four intervals with distinctive changes in Pb/Ca, which on the whole ranges from 10 to 40 nmol mol−1. Specifically, Pb/Ca increased gradually after being relatively stable during the 1950s and reached a plateau of ~24 nmol mol−1 between the late 1970s and early 1980s. The ratio then continued to rise, peaking at ~40 nmol mol−1 in the mid 2000s, followed by a decline until 2016, eventually reaching a level similar to that observed in the 1960s. Similarly on the isotope side, both 206Pb/207Pb and 208Pb/206Pb were remarkably stable during the 1950s, ranging from 1.1719 to 1.1764 and 2.0882 to 2.0921, respectively. From the 1960s to early 1980s, the isotope ratios display a large variability, ranging from 1.1524 to 1.1715 for 206Pb/207Pb and 2.0938 to 2.1106 for 208Pb/206Pb. Between the early 1980s and the mid-2000s, 206Pb/207Pb demonstrated relative stability, with an average of 1.163 ± 0.001 (n = 22). The ratio then increased and stabilized at 1.167 ± 0.001 (n = 6) in the 2010s. In contrast, 208Pb/206Pb displayed a fluctuating pattern. The ratio increased nearly monotonically from 2.0887 in 1984 to 2.1120 around 2005, but then decreased to 2.0997 in 2016.
The time series is divided into four distinct periods. The phase-out of Pb gasoline in China in 2000 is delineated by a grey dashed line. The error bars (1σ) of the Pb/Ca indicate the standard deviation of the mean for triplicate measurements, and Pb isotope ratios were determined through single measurements, with error bars representing the 2σ uncertainty. The error bars (2σ) shown in the panels of Pb isotope ratios represent the analytical precision of the NIST SRM981 values at a concentration comparable to the samples, with precisions of 0.00025 for 206Pb/207Pb and 0.00074 for 208Pb/206Pb.
Discussion
Atmospheric Pb deposition presented in the Dongsha coral
Dongsha Atoll is influenced by the southwest monsoon in summer and the northeast monsoon in winter. The meteorological dynamics reveals a dominance in aerosols sourced not only from nearby areas, such as South China (e.g., Guangdong and Hong Kong) and Taiwan, but also from distant regions (e.g., East and North China, and Japan) via the long-range atmospheric transport (Fig. 1, Supplementary Fig. S1). However, the aerosol contribution from Southeast Asia remains secondary because aerosols are more readily scavenged from the atmosphere by summer monsoon rainfall in the region39,40. Moreover, owing to its remote ___location from coastal areas (about 340 km) and surrounding deep water (about 1300 m in depth) (Fig. 1), Dongsha experiences minimal localized Pb contributions from land carried by surface runoff, as well as a subtle impact from coastal water-particle exchange38. Consequently, Dongsha represents an open ocean environment where atmospheric sources predominate in the Pb in seawater.
Pb has a short residence time in the atmosphere and in surface seawater, about one week and 2–4 years, respectively24,29,30. Thus, Pb in seawater can effectively represent changes in atmospheric Pb emissions. Once seawater Pb is absorbed by corals during their skeleton accretion, Pb isotope signatures can then be well preserved. For example, the Pb isotope ratios in Dongsha coral (206Pb/207Pb = 1.1608 ± 0.0002 and 208Pb/207Pb = 2.4485 ± 0.0004 in 2000, 2σ, Supplementary Fig. S2) closely match the reported values in surface seawater collected near the coral site in 2000 (206Pb/207Pb = 1.1619 ± 0.0003; 208Pb/207Pb = 2.4472 ± 0.0004, 2σ)24. This tight resemblance corroborates that the Dongsha record can indeed represent the Pb change in surface seawater. However, Pb concentration in corals alone is not sufficient to infer the source of Pb pollution. We hereby employ both Pb concentration and isotopic ratios in Dongsha coral to identify and potentially apportion the Pb sources in atmospheric Pb deposition over the past 60 years (Fig. 3).
Given the changing sources of Pb over time, we divide the record into four periods: (I) 1953-1960, (II) 1961-early 1980s, (III) mid 1980s-mid 2000s, and (IV) mid 2000s-2016. The red line represents a Chinese Pb isotopic signature, delineating the relationship between Pb/Zn ores (blue area) and coals (yellow area) found within China16. The blue dashed line represents a binary mixing line between the typical Australian Pb (by average, 206Pb/207Pb = 1.041, 208Pb/206Pb = 2.225) and U.S. Pb (by average, 206Pb/207Pb = 1.200, 208Pb/206Pb = 2.050) ores used in Pb gasoline additives44. In the lower left corner, the triple-isotope plot shows broad coverages of Pb isotopes in different sources from China (namely, Pb/Zn ores and coals) together with the Australian-US Pb mixing line and Chinese Pb line.
The early Pb pollution stage (1953–1960)
During the period from 1953 to 1960, the Pb/Ca ratio in Dongsha coral remained consistently low, averaging 11.2 ± 1.4 nmol mol-1 (n = 8, Fig. 2). Meanwhile, the isotopic compositions of the Dongsha coral exhibited remarkable stability, with 206Pb/207Pb at the highest value of 1.174 ± 0.001 and 208Pb/206Pb clustering at the lowest value of 2.091 ± 0.001, respectively, within the entire dataset (Fig. 2). These values deviate quite substantially from those of marine sediments from the SCS (206Pb/207Pb = 1.194 ± 0.014, 208Pb/206Pb = 2.082 ± 0.013, Supplementary Fig. S3), which are mostly sourced from terrigenous materials41, and the Pb isotope ratios in other natural sources from China reported in literature (Supplementary Fig. S3). Instead, the Pb isotope values are located slightly toward the Australian-US leaded gasoline mixing line in the triple-isotope plot (Fig. 3), suggesting that anthropogenic Pb had already left footprints in SCS seawater as early as in the 1950s.
During this time period, countries in the region gradually recovered from World War II. The initiation of industrial development and leaded gasoline consumption increased Pb pollution in the environment, evidenced by relatively elevated Pb concentrations in corals from the western SCS (refs. 23,37). Limited literature on Pb isotopes in environmental samples and gasoline Pb emission in the region42,43, however, prevents source apportionment of the anthropogenic Pb. Nevertheless, Dongsha Pb/Ca remains the lowest during the 1950s, which suggests that the source of Pb during this period should be predominantly natural and the least impacted by human activities in the record.
Rising contribution from gasoline Pb (1961–early 1980s)
After 1960, the Dongsha coral exhibited a gradual increase in Pb/Ca, elevating to approximately 25 nmol mol-1 in the early 1980s (Fig. 2). Similar Pb/Ca trajectories and magnitudes also appeared in other corals from the region, such as Nha Trang in the western SCS (ref. 23) and Phuket in the eastern Andaman Sea22 (Supplementary Fig. S4). This time interval aligns with the early phases of Asian economic development, marked by a surge in leaded gasoline consumption across the region24, including Taiwan, Hong Kong, and perhaps Japan as well21. Therefore, the rise in coral Pb/Ca can mostly be attributed to the widespread and increasing use of leaded gasoline in the region.
This attribution is supported by the changes in Pb isotopic composition in Dongsha coral. Between 1961 and the early 1980s, Pb isotopes registered the largest variability within the entire record, with 206Pb/207Pb and 208Pb/206Pb ranging from 1.1524 to 1.1715 and from 2.0938 to 2.1106, respectively (Fig. 2). These ratios are substantially different from those during the early pollution stage (i.e., 1953–1960, when 206Pb/207Pb = 1.174 ± 0.001, 208Pb/206Pb = 2.091 ± 0.001, n = 8) and shift further away from the values of natural terrestrial sources in the triple-isotope plot, and thus, support the growing contributions from anthropogenic sources (Fig. 3). Such variability in Pb isotope ratios most likely resulted from different Pb additives that were applied into gasolines during this time interval. Alkyllead produced from both Australian and US Pb ores was extensively used as an additive in gasolines consumed in the region, and they have distinctively different isotope signatures (by average, 206Pb/207Pb = 1.041, 208Pb/206Pb = 2.225 in typical Australian Pb; 206Pb/207Pb = 1.200, 208Pb/206Pb = 2.050 in typical US Pb)44,45. Moreover, local Pb ores were also extracted to produce Pb additives in domestic leaded gasolines45. This probably explains the observation that the Pb isotope compositions in Dongsha coral do not align well with the blend of Australian and U.S. Pb (Fig. 3).
Recently, Bi et al.16 reported an updated Chinese Pb line, based on more extensive isotope analyses on Chinese Pb/Zn ores and coals. The line is characterized by higher 206Pb/207Pb and 208Pb/206Pb relative to the Australian–US Pb line (Fig. 3). We found that, during this time, the Pb isotopic compositions in Dongsha coral predominantly fall within the spectrum between the blend of Australian–US Pb and the Chinese Pb line (Fig. 3), supporting the contributions from Chinese Pb sources, particularly from Chinese domestic Pb/Zn ores16. However, the Chinese economic development had remained mostly stagnant until the late 1970s when the “Open Door” policy was initiated. In the triple-isotope plot, 208Pb/206Pb in Dongsha coral is lower, at a given 206Pb/207Pb value, than that in Chinese urban aerosols collected before the leaded gasoline ban in 2000 (Supplementary Fig. S5). The observation suggests that the atmospheric Pb contribution from Mainland China, particularly that via long-range atmospheric transport, should have been minor before the early 1980s. The atmospheric Pb deposition at Dongsha Atoll could have been sourced from Japan during this time period. Japan underwent a rapid economic growth and heavy industrialization after the 1950s, leading to a sharp increase in Pb emissions that peaked in the late 1960s (refs. 21,46). However, the Pb contribution from Japan should also remain low because of the rainfall scavenging by winter monsoons during the long-range transport across the western Pacific47.
Instead, the atmospheric Pb deposition at Dongsha Atoll was probably dominated by nearby sources, such as from Taiwan and Hong Kong. The two regions have gained rapid industrialization and economic growth since the 1960s (refs. 48,49). The Gross Domestic Products (GDPs) in Taiwan and Hong Kong increased about tenfold and fourfold, respectively, between the 1960s and the early 1980s (data from the Federal Reserve Economic Data (https://fred.stlouisfed.org)). Accordingly, the number of vehicles and gasoline consumptions should have increased. During this time interval, leaded gasoline was the primary fuel product for vehicles, and the Pb content in petrol could have reached 0.84 g L−1 50,51. Being adjacent to Dongsha, the Pb emission from the gasolines consumed in Taiwan and Hong Kong43,52, with Pb additives mostly sourced from Australian and US ores, should have dominated in the coral Pb isotope signature (Supplementary Fig. S6). The substantial differences in the Pb isotopes of the gasoline additives from the Australian and US ores44, together with local-sourced Pb additives are likely responsible for the large fluctuations observed in Dongsha isotope ratios. The complexly sourced Pb may also have contributed to the large discrepancies in Pb isotopic compositions observed among different coral records in the region22,23 (Supplementary Fig. S4).
Transition to Chinese Pb dominance (mid 1980s–mid 2000s)
Since the late 1970s and early 1980s, China has experienced a dramatic soaring in its industrial development and economic growth. Accompanying the economy growth, Pb emission from automobile exhaust had nearly doubled in China until its complete leaded gasoline phase-out in 2000 (ref. 24). Meanwhile, the rapid economic expansion has been heavily reliant on coal consumption and metal smelting, which also released an enormous amount of heavy metals, including Pb, into the environment. It was reported that, as early as the 1990s, China has already become a major Pb emitter in the world45. Therefore, both gasoline-sourced and industrial-sourced Pb emissions can be transported from China, particularly its eastern industrial region, downwind to the SCS and subsequently be incorporated into coral skeletons23. Correspondingly, the Dongsha coral experienced a rapid increase in Pb/Ca after the early 1980s, peaking in 2004 with an annual rate of 1.0 nmol mol−1. This surge was notably sharp compared to the preceding period between 1961 and the early 1980s, where the rate was 0.4 nmol mol-1 per year.
The argument of notable Pb contribution from mainland China can be supported by Pb isotope ratios. During this time interval, in addition to the alkyllead imported from UK53, the majority of Pb additives in gasolines consumed in China were, in fact, manufactured from domestic Pb/Zn ores, which is characterised by higher 208Pb (ref. 16). Correspondingly, we observed relatively stable 206Pb/207Pb in Dongsha record at values of 1.163 ± 0.001 (1984–2004, n = 21), but a nearly monotonical increase in 208Pb/206Pb, progressing from 2.089 to 2.109 (Fig. 2). Note that Chinese coal is also characterized by higher 208Pb/206Pb ratios compared to those from other regions16,17,45. The coal combustion associated with soared industrial activities in China must have contributed to the rising Pb/Ca in Dongsha coral as well. However, the magnitude of atmospheric Pb emission from coal combustion was much smaller compared to the gasoline Pb emission16,24. Thus, in the triple-isotope plot (Supplementary Fig. S5), the isotopic composition of Dongsha coral is overall consistent with the values of urban aerosols collected before the leaded gasoline phase-out, which was dominated by vehicle fuel emissions; and furthermore, the isotopic ratios shifted toward the Chinese Pb/Zn ore values rather than Chinese coal values16 (Fig. 3).
The dominance of Pb emissions from China, particularly from its eastern region, continuously grew over time. By the mid 2000s, not only did the Pb/Ca reach its maximum level in the Dongsha coral (39 nmol mol-1), but also the Pb isotope values approached the highest 208Pb/206Pb in the record, nearest to the Chinese Pb line (Figs. 2, 3). The influence of Chinese Pb emissions also became regional, aided by long-range atmospheric circulations. For example, Pb isotopic compositions in the coral records from Nha Trang23 and Phuket22 located in the downwind of the Asian winter monsoon, show a converging trend toward the values in the Dongsha coral by decreasing in 206Pb/207Pb but increasing in 208Pb/206Pb (Supplementary Fig. S4). Similarly, via the transport by the westerlies, Chinese Pb emissions made broader footprints, influencing the Pb isotopes of surface seawater in the Northwestern Pacific54,55, and the Kuroshio56 (Supplementary Fig. S7), and even the aerosol deposits in the western United States13 and Canada12.
The influence of Chinese policies (mid 2000s–2016)
After 2004, the Pb/Ca in Dongsha coral exhibited a steady decline from 38 nmol mol-1 to 16 nmol mol−1, approaching the Pb level of 11 nmol mol−1 occurred during the early pollution stage (Fig. 2). A similar decreasing trend is observable in the coral records from Nha Trang23 and Phuket22, suggesting the regional significance of Pb decline (Supplementary Fig. S4). Simultaneously, in Dongsha coral, 206Pb/207Pb increased from 1.1630 in 2005 to 1.1692 in 2011, while the 208Pb/206Pb decreased from 2.1120 in 2005 to 2.0965 in 2011 (Fig. 2).
Numerous inventory-based studies indicated that, after China had completely phased out leaded gasoline in 2000, atmospheric Pb emission in China dramatically decreased57,58. The Pb emissions subsequently resumed to rise, thanks to soaring industrial activities which involve heightened coal combustion and ore smelting, and to a less extent, the rising consumptions of unleaded gasolines. For instance, the coal consumption in the region has been steadily increasing, and China, in particular, has witnessed a dramatic increase in coal combustion until reaching a plateau of 4.3 × 106 million metric tonnes around 2013 (Historical coal consumption data were sourced from the U.S. Energy Information Administration (EIA) website: https://www.eia.gov/international/data/world) (Fig. 4a). Correspondingly, Li et al.57 estimated that after the leaded gasoline completely phased out in 2000, atmospheric Pb emission from industrial activities (e.g., coal combustion and non-ferrous smelting) has nearly quadrupled by 2009, and the majority of this emission is associated with coal utilization.
a The black dot shows Dongsha coral data from this study. Historical coal consumptions in China and India (million metric tonnes, Mmt, dashed lines), and sulfur dioxide (SO2) emissions in China (kiloton, kt, red line) are also shown. Notably, the variations in national SO2 emissions in China exhibit a strong correlation with the Pb/Ca ratios in the Dongsha coral (r = 0.94, p < 0.001, n = 12, 2005–2016). b The white dot, square, and triangle denote coral records from the tropical Indian Ocean— Chagos24, Diego22, Salomon22, and Lakshadweep76, respectively. The grey dots represent Pb records from (c) Antarctic ice cores75, (d) Bermuda coral73, and (e) Greenland ice cores74. Greenland and Antarctic ice core records refer to the geometric mean of the atmospheric Pb flux reconstructed from a panel of ice cores in Southern Greenland (n = 13) and Antarctica (n = 16). Coral records are presented in terms of Pb/Ca. Emission inventory data for the USA and Europe are from Boyle et al.83, while data for other countries are from Lee et al.24 and Chen et al.77. Historical coal consumption data were obtained from the U.S. Energy Information Administration (EIA) website (https://www.eia.gov/international/data/world). SO2 emission of China were sourced from the Multi-resolution Emission Inventory model for Climate and air pollution research (MEIC) website (http://www.meicmodel.org).
The shift in the dominant sources of atmospheric Pb emission is clearly observable in the triple-isotope plot (Fig. 3), particularly if we take into account the Pb residence time in seawater. After the mid 2000s, the Pb isotopic composition gradually shifted away from the ___domain of Chinese Pb/Zn ores (206Pb/207Pb = 1.140 ± 0.055, 208Pb/206Pb = 2.149 ± 0.073) toward the ___domain of Chinese coals (206Pb/207Pb = 1.188 ± 0.030, 208Pb/206Pb = 2.089 ± 0.049, but by weighted mean, 206Pb/207Pb = 1.178-1.182, 208Pb/206Pb = 2.099-2.106), perhaps accompanied by a relatively increasing contribution of natural sources (206Pb/207Pb = 1.224 ± 0.060, 208Pb/206Pb = 2.031 ± 0.095)16 (Supplementary Figs. S3 and S8). However, the continuous decrease of Pb/Ca in the coral record post the mid 2000s is at odds with inventory-based studies which suggest a steady increase in atmospheric Pb emission at least until 2009, even though the rising magnitude of Pb emission from coal combustion and ore smelting remained lower than the emission amount from leaded gasoline in the earlier decades. Such discrepancy is likely attributable to the continuous technological advancement and improved management strategies to control air pollutions, such as the installation of devices to decrease sulfur dioxide (SO2) emission58 (Fig. 4a). Being one of the chalcophile elements, Pb is closely affiliated with S in sulfide ores and coals, particularly in Chinese coals59. During coal combustion, Pb is mostly emitted in the particulate forms of PbSO4 or PbO and becomes enriched in fly ash, which can be effectively removed together with other harmful heavy metals during the process of scrubbing for SO2 capture60,61. Thus, applying new technologies and strengthening policies on air pollution controls may bring co-benefits to reduce the amounts of anthropogenic hazardous elements in atmospheric emissions.
As early as 2005, China initiated a series of policies to reduce pollutant emissions and improve energy efficiency. Under these policies, China retired a considerable number of old and less-efficient coal-fired power plants and started to implement new technologies, such as installing flue gas desulfurization (FGD) devices in new thermal power plants for electricity generation and heat production62. Despite some arguments on their effectiveness63,64, the policies on SO2 emission abatement should benefit the reduction of Pb emitted to the atmosphere. Indeed, in line with the decrease of the national SO2 emissions in China by 15% from 2005 to 2010 (refs. 65,66), Pb/Ca in the Dongsha coral dropped parallel from 34 nmol mol−1 to 27 nmol mol−1 (Fig. 4a).
China continued its stringent policies on environmental protection, particularly on aerosol emission control during the 12th Five-Year Plan (12th FYP) period (2011–2015) (ref. 67). For example, in 2012, the Ministry of Ecology and Environment of China revised the threshold for acceptable ambient air quality to 500 ng m−3 of Pb (National Ambient Air Quality Standards (GB 3095–2012)) (ref. 68). This revision marked a dramatic improvement from the previous standard of 1000 ng m−3 established in 1996 (GB 3095-1996). In 2013, China implemented the ‘Air Pollution Prevention and Control Action Plan’ (State Council, Air Pollution Prevention and Control Action Plan (Guo Fa [2013] No. 37, September 10, 2013) (in Chinese), English translation available on the Clean Air Alliance of China website). These comprehensive and stringent policies encompassed a suite of targeted actions aimed at environmental protection, leading to remarkable improvements in ambient air quality across the country69,70. As a consequence, SO2 emissions further decreased by approximately 50%, and Pb/Ca in the Dongsha coral declined to 16 nmol mol−1 by 2016 (Fig. 4a). The decline in Pb concentrations recorded in our Dongsha coral, therefore, serves as evidence for the efficacy of these atmospheric prevention and control policies. With the continued implementation of efficient control interventions, it is anticipated that the Pb emission to the regional atmosphere will continue to decrease, while the relative contribution of naturally sourced Pb will further increase in the future, akin to the observations in the Atlantic Ocean71,72.
Regional and global implications from Pb records
Our Dongsha Pb record can provide regional and even global implications on policy effectiveness to control Pb emissions. Here, we selected Pb records from remote sites, such as Bermuda in the Atlantic Ocean73, Greenland74, Antarctica75, and the tropical Indian Ocean (i.e., the Chagos archipelago22,24 and Lakshadweep76), alongside our record from Dongsha Atoll, for comparative analysis with automotive emission inventories12,24,54,73,77 (Fig. 4). Most of these records are derived from sites geographically distant from Pb emission sources, making them representative of atmospheric Pb deposition on a regional scale. The fluctuations in Pb levels within these records are then mostly influenced by both the regional amount of Pb emissions and the aerosol transport facilitated by atmospheric circulations. Moreover, these records bear robust chronological controls with annual or higher resolution, allowing them to effectively register changes in atmospheric Pb deposition.
While it may be challenging to pinpoint the precise origin and pathway of aerosol transport, the comparisons between the Pb records and the regional Pb emission amounts reveal that the temporal variations of Pb in the Greenland (r2 = 0.74, p < 0.001, Fig. 4e), Bermuda (r2 = 0.78, p < 0.001, Fig. 4d), and Antarctica (r2 = 0.50, p < 0.001, Fig. 4c) records can be reasonably attributed to regional automotive Pb emissions, and atmospheric Pb deposition became substantially lower after phasing out leaded gasoline in the region. In contrast, the Dongsha record (r2 = 0.03, p = 0.291, Fig. 4a) shows a trend not solely attributed to automotive emissions, indicating persistent atmospheric Pb deposition post-1990s gasoline phase-out. For the four coral records from the tropical Indian Ocean (Fig. 4b), despite their discrepancies in Pb/Ca values, they all display an overall increasing trend, continuously through 2013, the youngest timing in the corals76. Therefore, these records reveal increasing influence of Pb emissions from industrial activities in regional countries, India in particular, long after phasing out leaded gasolines.
Strikingly, Dongsha, Chagos, and Lakshadweep islands are all located in regions adjacent to many low- and middle-income countries, where elevated blood Pb levels in children have been widely reported8. The contrast observed here not only underscores the increasing impact of industrial Pb emissions in Asia and around the tropical Indian Ocean but also urges imperative measures to continuously monitor, prevent, and mitigate industrial Pb emissions in developing countries, even in the wake of the ban on leaded gasoline. As shown in our Dongsha record, a discernible reduction in Pb/Ca can result from the implementation of stringent environmental policies in China. We, therefore, underscore the critical need for other developing countries to implement and strengthen environmental regulations, particularly those concerning the emissions of SO2 and heavy metals from coal-fired power plants to mitigate environmental Pb exposure and its associated severely detrimental health impacts.
Materials and Methods
Coral collection and sub-sampling procedures
In August 2016, we collected a 50-cm long Porites sp. coral core (DS86) at 8.6 metres water depth from Dongsha Atoll, SCS (20.62°N, 116.7°E) (Fig. 1). The core was sectioned into 7 mm-thick slabs along its maximum growth axes, followed by X-ray examination (Supplementary Fig. S9). Subsequently, we used a diamond-coated wire saw to cut samples, approximately 0.5–1.0 g, from each annual band of the coral slabs. The samples were then cleaned following the procedure adapted from the Shen and Boyle33 method to remove surface contaminants and organic materials in the corals. Initially, coral samples were crushed into small fragments for cleaning (in a diameter of 2–4 mm for primary cleaning and then in a diameter of 280–700 μm for final cleaning). The extensive cleaning process comprised alternating treatments with NaOH-H2O2 oxidant, hydrazine-based reductant (ammonia, hydrazine, and citric acid), and HNO3. After the cleaning, three subsamples, approximately 50 μg each, were randomly picked from the crushed coral samples for subsequent Pb and Ca concentration analyses. The remaining crushed coral samples were reserved for Pb isotope analysis.
Coral chronology
The X-ray image displays clear annual density bands on Dongsha coral DS86, which were used to determine its chronology (Supplementary Fig. S9). To ensure a robust age model and minimize chronology uncertainty, we further tuned the age model based on the Sr/Ca record from the coral, published by Lin et al.78. Specifically, we measured the distance between Sr/Ca maxima, representing each December, with each distance considered one year in the age model. Disparities in age estimates for Sr/Ca were minimal, less than two months.
Chemical procedures and measurements for Pb concentrations
Labware cleaning, reagent preparation, and all chemical procedures for Pb concentration and Pb isotopic analyses were carried out in a metal-free class-1000 clean room with a class-100 working bench at the Earth Observatory of Singapore (EOS), Nanyang Technological University (NTU). JT Baker ultrapure reagents (e.g., 8 M HBr, 14 M HNO3, and 12 M HCl) were used for all chemical procedures. A Milli-Q water system provided ultra-pure water (>18.2 MΩ·cm) for cleaning labware and reagent preparation.
For Pb concentration measurements, each subsample was dissolved in 1 mL of 5% HNO3 using acid-cleaned 1.5 mL polyethylene microcentrifuge tubes. Pb/Ca was measured on an ELEMENT 2 high-resolution inductively coupled plasma mass spectrometer (HR-ICP-MS, Thermo Fisher Scientific Inc., Germany). To mitigate the influence of background, the Pb blank from the machine was reduced with 5% HNO3, ensuring that relative blank values did not exceed 1‰ for each measured sample. For optimal precision, the Pb concentration of individual samples was controlled at around 1.0 ppb. To assess data accuracy, we used certified reference materials, including the coral powder reference JCp-1 and an in-house multi-element solution standard, with duplicate tests showing deviations of less than 3%. To externally correct Pb concentrations in subsamples, a 1.0 ppb 71 A multi-element standard (Inorganic Ventures) was used.
Chemical procedures and measurement for Pb isotopes
To prepare samples for Pb isotope analysis, we followed the procedures outlined in Kraus and Moore79 and Strelow80 for Pb purification. Each sample, approximately 0.3 g in weight, was dissolved in 1 mL of 7 N HNO3 and the solution was dried down at 150 °C. Then, 1 mL of 6 M HCl and two drops of HClO4 were added to ensure thorough digestion and removal of organic materials from the sample solution. Subsequently, the samples were dried again. Before proceeding with Pb purification and collection, the samples were re-dissolved using 200 μL of 1.1 N HBr. Pb separation procedure involved an anion exchange column (Bio-Rad AG1-X8, 200–400 mesh). The AG1-X8 resin was first loaded into a small Teflon column (2 cm long with a 4 mm i.d.) and cleaned by passing 1200 μL 6 N HCl followed by 400 μL 6 N HCl. The resin was then conditioned with 400 μL 1.1 N HBr before loading the sample in 200 μL 1.1 N HBr. After transferring the sample, 150 μL 1.1 N HBr was added to the resin, followed by 400 μL 1.1 N HBr and 400 μL 2 N HCl to elute the matrix, while Pb remained in the column. Pb was then eluted by 500 μL 6 N HCl and collected in a 7 mL Teflon beaker. Two more drops of HClO4 were added to eliminate organic material from the sample solution, which was then dried at 185 °C. Finally, the Pb fraction was dissolved in 1% HNO3 (+0.1% HF) for the measurement of Pb isotopic compositions.
Measurements on Pb isotopic composition (204Pb, 206Pb, 207Pb, and 208Pb) were conducted using a Neptune Plus multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS, Thermo Fisher Scientific Inc., Germany) housed at EOS, NTU. To ensure accuracy and reliability, we employed a calibration process to account for mass fractionation, isobaric interferences, procedural and instrumental blanks, tailing error, and accuracy adjustment. Our sample solutions were prepared with the 208Pb signal always above 200 mV during the measurement. 208Pb in chemistry procedural blanks was consistently measured below 1‰ in measured sample signals, that is, less than 0.2 mV with a similar amount of solution. To minimize Pb memory effects, we performed fast and rigorous cleaning on the sample introduction system, alternating between 0.5 N HCl and 0.2 N HNO3 before and after analysing individual samples and standards. A 1% HNO3 wash solution was then employed to reduce the baseline of 208Pb to less than 0.2 mV, ensuring that the relative blank values for each measured sample remained below 1‰. We applied a thallium (Tl) doping method, involving 205Tl and 203Tl, during Pb isotopic composition measurements to correct mass discrimination and drift. We also applied a standard-sample bracketing method, with a 0.5 ppb solution of Pb standard reference material (NIST SRM981) for analysis to correct instrumental drift. The measured ratios for 206Pb/207Pb and 208Pb/206Pb for the NBS981 were 1.09342 ± 0.00025 and 2.16634 ± 0.00074 (n = 203), respectively, which are in good agreement with the certified ratios (206Pb/207Pb: 1.09333 ± 0.00033 and 208Pb/206Pb: 2.1681 ± 0.0008). In each working session, our measured data underwent corrections for blank, instrumental mass bias, and mercury isotope interference. Calibration procedures followed the established protocols outlined in Reuer et al.81 and Boyle et al.82. The quality of the Pb isotope data from our laboratory is exemplified in Chen et al.38. For example, for the Pb isotopes of NIST SRM981 at a comparable concentration, we obtained standard deviations (n > 200) within 104 ppm for 206Pb/207Pb and within 329 ppm for 208Pb/206Pb. In this study, our focus is solely on interpreting the 206Pb, 207Pb, and 208Pb data, excluding 204Pb, for the following reasons: (a) The 204Pb signals in most samples were low and lacked the expected accuracy; (b) we report 206Pb/207Pb due to its traditional use in early environmental Pb studies; and (c) since the temporal variability of 208Pb/207Pb and 206Pb/207Pb in these samples is similar (Supplementary Fig. S2), we present 208Pb/206Pb instead of 208Pb/207Pb for more informative insights. Additionally, we maintain the use of 206Pb/207Pb and 208Pb/206Pb for the isotope ratio comparison plot, as this method is widely recognized and accepted in the field of anthropogenic Pb isotope research.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study have been deposited in a Zenodo repository and are accessible at https://zenodo.org/records/15314902.
References
Bellinger, D. C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 20, 172–177 (2008).
Needleman, H. L. & Bellinger, D. The health effects of low level exposure to lead. Annu. Rev. Public Health 12, 111–140 (1991).
Huang, H. et al. Exposure sources, intake pathways and accumulation of Pb in human blood. Soil Secur. 100150 (2024).
Collin, M. S. et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 7, 100094 (2022).
Thomas, V. M. The elimination of lead in gasoline. Annu. Rev. Energy Environ. 20, 301–324 (1995).
Needleman, H. L. The removal of lead from gasoline: historical and personal reflections. Environ. Res. 84, 20–35 (2000).
Angrand, R. C., Collins, G., Landrigan, P. J. & Thomas, V. M. Relation of blood lead levels and lead in gasoline: an updated systematic review. Environ. Heal. 21, 138 (2022).
Rees, N. & Fuller, R. The toxic truth: children’s exposure to lead pollution undermines a generation of future potential. (UNICEF, 2020).
Bai, X. et al. Present knowledge and future perspectives of atmospheric emission inventories of toxic trace elements: a critical review. Environ. Sci. Technol. 57, 1551–1567 (2023).
Zhu, C., Tian, H. & Hao, J. Global anthropogenic atmospheric emission inventory of twelve typical hazardous trace elements, 1995–2012. Atmos. Environ. 220, 117061 (2020).
Ohara, T. et al. An Asian emission inventory of anthropogenic emission sources for the period 1980–2020. Atmos. Chem. Phys. 7, 4419–4444 (2007).
Koffman, B. G. et al. Provenance of anthropogenic Pb and atmospheric dust to Northwestern North America. Environ. Sci. Technol. 56, 13107–13118 (2022).
Ewing, S. A. et al. Pb isotopes as an indicator of the Asian contribution to particulate air pollution in urban California. Environ. Sci. Technol. 44, 8911–8916 (2010).
Cui, Q. et al. Trends in elemental Pb concentrations within atmospheric PM2.5 and associated risk to human health in major cities of China. Environ. Pollut. 121036 (2023).
Xu, H. et al. Seasonal and annual variations in atmospheric Hg and Pb isotopes in Xi’an, China. Environ. Sci. Technol. 51, 3759–3766 (2017).
Bi, X.-Y. et al. Lead isotopic compositions of selected coals, Pb/Zn ores and fuels in China and the application for source tracing. Environ. Sci. Technol. 51, 13502–13508 (2017).
Wang, Z. et al. Isotopic signatures and outputs of lead from coal fly ash disposal in China, India, and the United States. Environ. Sci. Technol. 57, 12259–12269 (2023).
Tian, H. Z. et al. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 15, 10127–10147 (2015).
Wan, D. et al. Spatiotemporal trends of atmospheric Pb over the last century across inland China. Sci. Total Environ. 729, 138399 (2020).
Wang, T. et al. Anthropogenically-induced atmospheric Pb cycle in low-latitude Asia since the Industrial Revolution recorded by high-resolution stalagmites. Glob. Planet. Change 232, 104337 (2024).
Inoue, M. & Tanimizu, M. Anthropogenic lead inputs to the western Pacific during the 20th century. Sci. Total Environ. 406, 123–130 (2008).
Qian, N. et al. Spatial-temporal variability and sources of Lead (Pb) in the Indian Ocean and Asian Marginal Seas. J. Geophys. Res. Ocean. 128, e2022JC019222 (2023).
Chen, M., Goodkin, N. F., Boyle, E. A., Switzer, A. D. & Bolton, A. Lead in the western South China Sea: Evidence of atmospheric deposition and upwelling. Geophys. Res. Lett. 43, 4490–4499 (2016).
Lee, J.-M. et al. Coral-based history of lead and lead isotopes of the surface Indian Ocean since the mid-20th century. Earth Planet. Sci. Lett. 398, 37–47 (2014).
Inoue, M. et al. Distribution and temporal changes of lead in the surface seawater in the western Pacific and adjacent seas derived from coral skeletons. Environ. Pollut. 144, 1045–1052 (2006).
Dodge, R. E. & Gilbert, T. R. Chronology of lead pollution contained in banded coral skeletons. Mar. Biol. 82, 9–13 (1984).
Vielzeuf, D. et al. Growth kinetics and distribution of trace elements in precious corals. Front. Earth Sci. 6, 167 (2018).
Flegal, A. R. Lead in tropical marine systems: A review. Sci. Total Environ. 58, 1–8 (1986).
Nozaki, Y. & Tsunogai, S. 226Ra, 210Pb and 210Po disequilibria in the western North Pacific. Earth Planet. Sci. Lett. 32, 313–321 (1976).
Bacon, M. P., Spencer, D. W. & Brewer, P. G. 210Pb/226Ra and 210Po/210Pb disequilibria in seawater and suspended particulate matter. Earth Planet. Sci. Lett. 32, 277–296 (1976).
Chung, Y. 210Pb in the western Indian Ocean: distribution, disequilibrium, and partitioning between dissolved and particulate phases. Earth Planet. Sci. Lett. 85, 28–40 (1987).
Cochran, J. K., Bacon, M. P., Krishnaswami, S. & Turekian, K. K. 210Po and210Pb distributions in the central and eastern Indian Ocean. Earth Planet. Sci. Lett. 65, 433–452 (1983).
Shen, G. T. & Boyle, E. A. Lead in corals: reconstruction of historical industrial fluxes to the surface ocean. Earth Planet. Sci. Lett. 82, 289–304 (1987).
Flegal, A. R., Gallon, C., Ganguli, P. M. & Conaway, C. H. All the lead in China. Crit. Rev. Environ. Sci. Technol. 43, 1869–1944 (2013).
Zhu, Z. et al. Spatial and seasonal characteristics of dissolved heavy metals in the seawater of Beibu Gulf, the northern South China Sea. Front. Mar. Sci. 9, 996202 (2022).
Chen, T.-R. et al. Heavy metal pollution recorded in Porites corals from Daya Bay, northern South China Sea. Mar. Environ. Res. 70, 318–326 (2010).
Wang, B.-S. et al. Temporal distributions of anthropogenic Al, Zn and Pb in Hong Kong Porites coral during the last two centuries. Mar. Pollut. Bull. 63, 508–515 (2011).
Chen, M. et al. Boundary exchange completes the marine Pb cycle jigsaw. Proc. Natl. Acad. Sci. 120, e2213163120 (2023).
Chate, D. M. et al. Scavenging of aerosols and their chemical species by rain. Atmos. Environ. 37, 2477–2484 (2003).
He, J. & Balasubramanian, R. Rain-aerosol coupling in the tropical atmosphere of Southeast Asia: distribution and scavenging ratios of major ionic species. J. Atmos. Chem. 60, 205–220 (2008).
Zhu, L. et al. Source and distribution of lead in the surface sediments from the South China Sea as derived from Pb isotopes. Mar. Pollut. Bull. 60, 2144–2153 (2010).
Wong, M. H. A review on lead contamination of Hong Kong’s environment. Lead, Mercur. cadmium Arsen. Environ. Ch. 14, 217–223 (1987).
Duzgoren-Aydin, N. S., Li, X. D. & Wong, S. C. Lead contamination and isotope signatures in the urban environment of Hong Kong. Environ. Int. 30, 209–217 (2004).
Mukai, H. et al. Characterization of sources of lead in the urban air of Asia using ratios of stable lead isotopes. Environ. Sci. Technol. 27, 1347–1356 (1993).
Bollhöfer, A. & Rosman, K. J. R. Isotopic source signatures for atmospheric lead: the Northern Hemisphere. Geochim. Cosmochim. Acta 65, 1727–1740 (2001).
Hirao, Y. et al. Lead isotope ratios in Tokyo Bay sediments and their implications in the lead consumption of Japanese industries. Geochem. J. 20, 1–15 (1986).
Hosono, T., Alvarez, K. & Kuwae, M. Lead isotope ratios in six lake sediment cores from Japan Archipelago: Historical record of trans-boundary pollution sources. Sci. Total Environ. 559, 24–37 (2016).
Ho, S. P. S. Economics, economic bureaucracy, and Taiwan’s economic development. Pac. Aff. 60, 226–247 (1987).
Howe, C. Growth, Public Policy and Hong Kong’Economic Relationship with China. China Q 95, 512–533 (1983).
Ho, Y. B. The effect of Pb reduction in petrol on the Pb content of kerbside dust in Hong Kong. Sci. Total Environ. 93, 411–418 (1990).
Yao, P.-H. et al. Lead isotope characterization of petroleum fuels in Taipei, Taiwan. Int. J. Environ. Res. Public Health 12, 4602–4616 (2015).
Chow, T. J., Snyder, C. B., & Earl, J. L. Isotope ratios of lead as pollutant source indicators. Proc. Symp. Isot. Ratios Pollut. Source Behav. 95–108 (Vienna Int. At. Energy Agency, 1975).
Cheng, H. & Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: a review. Environ. Pollut. 158, 1134–1146 (2010).
Zurbrick, C. M., Gallon, C. & Flegal, A. R. Historic and industrial lead within the Northwest Pacific Ocean evidenced by lead isotopes in seawater. Environ. Sci. Technol. 51, 1203–1212 (2017).
Chien, C.-T., Ho, T.-Y., Sanborn, M. E., Yin, Q.-Z. & Paytan, A. Lead concentrations and isotopic compositions in the Western Philippine Sea. Mar. Chem. 189, 10–16 (2017).
Chen, M. et al. Dissolved lead (Pb) concentrations and Pb isotope ratios along the East China Sea and Kuroshio transect—evidence for isopycnal transport and particle exchange. J. Geophys. Res. Ocean. 128, e2022JC019423 (2023).
Li, Q., Cheng, H., Zhou, T., Lin, C. & Guo, S. The estimated atmospheric lead emissions in China, 1990–2009. Atmos. Environ. 60, 1–8 (2012).
Tian, H. et al. Atmospheric emission inventory of hazardous trace elements from China’s coal-fired power plants– temporal trends and spatial variation characteristics. Environ. Sci. Technol. 48, 3575–3582 (2014).
Tian, H. Z. et al. A review of key hazardous trace elements in Chinese coals: abundance, occurrence, behavior during coal combustion and their environmental impacts. Energy Fuels 27, 601–614 (2013).
Zhao, S. et al. Emission characteristic and transformation mechanism of hazardous trace elements in a coal-fired power plant. Fuel 214, 597–606 (2018).
Wang, Q. et al. Effective removal of trace-level toxic metals from flue gas desulfurization wastewater using SiO2 supported hydrogel sorbent. Mater. Today Sustain. 21, 100324 (2023).
Xia, Y., Zhao, Y. & Nielsen, C. P. Benefits of China’s efforts in gaseous pollutant control indicated by the bottom-up emissions and satellite observations 2000–2014. Atmos. Environ. 136, 43–53 (2016).
Hanif, M. A., Ibrahim, N. & Abdul Jalil, A. Sulfur dioxide removal: An overview of regenerative flue gas desulfurization and factors affecting desulfurization capacity and sorbent regeneration. Environ. Sci. Pollut. Res 27, 27515–27540 (2020).
Punshon, T., Knox, A. S., Adriano, D. C., Seaman, J. C. & Weber, J. T. Flue gas desulfurization (FGD) residue: potential applications and environmental issues. Biogeochem. Trace Elem. coal coal Combust. Byprod. 7–28 (1999).
Zhao, Y., Zhang, J. & Nielsen, C. P. The effects of recent control policies on trends in emissions of anthropogenic atmospheric pollutants and CO2 in China. Atmos. Chem. Phys. 13, 487–508 (2013).
Zhao, Y. et al. Decline in bulk deposition of air pollutants in China lags behind reductions in emissions. Nat. Geosci. 15, 190–195 (2022).
Zheng, W. & Walsh, P. P. Economic growth, urbanization and energy consumption—A provincial level analysis of China. Energy Econ. 80, 153–162 (2019).
Wu, R. et al. Are current Chinese national ambient air quality standards on 24-hour averages for particulate matter sufficient to protect public health?. J. Environ. Sci. 71, 67–75 (2018).
Wu, W. Is air pollution joint prevention and control effective in China—evidence from “Air Pollution Prevention and Control Action Plan. Environ. Sci. Pollut. Res. 30, 122405–122419 (2023).
Wang, Z. et al. Prospect of China’s ambient air quality standards. J. Environ. Sci. 123, 255–269 (2023).
Olivelli, A. et al. Decline of anthropogenic lead in South Atlantic Ocean surface waters from 1990 to 2011: New constraints from concentration and isotope data. Mar. Pollut. Bull. 189, 114798 (2023).
Bridgestock, L. et al. Return of naturally sourced Pb to Atlantic surface waters. Nat. Commun. 7, 12921 (2016).
Kelly, A. E., Reuer, M. K., Goodkin, N. F. & Boyle, E. A. Lead concentrations and isotopes in corals and water near Bermuda, 1780–2000. Earth Planet. Sci. Lett. 283, 93–100 (2009).
McConnell, J. R. et al. Pervasive Arctic lead pollution suggests substantial growth in medieval silver production modulated by plague, climate, and conflict. Proc. Natl. Acad. Sci. 116, 14910–14915 (2019).
McConnell, J. R. et al. Antarctic-wide array of high-resolution ice core records reveals pervasive lead pollution began in 1889 and persists today. Sci. Rep. 4, 5848 (2014).
Rahaman, W. et al. Anthropogenic Lead (Pb) deposition history of the western Indian Ocean from coral-based Pb/Ca ratio and Pb isotope records. Sci. Total Environ. 955, 177312 (2024).
Chen, M. et al. Monsoonal variations of lead (Pb) in coastal waters around Singapore. Mar. Pollut. Bull. 179, 113654 (2022).
Lin, K. et al. Influences of East Asian Winter Monsoon and El Niño-Southern Oscillation Variability on the Kuroshio Intrusion to the South China Sea Over the Past 60 Years. Geophys. Res. Lett. 51, e2023GL104155 (2024).
Kraus, K. A. & Moore, G. E. Anion exchange studies. VI. 1, 2 The divalent transition elements manganese to zinc in hydrochloric acid. J. Am. Chem. Soc. 75, 1460–1462 (1953).
Strelow, F. W. E. Distribution coefficients and anion exchange behavior of some elements in hydrobromic-nitric acid mixtures. Anal. Chem. 50, 1359–1361 (1978).
Reuer, M. K., Boyle, E. A. & Grant, B. C. Lead isotope analysis of marine carbonates and seawater by multiple collector ICP-MS. Chem. Geol. 200, 137–153 (2003).
Boyle, E. A. et al. GEOTRACES IC1 (BATS) contamination-prone trace element isotopes Cd, Fe, Pb, Zn, Cu, and Mo intercalibration. Limnol. Oceanogr. Methods 10, 653–665 (2012).
Boyle, E. A. et al. Anthropogenic lead emissions in the ocean: The evolving global experiment. Oceanography 27, 69–75 (2014).
Acknowledgements
We thank Dr. Ming-Shiou Jeng for his help in collecting the Dongsha coral core. This work has been possible thanks to the support of the Earth Observatory of Singapore via its funding from the National Research Foundation Singapore (NRF) and the Singapore Ministry of Education (MOE) under the Research Centres of Excellence initiative. The study was also financially supported by the National Science and Technology Council (NSTC), Taiwan, under grant numbers NSTC112-2116-M-001-033 and NSTC112-2116-M-001-003 to Y.-G.C. X.W. acknowledges the financial support from the Singapore Ministry of Education under grant numbers MOE-T2EP10122-0006 and MOE-MOET32022-0006. This work comprises the Earth Observatory of Singapore contribution no. 652.
Author information
Authors and Affiliations
Contributions
K.L., M.C., and X.W. designed the original study. K.L. and M.C. conducted the formal analysis, investigation, and data visualization. K.L., M.C., J.W., and C.S. developed the methodology and performed statistical analysis. K.L., M.C., and X.W. led the manuscript writing. Y.-G.C. and X.W. supervised the project. K.L. and X.W. performed validation. All authors contributed to the writing and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Arianna Olivelli and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, K., Chen, M., Wang, J. et al. Coral records indicate six-decade rise and fall of atmospheric lead emission in China. Commun Earth Environ 6, 376 (2025). https://doi.org/10.1038/s43247-025-02363-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02363-5