Abstract
Pollution by heavy metals (Cu, Zn, Cd, Cr) in global inland waters poses significant threats to freshwater biodiversity and ecosystem function, yet the global patterns and drivers of heavy metals remain poorly explored. Here, assessing using 11,573 data points collected from 514 peer-reviewed publications, key advances include: (1) quantification of median concentrations in global inland waters (Cu: 8.38, Zn: 30.00, Cd: 0.53, and Cr: 7.00 μg L−1), providing global-scale reference values to contextualize local water quality assessments; (2) anthropogenic activities, temperature, actual evapotranspiration, precipitation and runoff dominantly control the concentrations of heavy metals in inland waters, and (3) global heavy metal pollution hotspots were established, revealing significantly elevated concentrations in West and South Asia and Africa, followed by South America. Our study provides a comprehensive analysis of inland water for Cu, Zn, Cd, and Cr, offering a scientific foundation for targeting pollution control in vulnerable regions under climate change.
Similar content being viewed by others
Introduction
Inland waters are essential for supplying water resources, providing habitats, and supporting biodiversity (e.g., aquatic plants, fish, macroinvertebrates, and microbial communities)1, all of which contribute to ecosystem stability and water quality regulation. Global inland waters cover a total surface area of 3.62 × 106 km2, including rivers, lakes, reservoirs, and streams2, playing a vital role in the transport of substances within ecosystems. However, an increasing population and the development of economy have greatly exacerbated the deterioration in water quality, containing accumulation of heavy metals3. Excessive environmental pollution has caused a huge burden on inland water ecosystems due to their complexity, fragility, and limited ability of self-purification.
In the past years, as the most persistent pollution in aqueous environment, heavy metals have received growing attentions4,5,6,7,8, due to their solubility, toxicity, concentrations, and slow decomposition in natural environments3,6. Copper (Cu) and zinc (Zn) are the most common in nature, and high concentrations can also pose great risks, while cadmium (Cd), chromium (Cr) are classical heavy metals with high toxicity in the environment9. For example, previous study evidenced the lethal impact of Zn concentrations on the crustaceans Temora stylifera, and acute effect of Cu concentrations on the mollusks Corbicula fluminea10. Exceeding Cd can damage kidney through injuring proximal tubules and lead to osteoporosis by intervening with calcium metabolism in animals11.
Heavy metals originated from both anthropogenic activities and natural processes in aquatic environments. Anthropogenic sources, including industrial and agricultural activities, for example, mining, smelting, disposal of unreasonable wastewater, and the use of fertilizer as well as pesticides containing heavy metals, and fossil fuel combination emissions, contribute significantly to inland waters10,12. However, the function of some natural conditions could not be ignored. Atmospheric deposition, geologic weathering, and soil erosion are the partial reasons leading to the pollution by heavy metals in inland waters. Additionally, precipitation frequency and intensity can directly or indirectly affect heavy metal pollution levels. Increased rainfall may enhance the transport of heavy metals from anthropogenic sources to rivers and lakes, while simultaneously influencing their concentrations through processes such as water body dilution, altered surface runoff patterns, and enhanced soil erosion13,14,15. Additionally, changes in hydrometeorological factors, for example, sudden increases in flow or turbulence caused by heavy rainfall, may cause the resuspension of sediment heavy metals and the release of dissolved heavy metals16.
To date, huge amounts of studies are dedicated to pollutant levels, sources, and effects on aquatic organisms as well as humans. Furthermore, machine learning techniques have been widespread in predicting substances of global distribution17,18. Wei et al. have reported the global patterns of lead concentrations in inland waters8, whereas the other considerable heavy metals (Cu, Zn, Cd, Cr) should also be on the agenda. Therefore, here a database about Cu, Zn, Cd, and Cr concentrations from published literature before November 2023 in global inland waters was established. In the context of human activities and social development, our aims as follow: (1) to cognize the concentrations range of these four metals in global inland waters; (2) to quantify the effects of inland water ecosystem types, climatic seasons, monsoonal seasons, and land use types on the heavy metal pollution levels in inland waters; (3) to quantify the driving factors affecting the heavy metals; and (4) to predict the spatial distribution of heavy metal concentrations in global patterns.
Results
Distribution patterns of Cu, Zn, Cd, and Cr concentrations in inland water
Our database showed the abundant distribution of Cu, Zn, Cd, and Cr in inland waters across the globe with most studies in tropics and subtropics as well as a few in North America, Northern Europe and Australia (Fig. 1a). The density map of these four metals is shown in Fig. 1b, and concentrations in our database ranged from 0 to 183.00 × 103 μg L−¹ (median: 8.38) for Cu, 0 to 276.05 × 103 μg L⁻¹(median: 30) for Zn, 0 to 8.87 × 103 μg L⁻¹ (median: 0.53) for Cd, and 0 to 384.00 × 103 μg L⁻¹ (median: 7.00) for Cr.
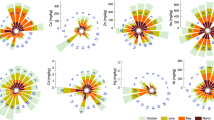
In addition, different seasonal classifications, ecosystem types, and land use types did impact on heavy metal concentrations in inland waters. Cu and Cr during dry season (Cu: 11.75, 95% CI: 5.13–26.30 μg L−1 and Cr: 8.71, 95% CI: 3.63–20.42 μg L−1) were significantly higher than those during wet season (Cu: 7.76, 95% CI: 0.14–0.97 μg L−1 and Cr: 5.13, 95% CI: 2.69–9.33 μg L−1) (Fig. 2 and Supplementary Table S2). The concentrations of heavy metals in inland waters were significantly higher during the pre-monsoon period compared to the monsoon and post-monsoon periods (Fig. 2 and Supplementary Table S2). Heavy metal concentrations showed no significant differences across ecosystems, except for Zn, which was significantly higher in rivers than in lakes (Fig. 2 and Supplementary Table S2). All land use types contributed to heavy metal emissions, with industry, agriculture, and residential areas having the dominant effects (Fig. 2 and Supplementary Table S2). Natural land was also an important source of heavy metals, especially Cu, Zn, and Cr (Fig. 2 and Supplementary Table S2). Additionally, the interaction of classification variables for the four metals showed seasonal dynamics, combined with ecosystem and land use types, collectively determining heavy metal concentrations in inland waters (marginal R2 of the interaction models > marginal R2 of the single models) (Supplementary Table S3). Industrial areas emitted significantly higher amounts of heavy metals (particularly Cu and Zn) into rivers compared to other land use types (Supplementary Table S3). Compared to the wet season, the concentrations of Cu and Cr in rivers during the dry season increased significantly by 94.98% (Supplementary Table S3). During the dry season, the concentrations of Cu and Cr in rivers were high and significantly higher than those in the reservoir by 272% and 316.87%, respectively (Supplementary Table S3). Additionally, during the dry season, the heavy metal contents in water bodies under various land use types were higher. Specifically, industrial areas accumulated Zn and Cd concentrations during the dry season were significantly higher than those during the wet season by 44.54% and 9.64%, respectively, whereas mixed land use accumulated Cu and Cr concentrations during the dry seasons by 28.82% and 139.88%, respectively. (Supplementary Table S3). Water pH showed a significantly negative correlation with heavy metal concentrations, except for Cr (Supplementary Fig. S2 and Table S4).
Black points indicate posterior means, and colored bands indicate 95% and 80% confidence interval. Unreported land use types are defined as undefined land, and multiple land refers to the combination of industry, residents, agriculture or tourist, and so on. Numbers in parentheses indicate the sample size at each level under the classification types.
Drivers of heavy metal concentrations in inland waters
Random Forest (RF) model revealed excellent performance in prediction of heavy metal concentrations in inland waters (R2: 0.85, 0.83, 0.87, and 0.89; RMSE: 0.34, 0.36, 0.22, and 0.32, respectively for Cu, Zn, Cd, and Cr) (Fig. 3b and Supplementary Table S9). To improve the interpretability of the RF model, we excluded mean annual precipitation (MAP) during the process of reducing factor collinearity, retaining actual evapotranspiration (AET). However, since MAP was a critical factor, we also discussed its role in subsequent analyses, given the high correlation between MAP and AET (Supplementary Fig. S15). According to relative variable importance from RF, we found that second treatment of wastewater treatment plants (WWTPs), MAT, AET, human development index (HDI), elevation, and land surface runoff basically drive the four heavy metal concentrations in global inland waters, but the degree of influence on different metals varies (Fig. 3a). Additionally, we also conducted linear relationships using Bayesian mixed-effects model and exhibited the faintly effects of river area, cropland, urban, pasture, and forest on the four metals to varying degrees (Supplementary Fig. S16). Partial dependence plots revealed the non-linear changing tendency between heavy metals and predictors (Supplementary Fig. S4–7).
a The relative importance of predictors; b Comparison of predicted and observed concentrations of heavy metals in inland waters after log transformation. In regression plot (b), the dashed line indicates the 1:1 line, the blue line indicates the regression line between predicted and observed values, and the R2 represents the evaluation of random forest models. MAT mean annual temperature, AET actual evapotranspiration, LSR land surface runoff, HDI human development index, Primary primary treatment of wastewater treatment plants (WWTPs), Second secondary treatment of WWTPs, and Tertiary tertiary treatment of WWTPs.
Global patterns of heavy metal concentrations in inland waters
We produced global pattern maps of Cu, Zn, Cd, and Cr concentrations based on RF model, which maps clearly captured their spatial differences (Fig. 4a–d). Our analysis revealed that the observations in the database were relatively evenly across latitudes with broad coverage (Fig. S18). We also explored latitudinal trends in heavy metal concentrations based on model predictions to identify overall trends (Fig. 4a–d). Overall, Asia and Africa are the most affected by pollution from heavy metals, followed by South America (Fig. 4). Pollution of heavy metals was predominantly distributed between 40°N and 20°S (Fig. 4). Specifically, with the similar tendency of Cu and Zn, the highest concentrations were predicted in South Asia (e.g., India, Nepal), West Asia (e.g., Iran), Africa (particularly the midportion, including the Niger and Nile River basins), and South America (Fig. 4a, b). For Cd and Cr with similar trends, the highest concentrations were predicted in Africa (especially the midportion) and South Asia (e.g., India, Nepal, and Iran) (Fig. 4c, d). Meantime, all the prediction uncertainties see Supplementary Fig. S8.
a Cu; b Zn; c Cd; d Cr (left: global distribution map; right: global latitudinal patterns, different color lines indicate the locally weighted regressions between latitude and four heavy metal concentrations in the predicted global map). The predicted value is the average value of the results of 100 iterations of model. Predicting heavy metal concentrations (μg L−1) after log transformation was reserved to draw the map, aiming to avoid skewed distribution. The spatial resolution of grid cells is 3 arcmin by 3 arcmin.
Analysis of dissolved heavy metals in inland waters
We comprised the total concentrations and dissolved concentrations of heavy metals and found the total concentrations higher than the dissolved concentrations for Cu and Zn (Supplementary Fig. S3 and Table S5). We conducted the same analysis for the dissolved heavy metals (Supplementary Fig. S9–14 and S17) and discovered that the performance of the dissolved heavy metals in comparison between seasons, ecosystem types, land use types, and pH was basically similar with the overall data (Supplementary Fig. S10 and Table S6–8). Although there were slight changes in the importance of rankings of the RF models, in which population counts showed stronger effects for dissolved Cu and Zn (Supplementary Fig. S12), the global predicted spatial distributions of dissolved heavy metal concentrations were consistent with those of the total data (Supplementary Fig. S13–14).
Discussion
Difference of heavy metal concentrations in inland waters
The median concentrations of Cu, Zn, Cd, and Cr exceeded their respective background values in inland waters with 1, 10, 0.07, and 0.5 μg L−1, respectively19, suggesting combined natural and anthropogenic sources of heavy metals to inland waters. The concentrations of Cu, Zn, and Cr in inland waters exhibited a wider range (spanning six orders of magnitude) than those of Cd. On the one hand, although heavy metals are naturally occurring elements in the Earth’s crust, their concentrations vary significantly across different regions. Pollution of heavy metals and exposure largely originated from anthropogenic activities liking mining and smelting operations, industrial production and utilization, as well as domestic and agricultural use of metals and metal-containing compounds20. The Cu and Zn generally occurred in the most common rock-forming minerals, and Cr existed in rocks such as ilmenite and magnetite, whereas Cd is not very common in such rocks21, which is one of the reasons to led the difference between the four heavy metal concentrations. On the other hand, the wide range of uses for metals determines the amount of their artificial mining. For example, Cu and Zn are widely applied in many industrials, including automotive, aerospace, construction, electricity, energy, and mechanical engineering, thanks to excellent properties in physical, chemical, thermal, electrical, and isolating22. Enriching in ultramafic rocks, chromite is the primary commercial sources of Cr23, while Cd, a highly toxic heavy metal, is generally associated with ferrous and non-ferrous metal ores and mainly released to the surface environment through mining as well as milling activities24. Notably, although Cu and Zn are more abundant in Earth’s crust, Cr still exhibits a wide concentration range, suggesting that Cr emissions deserve greater attention. It is reported that Cr production has doubled decade since 1900, with by 1984 production reached 10.16 million metric tons, mainly used in metallurgical (67%), refractories (18%), and chemical (15%)23.
Drivers of heavy metal concentrations in global inland water
Anthropogenic activities (the dominant source of heavy metal emissions), together with natural factors such as climate (e.g., MAT, AET, precipitation), topography (e.g., elevation), and hydrology (e.g., surface runoff), collectively determine heavy metal concentrations in inland waters. Mining, coal combustion, effluent discharge, and waste disposal are the major industrial activities that contribute to the release of heavy metals into the environment21. As essential freshwater sources, rivers receive significant wastewater inputs, and their flow spreads heavy metal pollution through inland waters. Previous study indicated that mining and manufacturing were exposed to be pivotal factors of heavy metal concentrations in global rivers and lakes from 1970 to 20176. During the extraction of low-concentration metal ores, large amounts of waste rocks are generated, and heavy metals (such as Cu and Cd) in those rocks are dissolved by acid mine drainage, resulting in serious heavy metal pollution21. Additionally, Cu, Zn, and Cd contents discharged due to Cu mine or smelter plants are also significant12,25, and Cd is also one of the byproducts of Zn production26. Notably, high-temperature processes, such as coal and oil combustion in electric power stations and heat and industrial plants, gasoline combustion, roasting and smelting of ores in nonferrous metal smelters, and refuse incineration, directly release significant amounts of heavy metal contents into environmental systems, including atmospheric, aquatic, and terrestrial ecosystems27. Cd and Zn are common heavy metals in coal residues21. These pollutants, initially released into the atmosphere and deposited on land surfaces, are subsequently transported to inland waters through land surface runoff and wet and dry deposition.
The physical geographic environment constitutes an interconnected system with mutually interacting components28. The contributions of land surface runoff and precipitation to heavy metals in surface waters are interdependent. The mechanism by which precipitation influences the concentrations of heavy metals in the water bodies involves a complex combination of factors, including land use types, rainfall intensity, ecosystem types, and sampling frequency. Primarily, urban, mining, and agricultural areas containing high levels of heavy metals can easily discharge these metals into adjacent aquatic ecosystems through runoff during rainfall, especially heavy rain29,30,31. Increases in the amount or intensity of precipitation can enhance dissolution of metal carbonates and metal sulfides in sediments, which can then promote the release of heavy metals32, and may also improve the water volume, resulting in dilution of heavy metal concentrations3. First post-drought rain concentrates metals, while prolonged flow dilutes them, increasing export potential33. Additionally, precipitation alters the soil structure and leaches heavy metals from soil34, promoting their migration into adjacent water, especially during heavy rainfall events. Notably, the snow or ice covers with higher heavy metal concentrations may serve as an additional pathway for heavy metals input to freshwater ecosystems35,36. The acidification of the environment is also a factor in climate change. For example, acid rain would promote average annual leaching fluxes of Cu, Zn, and Cd in mine tailing37 and increase their solubility in the sediments and soils38,39, then aggravating their concentrations in the inland waters. Under lower pH conditions, the increased competition between metal ions and protons (H+) for binding sites on organic ligands and mineral surfaces leads to the dissociation of metal complexes, thereby releasing free metal ions into the aqueous solution40. Indeed, our study also indicated the strong negative relationship between pH and Cu, Zn, and Cd concentrations in inland waters, except Cr. Owing to the complex forms of Cr in waters, mainly including Cr(III) and Cr(VI), it can easily transform between these forms under changes in pH and redox conditions41.
Climate change accelerates the leach and load of heavy metals in surface resources due to increased runoff42, with rainfall being the main driving factor. Land surface runoff was considered as a significant source of heavy metals from land to waters15,43, mainly including urban runoff and agriculture runoff. Urban runoff is easy to takes away untreated heavy metals from land to aquatic environments, resulting from the rising imperviousness and intensive land use. The thin water layer near the soil or building material surface transfers dissolved metals to runoff, rapidly carrying pollutants away from the catchment44. Taka et al. indicated that the annual Cu and Zn loads in major studied urbanized catchments were 86 times than that to the rural site45. Research indicated that roads with more than 5000 vehicles per day are more likely to overflow with heavy metal pollution than highways46. The rising use of Zn in safety fences and galvanized car parts for corrosion prevention is a key anthropogenic source of Zn pollution in runoff46. In addition, runoff enhanced by melting thoroughly excavated heavy metal concentrations in the waters. Westerlund et al. found that particle sizes and total suspended solids during the melt period showed a strong correlation with total concentrations of heavy metals, whereas this relationship was less significant during the rain period47. Previous studies calculated the Cu and Zn concentrations in leaching water from agricultural soils in the Netherlands and found that surface water concentrations of Cu and Zn often exceeded critical values48. However, a recent study indicated that 14–17% of cropland is influenced by toxic metal pollution in global49. In cropland in most countries, the sources of heavy metals include natural sources, mining, smelting, agrochemicals and sewage sludge applications, and livestock manure uses50. For example, the main concerns of Cu, Zn, Cd, and Cr also occur in the use of sewage sludge for soil managements21.
This non-liner relationship between MAT and heavy metal concentrations in inland waters could be explained in diverse ways, directly or indirectly. Elevated temperatures enhance evapotranspiration, leading to water volume reduction and consequent heavy metal concentrations, while the extent of this process varies in different freshwater ecosystems. Unlike lakes and reservoirs, flow velocity of rivers will regulate water temperatures51, then affecting evapotranspiration. Additionally, rising temperatures exert significant influence on gas solubility, precipitation reactions, sorption reactions, and biological activity52, then influencing the heavy metal concentrations. Primarily, rising temperature will decrease the solubility of O2 (which influences redox potential) and CO2 (which influences pH), then may impact the process of oxidation in mine33. Decreasing dissolved oxygen also changes the heavy metal concentrations in water. Zn was released from sediments under anoxic condition, but re-adsorbed by iron or manganese oxides when oxygenated53. Additionally, higher temperature would fasten the release rate of dissolved heavy metal concentrations from freshwater sediments54, because the desorption process is enhanced with rising temperature, increasing the ion release rate38. Temperature can also influence heavy metal concentrations in aquatic environments by regulating phytoplankton growth. The dynamics of Cu, Zn, and Cd in reservoir systems are strongly regulated by algal-derived organic matter, as phytoplankton can absorb and assimilate these metals from the aquatic environment, and when the algae die, they settle into the sediments along with the associated heavy metals55, which are then easily ingested by benthic invertebrates56. Notably, increased temperatures promote heavy metals to overflow from mining areas. The leaching of Cu and Cr from tailing in arctic region enhances with the rise temperature, because of accelerating in sulfide oxidation, chemical reactivity, and solubility of heavy metals57.
Noteworthily, natural processes under climate change, such as weathering, also contribute to the input of some heavy metals into natural water bodies. Studies indicated that climate change enhances the mobilization of natural metal sources in a high mountain catchment58. Rock weathering is dominantly regulated by critical climatic and topographic factors, particularly temperature and runoff. By analyzing globally distributed basalt watersheds, a previous study revealed that temperature, runoff, and elevation collectively govern chemical weathering rates, thereby modulating heavy metal accumulation patterns59. Heavy metals in waters with higher elevation are more likely associated with geographical background of catchment (e.g., lithological characteristics) and with soil weathering and leaching processes. Watersheds with steep river slopes in higher elevations will accelerate physical erosion and promote chemical weathering59. Todd et al. (2012) found that climate-driven permafrost thaw and falling groundwater levels increased dissolved Zn and ecologically concerning metals by 100–400% (400–2000 μg L−1) in an undisturbed alpine watershed over 30 years60. Among them, the decline in groundwater levels has caused more underground sulfide-containing rocks to be exposed to the air, enhancing oxidation process and elevating dissolved metal concentrations60.
HDI serves as a proxy for national development. In general, the higher the HDI, the lower the heavy metal concentrations, which involves latent environmental governance capabilities of country, including critical infrastructure such as related management policies. Indeed, inadequate management of heavy metals constitutes another major driver of contamination by heavy metals in inland water bodies. Study shows that as a vital metal of industrial society, the use of Zn increased unprecedentedly between 2000 and 2010, while waste management became the highest losses of Zn to the environment61. Higher HDI means superior education, economy, and health62, where areas that people will introduce policies to decrease the related emissions of heavy metals to a certain extent. For example, in order to control contamination level of Cd, the United Nations Economic Commission for Europe endorsed the Aarhus Protocol on the Heavy metals63, and Sweden limited the maximum Cd concentrations of 100 mg kg−1 in phosphatic fertilizers as well as imposed a tax on it between 5 and 100 mg kg−16. Conversely, although Indian Standard enacted the maximum limitation for total chromium in water supplies is 0.05 mg L−164, the huge population and unreasonable sanitary conditions are contributed to a certain heavy metal pollution. A lot of religious activities, for example, idol immersion, have led to significant increases in pollution of Zn and Cr in River Hooghly in India65. Compared to HDI, the stronger correlation of population counts with dissolved Cu or Zn concentrations underscored the predominant role of direct anthropogenic activities in inland water pollution, particularly in areas with intense human activities. Additionally, WWTPs were also considered as a potential source of heavy metals in inland waters. A previous study showed that a considerable amount of Cu, Zn, Cd, and Cr were removed in sewage sludge from municipal and industrial wastewater treatment plants66. Wastewater treatment rates among countries decrease with average income67, indicating that countries with lower economic development have relatively backward heavy metal control. Notably, our model showed that the secondary treatment in WWTPs can reduce the discharge of heavy metals into inland waters to a certain extent (Supplementary Fig. S4–7). Although tertiary treatment has a stronger removal effect on pollution, whereas, its penetration rate is currently low in most countries68. Therefore, secondary treatment should be established as the minimum global standard for heavy metal removal in WWTPs, offering optimal cost-benefit efficiency, particularly for low-income countries.
Global patterns of heavy metal concentrations in inland waters
On the whole, our predictions are similar to the global prediction of lead concentrations in inland waters8. Past studies identified continent-specific heavy metal pollution sources: Africa (waste discharge and rock weathering), North America (fertilizers, pesticides, and rock weathering), Europe (mining, manufacturing, and waste discharge), and Asia and South America (mining, manufacturing, rock weathering)6. In Africa, rapid population growth and high urbanization rates have led to recent urban expansion without proper planning or adequate waste disposal facilities69. Additionally, mining in sub-Saharan Africa has caused heavy metal pollution, as increasing mining, fugitive dust, toxic waste dumping and burning, and factories in residential areas, and lacking proper regulations of mineral-rich areas70. For example, Nairobi’s 30-acre landfill (more than 2000 tons per day) pollutes East Africa, while West Africa’s oil industry (pipeline leaks, spills, and sabotage) causes heavy metal contamination69. Previous studies showed that South Asian rivers, despite accounting for only about 9% of global river discharge, contribute disproportionately to dissolved heavy metal fluxes (e.g., 15 ± 1% of global Cu)71. As a country with the second largest population and rapid industrialization and urbanization, vehicular emissions and industrial emissions are the major sources of heavy metal pollution ambient air in Indian72, then entry aquatic waters by dry or wet deposition. Mining and related industries, as well as excessive use of pesticides in agricultural land resulted in the increase of heavy metal concentrations in Iranian water resources73. In Nepal, industrial activities and traffic areas contributed a considerable part of the source of heavy metals74.
Anthropogenic activities, increase temperature, change of rainfall pattern (containing increased rainfall intensify and frequent extreme rainfall), strengthened evapotranspiration, and improved runoff will promote the heavy metal pollution of related regions to varying degrees. Low-latitude regions are more vulnerable to change of temperature and precipitation, where intensified physical erosion and chemical weathering, along with increased land surface runoff, have resulted in elevated levels of heavy metal contamination. These areas demand greater attention from the management and control of heavy metal emissions. Notably, most of South America and Africa are located in a tropical rainforest climate75, which is more susceptible to changes in temperatures and precipitation, lead to higher heavy metal concentrations in aquatic environments from anthropogenic and natural sources.
Limitations and prospects
Although our study successfully captured global concentration ranges of Cu, Zn, Cd and Cr in inland waters, found that the concentrations of these metals in inland waters showed high model explanation under the interaction among different categories (seasonal types, land use types, and ecosystem types), conformed the main drivers of these metals in the waters, and understood the global patterns of concentrations of these metals, several limitations remain. The less distribution of observations of heavy metals, especially in the Americas, Russia, and Australia, may influence our accuracy of global prediction of heavy metals. The sampling data of different years in the database may cause the prediction model to deviate from the actual situation to a certain extent. The difference of Zn concentrations between rivers and lakes may have some impact on the accuracy of global prediction of Zn concentrations. Additionally, the significant difference between total concentrations and dissolved concentrations of heavy metals (Cu and Zn) may contribute to underestimating the global map of predicting concentrations (total) in inland water.
More attention from Cu, Zn, Cd, and Cr pollution in inland waters should be reinforced and several measures should be implemented. Above all, corresponding measures are formulated according to the sources of heavy metals in different regions, for instance, regions of industrials, agricultures, mixed land, or intensive traffic areas. Besides, the interaction suggests that different ecosystem types may need to be addressed seasonally. Industrial discharge controls should be prioritized during dry seasons for riverine systems to compensate for reduced dilution capacity. The mixed land combing several heavy metal sources should be better managed during the dry season. Before wet season, the management measures should be held in high regard, owing to the predicting importance of land surface runoff. Additionally, regions like Africa (midportion) and South Asia (e.g., India and Nepal) should prioritize monitoring pollution of heavy metals in water bodies, since climate (e.g., temperature, rainfall) may exacerbate release of heavy metals from both human activities and natural sources.
Heavy metal pollution in the waters should be alleviated. Introducing specific aquatic phytoremediation plants has been shown to have the potential to minimize the heavy metal concentrations from surface waters, for example, Cu, Zn, Cd in the contaminated water could be remedied by Miscanthus sp. Geodae-Uksae 176. Nanosized metal oxides are widely served as highly efficient adsorbents for heavy metals from water or wastewater, thanks to its fast kinetics, high capacity, and better adsorption properties for heavy metals in waters77. It is recommended that secondary treatment be the minimum standard for urban sewage treatment plants. Microalgae supplementation significantly enhances heavy metal removal efficiency in sterilized secondary effluent, achieving 81.7–94.1% reduction for copper and zinc within 10 days78. Their rapid biosorption capacity (observable within 6 h post-inoculation) makes them particularly suitable for wastewater treatment plants requiring quick heavy metal mitigation. Besides, sediments, another important heavy metal pollution sink and source, are necessary to explore heavy metal pollution of the global patterns, and more data reporting is crucial to additionally accurate predict the heavy metal pollution.
Areas with low latitude, which are more vulnerable to climate change, should be intensified policies related to management and control of heavy metal emissions with their local governments. Anthropogenic activities constitute the primary source of heavy metal emissions, whereas climate change (particularly through temperature and precipitation changes) exacerbates heavy metal mobilization from both anthropogenic and natural sources into global inland waters. Further research should intend to explore how the increased temperature concretely enhances the heavy metal concentrations in global inland waters, and we also hope our research is useful for future studies.
Conclusion
In the context of human activities and social development, our study concentrated on (1) analyzing the differences in heavy metal concentrations across freshwater ecosystems, seasons, and land use types; (2) identifying the key drivers affecting heavy metal distributions in global inland waters; and (3) predicting the global spatial patterns of heavy metal concentrations. The results affirmed the combined sources from nature and anthropogenic activities. Anthropogenic activities dominated heavy metal discharge, while climate factors (e.g., temperature, precipitation, and evapotranspiration) further exacerbate heavy metal concentrations in global inland waters from anthropogenic sources and natural processes through land surface runoff. The secondary treatment of wastewater treatment plants (WWTPs) reduces the concentrations of heavy metals in sewage to a certain extent.
Additionally, RF model was used to predict the Cu, Zn, Cd, and Cr concentrations in global patterns and found that the concentrations were predominantly distributed between 40°N and 20°S. The models predicted higher concentrations of Cu and Zn in regions including South Asia, West Asia, Africa (especially in midportion), and South America, while higher levels of Cd and Cr were projected primarily in Africa and South Asia. A series of measures should be implemented to address heavy metal pollution in inland waters. Secondary treatment should be established as the minimum global standard for heavy metal removal in WWTPs, particularly for low-income countries. In addition to managing heavy metal emissions from urban, transportation, industrial, and agricultural sources, region- and season-specific control strategies should be adopted. For instance, enhanced monitoring and regulation of heavy metal emissions should be enforced in urban areas, transportation networks, and industrial zones during the dry season, when pollutant accumulation tends to be more severe. We hope this study will facilitate future research to thoroughly investigate how climate variability influences heavy metal concentrations in global inland waters.
Materials and methods
Data sources and processing
We searched for data from peer-reviewed publications from Web of Science and China National Knowledge Infrastructure (CNKI) using following keywords: (“heavy metal OR copper OR zinc OR cadmium OR chromium”) AND (river OR stream OR fluvial OR lake OR water* OR inland water). Several criteria were chosen to meet eligible data: (i) at least one of the four heavy metals was reported in inland waters; (ii) the study samples must be in inland waters, including rivers (or describe as streams), lakes, reservoirs, ponds and so on, whereas we excluded estuaries owing to considerable effects from seawater; (iii) surface water samples were collected under national conditions and excluded groundwater; (iv) concentrations are uniformly converted into μg L−1. We acquired data from text, digitized graphs, tables, and supplementary materials. According to these criteria, we obtained 3301, 3213, 2875, and 2184 observations of Cu, Zn, Cd, and Cr in inland waters, respectively, with a total of 514 articles (Fig. 1a and Supplementary Notes S2). After collecting surface water, the water samples were directly subjected to digestion processes to determine the total concentrations of heavy metals, or the water samples were directly filtered at 0.45 μm membrane to determine the dissolved concentrations of heavy metals. All the heavy metal concentrations of collecting data were divided into three forms: total concentration, dissolved concentration, and unreported category. Additionally, to enable cross-regional comparisons, we divided the sampling season into two categories according to the seasons and sampling time mentioned in the publications based on precipitation: climatic season (dry and wet season) and monsoonal season (pre-monsoon, monsoon, and post-monsoon). We also divide ecosystems into rivers, lakes, and reservoirs to explore the differences in heavy metal concentrations among ecosystem types. Land use types were categorized into industrial, agricultural, residential, touristic, natural, multiple, and undefined. Here, “natural” indicated undisturbed areas, “multiple” referred to mixed-use zones (e.g., industrial-agricultural-residential), and “undefined” covered cases with missing land use data in the literature.
Statistical modeling
All data were executed in R version 4.4.279. The overall process is shown in the supplementary file (Supplementary Fig. S1). We employed Bayesian mixed-effects models to examine differences in heavy metal concentrations across different divisions and used Random Forest (RF) models to predict spatial patterns of heavy metal concentrations in global inland waters. In Bayesian mixed-effects models, non-detects (zeros) were replaced with 10% of the minimum value, and extreme values (>99.5 threshold percentile) were winsorized, with the heavy metal concentrations distribution in Fig. 1b. We then performed log10 transformation to fit a Gaussian distribution. In RF models, the log10 transformation was directly applied to the model training to retain the true extreme values.
We conducted a series of analysis for heavy metal concentrations (including three forms). Above all, we conducted Bayesian mixed-effects models in the brms package in R80 to evaluate the difference between four heavy metals and the effects of season division, land use types, and ecosystem types on heavy mental concentrations in inland water. Meanwhile, we also conducted interaction models between classification variables for each heavy metal. We fitted and generated each model with random effects of study identity and sampling year to eliminate unnecessary interference. We fitted each model using 4 parallel chains of 10,000 iterations with a total of 36,000 poster samples. For Cu, Zn, Cd, and Cr concentrations with Gaussian distribution in the brms package80. We specified information Cauchy priors (μ = 0, σ = 1) for the random effects and normal priors (μ = 0, σ = 1) for fixed effects. We evaluated the trace plots and the Gelman-Rubin statistic, which was <1.1, to ensure convergence. We revealed posterior mean and 95% and 80% credible intervals (CI). We also calculated the conditional R2 (variance explained by fixed and random factors) and marginal R2 (variance explained by random factors). Next, we will start the RF model and prediction.
Variable selection
Predicting the four heavy metal concentrations in global inland waters basing machine learning is another considerable purpose of this article. When starting RF modeling, we first need to select predictors. This study resampled all variables in EPSG:4326 (World Geodetic System 1984) at 0.05° grid resolution. When selecting potential influencing factors, we considered: (1) anthropogenic activities and natural conditions associated with heavy metal sources, and (2) key hydrological processes that may affect metal transport and transformation. These factors were identified through a comprehensive review of published global datasets. Consequently, we selected 24 potentially impacted predictors (including 4 hydrology predictors, 3 physiography predictors, 3 climate predictors, 4 landcover predictors, 2 soils-geology predictors, and 8 anthropogenic predictors) that were extracted from the HydroSHEDS data81(https://www.hydrosheds.org) and wastewater treatment68 (Table S1). To avoid collinearity, we eliminated predictors with low contributions one by one until the variance inflation factors (VIFs) of all predictors were less than five, and there were 21 remaining predictors for the heavy metals. Notably, precipitation was excluded in this process, due to the high correlation with act evapotranspiration (Supplementary Fig. S15). Given precipitation was considered as a significant diver, we also discussed precipitation in detail in the Discussion.
Predicting model
We conducted RF regression model based on randomForest package82 to modeling for heavy metal concentrations in inland waters. The train function from the R package “caret” was used to optimize the model parameters of the four machine learning models83. We used repeated random 10-fold cross-validation for RF models of four heavy metals, respectively. The process was trained 10 times, each time using 90% of the data for training and leaving the remaining 10% for validation. Finally, we selected the best parameters to fit the RF model. Root-mean-square error (RMSE) and R2_Accuracy is committed to quantify model predictive performance and accuracy. R2_Accuracy is regarded as the amount of variation explained by the model, and its calculation formula is as follows:
where SSE is the sum of the square error between observation and prediction, and SST is the total sum of squares. A model produced the lowest RMSE and highest R2_Accuracy, which could be identified as the best model with the highest predictive power. All the results of models see Supplementary Table S9.
Based on the optimal RF model, we evaluated the importance of predictors using the “caret” package83. The normalized variable importance scores, scaled between 0% and 100%, revealed the relative influence of each predictor on the model’s outcomes. Additionally, we also used the Bayesian mixed-effects model (linear model) to assist in verifying the impact of predictors on the dependent variable (Supplementary Figs. S16–17). The important results obtained by the two models are basically the same.
Global prediction and coefficient of variation
We extracted data related to the predictors of heavy metals for the global scale, excluding glaciers and deserts. These data were performed predicted global spatial distribution of the four metals based on their, respectively, optimal models, which prediction process was conducted 100 iterations randomly. Afterwards, we generated 100 global maps associated with heavy metals, and by averaging the outcomes across these 100 iterations, we derived the per-pixel mean values and standard deviations. Subsequently, we computed the coefficient of variation, defined as the ratio of the standard deviation to the mean value.
Sensitive analysis
According to Bayesian mixed-effects models, we conducted a comparison between the total concentrations and dissolved concentrations of Cu, Zn, Cd, and Cr in the database, and we found that there is significance between total concentrations and dissolved concentrations of Cu and Zn (Supplementary Fig. S3 and Table S5). Then we performed all the analyzing processes based on dissolved Cu and Zn concentrations to further discussion.
Data availability
Raw data were deposited in figshare with a (https://doi.org/10.6084/m9.figshare.29338148.v1).
Code availability
R code for generation of the results was deposited in figshare with a (https://doi.org/10.6084/m9.figshare.29338148.v1).
References
Palmer, S. C. J., Kutser, T. & Hunter, P. D. Remote sensing of inland waters: challenges, progress and future directions. Remote Sens. Environ. 157, 1–8 (2015).
Raymond, P. A. et al. Global carbon dioxide emissions from inland waters. Nature 503, 355–359 (2013).
Varol, M. Dissolved heavy metal concentrations of the Kralkızı, Dicle and Batman dam reservoirs in the Tigris River basin, Turkey. Chemosphere 93, 954–962 (2013).
Pekey, H., Karakaş, D. & Bakogˇlu, M. Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar. Pollut. Bull. 49, 809–818 (2004).
Li, S., Xu, Z., Cheng, X. & Zhang, Q. Dissolved trace elements and heavy metals in the Danjiangkou Reservoir, China. Environ. Geol. 55, 977–983 (2008).
Li, Y. et al. Trends and health risks of dissolved heavy metal pollution in global river and lake water from 1970 to 2017. Rev. Environ. Contamination Toxicol. 251, 1–24 (2019).
Arslan, Ş & Avşar, Ö Assessment of heavy metal pollution in Köyceğiz-Dalyan coastal lagoon watershed (Muğla), SW Turkey. Arab J. Geosci. 13, 719 (2020).
Wei, S. et al. Global patterns and drivers of lead concentration in inland waters. J. Hazard. Mater. 460, 132455 (2023).
Ali, H., Khan, E. & Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 1–14 (2019).
Tang, Z. et al. Ecological risk assessment of aquatic organisms induced by heavy metals in the estuarine waters of the Pearl River. Sci. Rep. 13, 9145 (2023).
Tai, Y.-T. et al. The preferential accumulation of cadmium ions among various tissues in mice. Toxicol. Rep. 9, 111–119 (2022).
Varol, M. & Şen, B. Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. CATENA 92, 1–10 (2012).
Punia, A. Role of temperature, wind, and precipitation in heavy metal contamination at copper mines: a review. Environ. Sci. Pollut. Res. 28, 4056–4072 (2021).
Rothwell, J. J., Evans, M. G., Daniels, S. M. & Allott, T. E. H. Baseflow and stormflow metal concentrations in streams draining contaminated peat moorlands in the Peak District National Park (UK). J. Hydrol. 341, 90–104 (2007).
Zhang, H. et al. Accumulation of arsenic, mercury and heavy metals in lacustrine sediment in relation to eutrophication: impacts of sources and climate change. Ecol. Indic. 93, 771–780 (2018).
Kalnejais, L. H., Martin, W. R. & Bothner, M. H. The release of dissolved nutrients and metals from coastal sediments due to resuspension. Mar. Chem. 121, 224–235 (2010).
He, X. et al. Global patterns and drivers of soil total phosphorus concentration. Earth Syst. Sci. Data 13, 5831–5846 (2021).
Cai, L. et al. Global models and predictions of plant diversity based on advanced machine learning techniques. N. Phytol. 237, 1432–1445 (2023).
Förstner, U. & Prosi, F. Heavy metal pollution in freshwater ecosystems. in Biological Aspects of Freshwater Pollution 129–161 (Elsevier, 1979). https://doi.org/10.1016/B978-0-08-023442-7.50011-6.
He, Z. L., Yang, X. E. & Stoffella, P. J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 19, 125–140 (2005).
Bradl, H. B. Chapter 1 Sources and origins of heavy metals. in Interface Science and Technology vol. 6 1–27 (Elsevier, 2005).
Ekman Nilsson, A. et al. A review of the carbon footprint of Cu and Zn production from primary and secondary sources. Minerals 7, 168 (2017).
Saha, R., Nandi, R. & Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 64, 1782–1806 (2011).
Du, B. et al. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 720, 137585 (2020).
Hu, N., Li, Z., Huang, P. & Tao, C. Distribution and mobility of metals in agricultural soils near a copper smelter in South China. Environ. Geochem Health 28, 19–26 (2006).
Li, H., Li, Y., Lee, M.-K., Liu, Z. & Miao, C. Spatiotemporal analysis of heavy metal water pollution in transitional China. Sustainability 7, 9067–9087 (2015).
Pacyna, J. M., Scholtz, M. T. & Arthur Li, Y.-F. Global budget of trace metal sources. Environ. Rev. 3, 145–159 (1995).
Xiao, W. et al. The easily overlooked effect of global warming: diffusion of heavy metals. Toxics 12, 400 (2024).
Mahbub, P., Ayoko, G. A., Goonetilleke, A., Egodawatta, P. & Kokot, S. Impacts of traffic and rainfall characteristics on heavy metals build-up and wash-off from urban roads. Environ. Sci. Technol. 44, 8904–8910 (2010).
Meite, F. et al. Impact of rainfall patterns and frequency on the export of pesticides and heavy-metals from agricultural soils. Sci. Total Environ. 616–617, 500–509 (2018).
Wang, P., Sun, Z., Hu, Y. & Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 695, 133893 (2019).
Nedrich, S. M. & Burton, G. A. Indirect effects of climate change on zinc cycling in sediments: the role of changing water levels. Environ. Toxic. Chem. 36, 2456–2464 (2017).
Clemente, J. S. & Huntsman, P. Potential climate change effects on the geochemical stability of waste and mobility of elements in receiving environments for Canadian metal mines south of 60°N. Environ. Rev. 27, 478–518 (2019).
Zhu, H. et al. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Trans. Nonferrous Met. Soc. China 22, 1470–1477 (2012).
Wu, R. et al. Heavy metal levels and sources in suspended particulate matters of the glacier watersheds in Northeast Tibetan Plateau. Front. Environ. Sci. 10, 918514 (2022).
Zhang, F. et al. Levels, inventory, and risk assessment of heavy metals in wetland ecosystem, northeast China: implications for snow cover monitoring. Water 13, 2161 (2021).
Li, W., Deng, Y., Wang, H., Hu, Y. & Cheng, H. Potential risk, leaching behavior and mechanism of heavy metals from mine tailings under acid rain. Chemosphere 350, 140995 (2024).
Zhao, S., Zhao, Y., Cui, Z., Zhang, H. & Zhang, J. Effect of pH, temperature, and salinity levels on heavy metal fraction in lake sediments. Toxics 12, 494 (2024).
Zheng, S., Zheng, X. & Chen, C. Leaching behavior of heavy metals and transformation of their speciation in polluted soil receiving simulated acid rain. PLoS ONE 7, e49664 (2012).
Nowrouzi, M., Mansouri, B., Nabizadeh, S. & Pourkhabbaz, A. Analysis of heavy metals concentration in water and sediment in the Hara biosphere reserve, southern Iran. Toxicol. Ind. health 30, 64–72 (2014).
Richard, F. C. & Bourg, A. C. M. Aqueous geochemistry of chromium: a review. Water Res. 25, 807–816 (1991).
Wijngaard, R. R., Van Der Perk, M., Van Der Grift, B., De Nijs, T. C. M. & Bierkens, M. F. P. The impact of climate change on metal transport in a lowland catchment. Water Air Soil Pollut. 228, 107 (2017).
Luo, M. et al. Effect of river-lake connectivity on heavy metal diffusion and source identification of heavy metals in the middle and lower reaches of the Yangtze River. J. Hazard. Mater. 416, 125818 (2021).
Zhang, T. et al. Rainfall runoff and dissolved pollutant transport processes over idealized urban catchments. Front. Earth Sci. 8, 305 (2020).
Taka, M. et al. Heavy metals from heavy land use? Spatio-temporal patterns of urban runoff metal loads. Sci. Total Environ. 817, 152855 (2022).
Huber, M., Welker, A. & Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: occurrence, influencing factors, and partitioning. Sci. Total Environ. 541, 895–919 (2016).
Westerlund, C. & Viklander, M. Particles and associated metals in road runoff during snowmelt and rainfall. Sci. Total Environ. 362, 143–156 (2006).
De Vries, W., Kros, J., Voogd, J. C. & Ros, G. H. Integrated assessment of agricultural practices on large scale losses of ammonia, greenhouse gases, nutrients and heavy metals to air and water. Sci. Total Environ. 857, 159220 (2023).
Hou, D. et al. Global soil pollution by toxic metals threatens agriculture and human health. https://doi.org/10.1126/science.adr5214 (2025).
Acosta, J. A., Faz, A. & Martinez-Martinez, S. Identification of heavy metal sources by multivariable analysis in a typical Mediterranean city (SE Spain). Environ. Monit. Assess. 169, 519–530 (2010).
Garner, G., Malcolm, I. A., Sadler, J. P. & Hannah, D. M. The role of riparian vegetation density, channel orientation and water velocity in determining river temperature dynamics. J. Hydrol. 553, 471–485 (2017).
Blowes, D. W. et al. The geochemistry of acid mine drainage. in Treatise on Geochemistry (eds. Holland, H. D. & Turekian, K. K.) vol. 11 131–190 (Elsevier Science, 2013).
Kang, M., Tian, Y., Peng, S. & Wang, M. Effect of dissolved oxygen and nutrient levels on heavy metal contents and fractions in river surface sediments. Sci. Total Environ. 648, 861–870 (2019).
Li, H., Shi, A., Li, M. & Zhang, X. Effect of pH, temperature, dissolved oxygen, and flow rate of overlying water on heavy metals release from storm sewer sediments. J. Chem. 2013, 1–11 (2013).
Duan, D., Ran, Y., Cheng, H., Chen, J. & Wan, G. Contamination trends of trace metals and coupling with algal productivity in sediment cores in Pearl River Delta, South China. Chemosphere 103, 35–43 (2014).
Webb, A. L., Hughes, K. A., Grand, M. M., Lohan, M. C. & Peck, L. S. Sources of elevated heavy metal concentrations in sediments and benthic marine invertebrates of the western Antarctic Peninsula. Sci. Total Environ. 698, 134268 (2020).
Fu, S. & Lu, J. Temperature-driven variation in the removal of heavy metals from contaminated tailings leaching in northern Norway. Environ. Monit. Assess. 191, 123 (2019).
Zaharescu, D. G., Hooda, P. S., Burghelea, C. I., Polyakov, V. & Palanca-Soler, A. Climate change enhances the mobilisation of naturally occurring metals in high altitude environments. Sci. Total Environ. 560–561, 73–81 (2016).
Wu, W., Qu, S., Nel, W. & Ji, J. The influence of natural weathering on the behavior of heavy metals in small basaltic watersheds: a comparative study from different regions in China. Chemosphere 262, 127897 (2021).
Todd, A. S. et al. Climate-change-driven deterioration of water quality in a mineralized watershed. Environ. Sci. Technol. 46, 9324–9332 (2012).
Meylan, G. & Reck, B. K. The anthropogenic cycle of zinc: status quo and perspectives. Resour. Conserv. Recycl. 123, 1–10 (2017).
Kummu, M., Taka, M. & Guillaume, J. H. A. Gridded global datasets for gross domestic product and human development index over 1990–2015. Sci. Data 5, 180004 (2018).
Duan, J. & Tan, J. Atmospheric heavy metals and Arsenic in China: situation, sources and control policies. Atmos. Environ. 74, 93–101 (2013).
Benazir, J. F., Suganthi, R., Rajvel, D., Pooja, M. P. & Mathithumilan, B. Bioremediation of chromium in tannery effluent by microbial consortia. Afr. J. Biotechnol. 9, 3140–3143 (2010).
Maiti, M., Chaudhuri, S. & Biswas, A. Activities of idol immersion leads to heavy metal contamination in river Hooghly in and around the city of Kolkata. J. Indian Chem. Soc. 98, 100223 (2021).
Wang, C., Hu, X., Chen, M. & Wu, Y. Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge from municipal and industrial wastewater treatment plants. J. Hazard. Mater. 119, 245–249 (2005).
Sato, T., Qadir, M., Yamamoto, S., Endo, T. & Zahoor, A. Global, regional, and country level need for data on wastewater generation, treatment, and use. Agric. Water Manag. 130, 1–13 (2013).
Van Puijenbroek, P. J. T. M. et al. Quantifying future sanitation scenarios and progress towards SDG targets in the shared socioeconomic pathways. J. Environ. Manag. 346, 118921 (2023).
Yabe, J., Ishizuka, M. & Umemura, T. Current levels of heavy metal pollution in Africa. J. Vet. Med. Sci. 72, 1257–1263 (2010).
Fasinu, P. S. & Orisakwe, O. E. Heavy metal pollution in sub-Saharan Africa and possible implications in cancer epidemiology. Asian Pac. J. Cancer Prev. 14, 3393–3402 (2013).
Samanta, S. & Dalai, T. K. Massive production of heavy metals in the Ganga (Hooghly) River estuary, India: global importance of solute-particle interaction and enhanced metal fluxes to the oceans. Geochim. et. Cosmochim. Acta 228, 243–258 (2018).
Suvarapu, L. N., Seo, Y.-K. & Baek, S.-O. Heavy metals in the Indian atmosphere: a review. Res. J. Chem. Environ. 18, 99–111 (2014).
Pirsaheb, M., Khamutian, R. & Pourhaghighat, S. Review of heavy metal concentrations in Iranian water resources. J. Health Life Sci. 1, 35–45 (2015).
Shakya, S. et al. Determination and contamination assessment of heavy metals in street dust from different types of land-use in Kathmandu district, Nepal. J. Inst. Sci. Tech. 24, 6–18 (2019).
Vineetha, G. et al. Impact of a catastrophic flood on the heavy metal pollution status and the concurrent responses of the bentho-pelagic community in a tropical monsoonal estuary. Mar. Pollut. Bull. 155, 111191 (2020).
Bang, J. et al. Phytoremediation of heavy metals in contaminated water and soil using Miscanthus sp. Goedae-Uksae 1. Int. J. Phytoremediat. 17, 515–520 (2015).
Hua, M. et al. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J. Hazard. Mater. 211–212, 317–331 (2012).
Chan, A., Salsali, H. & McBean, E. Heavy metal removal (copper and zinc) in secondary effluent from wastewater treatment plants by microalgae. ACS Sustain. Chem. Eng. 2, 130–137 (2014).
R Core Team, R. R: A Language and Environment For Statistical Computing (R Foundation for Statistical Computing, 2024).
Bürkner, P.-C. brms : An R package for Bayesian multilevel models using Stan. J. Stat. Soft. 80, 1–28 (2017).
Lehner, B. & Grill, G. Global river hydrography and network routing: baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 27, 2171–2186 (2013).
Liaw, A. & Wiener, M. Random forest: a classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comput. Sci. 2, 18–22 (2002).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Soft. 28, 1–26 (2008).
Acknowledgements
K.Y. was funded by the National Natural Science Foundation of China (32271633) and the Central-guided Local Science and Technology Development Fund Projects of Fujian Province (2023L3005), Y.P. received funds from the State Key Laboratory of Subtropical Silviculture (SKLSS-KF2024-02), the National Natural Science Foundation of China (32201342), and Natural Science Foundation of Fujian Province (2022J01642). F.W. was founded by the National Natural Science Foundation of China (32171641), and J.P. was funded by the Spanish MCIN (AEI/10.13039/501100011033) and the European Union NextGenerationEU/PRTR (TED2021-132627B-I00). We confirm that no sampling permissions were required for this study, as all data were extracted from previously published literature.
Author information
Authors and Affiliations
Contributions
K.Y., Q.W., and X.J. conceived the study. X.J. collected the raw data. K.Y., Q.W., and X.J. performed data analysis and wrote the first draft of the manuscript. J.P., J.S., Q.Y., and P.H. discussed results. Z.L., Y.P., Z.Z., and F.W. contributed to substantial revisions of the manuscript. J.Y., Z.C., X.P., and C.Y. contributed to the visualization, and all authors edited the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Fikret Ustaoğlu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Somaparna Ghosh. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, X., Wu, Q., Peñuelas, J. et al. Climate and anthropogenic activities control the concentrations of copper, zinc, cadmium and chromium in global inland waters. Commun Earth Environ 6, 520 (2025). https://doi.org/10.1038/s43247-025-02508-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02508-6