Abstract
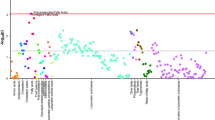
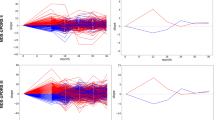
The early pathophysiology of Parkinson’s disease (PD) is poorly understood. We analyzed 2,920 Olink-measured plasma proteins in 51,804 UK Biobank participants, identifying 859 incident PD cases after 14.45 years. We found 38 PD-related proteins, with six of the top ten validated in the Parkinson’s Progression Markers Initiative (PPMI) cohort. ITGAV, HNMT and ITGAM showed consistent significant association (hazard ratio: 0.11–0.57, P = 6.90 × 10−24 to 2.10 × 10−11). Lipid metabolism dysfunction was evident 15 years before PD onset, and levels of BAG3, HPGDS, ITGAV and PEPD continuously decreased before diagnosis. These proteins were linked to prodromal symptoms and brain measures. Mendelian randomization suggested ITGAM and EGFR as potential causes of PD. A predictive model using machine learning combined the top 16 proteins and demographics, achieving high accuracy for 5-year (area under the curve (AUC) = 0.887) and over-5-year PD prediction (AUC = 0.816), outperforming demographic-only models. It was externally validated in PPMI (AUC = 0.802). Our findings reveal early peripheral pathophysiological changes in PD crucial for developing early biomarkers and precision therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data used in the present study are available from the UKB, with restrictions applied. Data were used under a license and are thus not publicly available. Access to the UKB data can be requested through the standard protocol (https://www.ukbiobank.ac.uk/register-apply/). The PPMI (Parkinson’s Progression Markers Initiative) database is publicly available, and researchers can apply for access. To request data, please refer to the PPMI Data User Guide at: https://www.ppmi-info.org/sites/default/files/docs/PPMI%20Data%20User%20Guide.pdf. This guide provides detailed instructions on how to apply for access and outlines the associated usage policies. We obtained protein quantitative trait loci (pQTLs) from the GWAS summary statistics in the UKB-PPP, which includes 34,557 participants of European ancestry (https://www.nature.com/articles/s41586-023-06592-6). GWAS summary statistics on PD were obtained from a meta-analysis conducted by the International Parkinson’s Disease Genomics Consortium with a total of 33,674 cases and 449,056 controls of European descent (https://gwas.mrcieu.ac.uk/datasets/ieu-b-7/).
Code availability
Analyses were performed using R software (v4.3.1) and Python (v3.9). We considered a two-tailed P value < 0.05 to be significant. Code is available at https://github.com/YiHanGan/PD-Proteomic-Project.
References
Yan, S. et al. Neuronally derived extracellular vesicle α-synuclein as a serum biomarker for individuals at risk of developing Parkinson disease. JAMA Neurol. 81, 59–68 (2024).
Bartl, M. et al. Blood markers of inflammation, neurodegeneration, and cardiovascular risk in early Parkinson’s disease. Mov. Disord. 38, 68–81 (2023).
El-Agnaf, O. M. et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 20, 419–425 (2006).
Emamzadeh, F. N. Role of apolipoproteins and alpha-synuclein in Parkinson’s disease. J. Mol. Neurosci. 62, 344–355 (2017).
Kowal, S. L., Dall, T. M., Chakrabarti, R., Storm, M. V. & Jain, A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disord. 28, 311–318 (2013).
Varadi, C. Clinical features of Parkinson’s disease: the evolution of critical symptoms. Biology (Basel) 9, 103 (2020).
Neikrug, A. B. et al. Parkinson’s disease and REM sleep behavior disorder result in increased non-motor symptoms. Sleep Med. 15, 959–966 (2014).
Chelliah, S. S., Bhuvanendran, S., Magalingam, K. B., Kamarudin, M. N. A. & Radhakrishnan, A. K. Identification of blood-based biomarkers for diagnosis and prognosis of Parkinson’s disease: a systematic review of proteomics studies. Ageing Res. Rev. 73, 101514 (2022).
Hu, L. et al. Integrated metabolomics and proteomics analysis reveals plasma lipid metabolic disturbance in patients with Parkinson’s disease. Front. Mol. Neurosci. 13, 80 (2020).
Khosousi, S. et al. Complement system changes in blood in Parkinson’s disease and progressive supranuclear palsy/corticobasal syndrome. Parkinsonism Relat. Disord. 108, 105313 (2023).
Posavi, M. et al. Characterization of Parkinson’s disease using blood-based biomarkers: a multicohort proteomic analysis. PLoS Med. 16, e1002931 (2019).
Abdi, I. Y. et al. Cross-sectional proteomic expression in Parkinson’s disease-related proteins in drug-naive patients vs healthy controls with longitudinal clinical follow-up. Neurobiol. Dis. 177, 105997 (2023).
Licker, V. et al. Proteomic analysis of human substantia nigra identifies novel candidates involved in Parkinson’s disease pathogenesis. Proteomics 14, 784–794 (2014).
Petyuk, V. A. et al. Proteomic profiling of the substantia nigra to identify determinants of Lewy body pathology and dopaminergic neuronal loss. J Proteome Res. 20, 2266–2282 (2021).
Downs, M., Sethi, M. K., Raghunathan, R., Layne, M. D. & Zaia, J. Matrisome changes in Parkinson’s disease. Anal. Bioanal. Chem. 414, 3005–3015 (2022).
Raghunathan, R., Hogan, J. D., Labadorf, A., Myers, R. H. & Zaia, J. A glycomics and proteomics study of aging and Parkinson’s disease in human brain. Sci. Rep. 10, 12804 (2020).
Yang, L. et al. An alpha-synuclein MRM assay with diagnostic potential for Parkinson’s disease and monitoring disease progression.Proteomics Clin. Appl. 11, 10.1002/prca.201700045 (2017).
Rotunno, M. S. et al. Cerebrospinal fluid proteomics implicates the granin family in Parkinson’s disease. Sci. Rep. 10, 2479 (2020).
Wang, Y. et al. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci. Transl. Med. 4, 121ra120 (2012).
Walker, K. A. et al. Large-scale plasma proteomic analysis identifies proteins and pathways associated with dementia risk. Nat. Aging 1, 473–489 (2021).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622, 329–338 (2023).
Yoshikawa, T., Nakamura, T. & Yanai, K. Histamine N-methyltransferase in the brain. Int. J. Mol. Sci. 20, 737 (2019).
Jiménez-Jiménez, F. J., Alonso-Navarro, H., García-Martín, E. & Agúndez, J. A. G. Thr105Ile (rs11558538) polymorphism in the histamine N-methyltransferase (HNMT) gene and risk for Parkinson disease: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 95, e4147 (2016).
Shan, L. et al. Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: a postmortem study. Neurobiol. Aging 33, 1488 (2012).
Pang, Y. P., Zheng, X. E. & Weinshilboum, R. M. Theoretical 3D model of histamine N-methyltransferase: insights into the effects of a genetic polymorphism on enzymatic activity and thermal stability. Biochem. Biophys. Res. Commun. 287, 204–208 (2001).
Hou, L. et al. Integrin CD11b mediates locus coeruleus noradrenergic neurodegeneration in a mouse Parkinson’s disease model. J. Neuroinflammation 17, 148 (2020).
Thimgan, M. S. et al. Cross-translational studies in human and Drosophila identify markers of sleep loss. PLoS One 8, e61016 (2013).
Allard, D. E. et al. Schwann cell-derived periostin promotes autoimmune peripheral polyneuropathy via macrophage recruitment. J. Clin. Invest. 128, 4727–4741 (2018).
Sharabi, Y., Vatine, G. D. & Ashkenazi, A. Parkinson’s disease outside the brain: targeting the autonomic nervous system. Lancet Neurol. 20, 868–876 (2021).
Lindestam Arlehamn, C. S. et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 11, 1875 (2020).
Karayel, O. et al. Proteome profiling of cerebrospinal fluid reveals biomarker candidates for Parkinson’s disease. Cell Rep. Med. 3, 100661 (2022).
Halloway, S. et al. Association of neurofilament light with the development and severity of Parkinson disease. Neurology 98, e2185–e2193 (2022).
Verma, A. K. et al. Plasma prolidase activity and oxidative stress in patients with Parkinson’s disease. Parkinsons Dis. 2015, 598028 (2015).
Bartl, M. et al. Lysosomal and synaptic dysfunction markers in longitudinal cerebrospinal fluid of de novo Parkinson’s disease. NPJ Parkinsons Dis. 10, 102 (2024).
Margadant, C. & Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 (2010).
Henderson, N. C. & Sheppard, D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochim. Biophys. Acta 1832, 891–896 (2013).
Henderson, N. C. et al. Targeting of ɑv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Zhong, L. et al. Runx2 activates hepatic stellate cells to promote liver fibrosis via transcriptionally regulating Itgav expression. Clin. Transl. Med. 13, e1316 (2023).
Terauchi, A. et al. The projection-specific signals that establish functionally segregated dopaminergic synapses. Cell 186, 3845–3861.e3824 (2023).
Yun, S. P. et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 24, 931–938 (2018).
Diniz, L. P. et al. α-synuclein oligomers enhance astrocyte-induced synapse formation through TGF-β1 signaling in a Parkinson’s disease model. J. Neurochem. 150, 138–157 (2019).
Monzani, E. et al. Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. Engl. 58, 6512–6527 (2019).
Zhao, X., Xiao, W. Z., Pu, X. P. & Zhong, L. J. Proteome analysis of the sera from Chinese Parkinson’s disease patients. Neurosci. Lett. 479, 175–179 (2010).
Sinclair, E. et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 12, 1592 (2021).
Fanning, S., Selkoe, D. & Dettmer, U. Vesicle trafficking and lipid metabolism in synucleinopathy. Acta Neuropathol. 141, 491–510 (2021).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673 (2022).
Droby, A. et al. The interplay between structural and functional connectivity in early stage Parkinson’s disease patients. J. Neurol. Sci. 442, 120452 (2022).
Slingerland, S. et al. Cholinergic innervation topography in GBA-associated de novo Parkinson’s disease patients. Brain 147, 900–910 (2024).
Walker, K. A. et al. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci. Transl. Med. 15, eadf5681 (2023).
Deming, Y. et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 11, eaau2291 (2019).
Jin, J. et al. Association between epidermal growth factor receptor gene polymorphisms and susceptibility to Parkinson’s disease. Neurosci. Lett. 736, 135273 (2020).
Urso, D., Batzu, L., Logroscino, G., Ray Chaudhuri, K. & Pereira, J. B. Neurofilament light predicts worse nonmotor symptoms and depression in Parkinson’s disease. Neurobiol. Dis. 185, 106237 (2023).
Wang, X. et al. The association of serum neurofilament light chains with early symptoms related to Parkinson’s disease: a cross-sectional study. J. Affect. Disord. 343, 144–152 (2023).
Diaz-Ortiz, M. E. et al. GPNMB confers risk for Parkinson’s disease through interaction with alpha-synuclein. Science 377, eabk0637 (2022).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020).
Williams, S. A. et al. Plasma protein patterns as comprehensive indicators of health. Nat. Med. 25, 1851–1857 (2019).
Oh, H. S. et al. Organ aging signatures in the plasma proteome track health and disease. Nature 624, 164–172 (2023).
Katz, D. H. et al. Proteomic profiling platforms head to head: Leveraging genetics and clinical traits to compare aptamer- and antibody-based methods. Sci. Adv. 8, eabm5164 (2022).
Collins, R. What makes UK Biobank special? Lancet 379, 1173–1174 (2012).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Eldjarn, G. H. et al. Large-scale plasma proteomics comparisons through genetics and disease associations. Nature 622, 348–358 (2023).
Dhindsa, R. S. et al. Rare variant associations with plasma protein levels in the UK Biobank. Nature 622, 339–347 (2023).
Wik, L. et al. Proximity extension assay in combination with next-generation sequencing for high-throughput proteome-wide analysis. Mol. Cell. Proteomics. 20, 100168 (2021).
Gelb, D. J., Oliver, E. & Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 56, 33–39 (1999).
Zheng, Z., Lv, Y., Rong, S., Sun, T. & Chen, L. Physical frailty, genetic predisposition, and incident Parkinson disease. JAMA Neurol. 80, 455–461 (2023).
Simonet, C. et al. Assessment of risk factors and early presentations of Parkinson disease in primary care in a diverse UK population. JAMA Neurol. 79, 359–369 (2022).
Wooten, G. F., Currie, L. J., Bovbjerg, V. E., Lee, J. K. & Patrie, J. Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639 (2004).
Van Den Eeden, S. K. et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 157, 1015–1022 (2003).
Najafi, F. et al. Association between socioeconomic status and Parkinson’s disease: findings from a large incident case-control study. BMJ Neurol. Open 5, e000386 (2023).
Hu, G. et al. Body mass index and the risk of Parkinson disease. Neurology 67, 1955–1959 (2006).
Morens, D. M., Grandinetti, A., Reed, D., White, L. R. & Ross, G. W. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology 45, 1041–1051 (1995).
Zhang, D., Jiang, H. & Xie, J. Alcohol intake and risk of Parkinson’s disease: a meta-analysis of observational studies. Mov. Disord. 29, 819–822 (2014).
de Jong, F. A., Howlett, G. J. & Schreiber, G. Messenger RNA levels of plasma proteins following fasting. Br. J. Nutr. 59, 81–86 (1988).
Enroth, S., Hallmans, G., Grankvist, K. & Gyllensten, U. Effects of long-term storage time and original sampling month on biobank plasma protein concentrations. EBioMedicine 12, 309–314 (2016).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Milà-Alomà, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med. 28, 1797–1801 (2022).
Milà-Alomà, M. et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer’s continuum. Alzheimers Dement. 16, 1358–1371 (2020).
Mila-Aloma, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-beta pathology in preclinical Alzheimer’s disease. Nat. Med. 28, 1797–1801 (2022).
Guo, Y. et al. The dynamics of plasma biomarkers across the Alzheimer’s continuum. Alzheimers Res. Ther. 15, 31 (2023).
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019).
Piehl, N. et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 185, 5028–5039 (2022).
Schalkamp, A. K., Peall, K. J., Harrison, N. A. & Sandor, C. Wearable movement-tracking data identify Parkinson’s disease years before clinical diagnosis. Nat. Med. 29, 2048–2056 (2023).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408 (2018).
LightGBM: a. highly efficient gradient boosting decision tree. In: Advances in Neural Information Processing Systems 30 (NIPS, 2017).
Lundberg, S. & Lee, S. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 30, 4765–4774 (2017).
Acknowledgements
We thank all the participants and researchers from the UKB and PPMI. This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600, J.-T.Y.), National Natural Science Foundation of China (92249305, J.-T.Y., 82472055 and 82071997, W.C., 82402381 and 82471940, J.Y.), National Key R&D Program of China (2023YFC3605400, W.C.), Research Start-up Fund of Huashan Hospital (2022QD002, J.-T.Y.), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001, J.-T.Y.), Shanghai Pujiang Talent Program (23PJD006, J.Y.), the National Postdoctoral Program for Innovative Talents (BX20240073, Y.G.) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University. PPMI is funded by the Michael J. Fox Foundation for Parkinson’s Research funding partners 4D Pharma, AbbVie, Acurex Therapeutics, Allergan, Amathus Therapeutics, ASAP, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, Dacapo Brain Science, Denali, The Edmond J. Safra Foundaiton, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily and Voyager Therapeutics. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.-T.Y. conceptualized and designed the study and interpreted data. Y.-H.G., L.Z.M., Y.Z., J.Y. and Y.H. collected, analyzed and interpreted the data and drafted the paper. All authors revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted following the Declaration of Helsinki. The UKB has research tissue bank approval from the North West Multi-Center Research Ethics Committee (11/NW/0382). Written informed consent was obtained from all participants. The present study was approved by the UKB under application number 19542.
Peer review
Peer review information
Nature Aging thanks Lucilla Parnetti, Yue Qi, Marcel Verbeek, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Baseline NPX levels of the 38 PD-associated proteins.
Violin plots display the baseline NPX levels (y axis) 38 PD-associated proteins compared between PD patients observed during follow-up and controls. The width of the violin reflects the density of the corresponding data. The box plots show the median (center line), the interquartile range (top and bottom edge), and the maximum and minimum (whiskers) of the NPX levels. The comparisons were conducted using two-sided wilcoxon test.
Extended Data Fig. 2 The expression of the PD-related proteins in the central nervous system (CNS) cell types.
Expression levels of the PD-associated-protein coding genes in different cell types of normal human brain. Brain snRNA-seq was retrieved from Garcia et al.1. 1. Garcia, F. J. et al. Single-cell dissection of the human brain vasculature. Nature 603, 893–899 (2022).
Extended Data Fig. 3 Functional enrichment analysis of cluster 1 and 2 proteins identified by protein trajectory clustering.
Top enriched GO biological pathways, GO molecular function pathways, and KEGG pathways were presented. The analyses were conducted using the clusterProfiler package (Hypergeometric test) and were adjusted for multiple testing using the Benjamini–Hochberg method. The shade of color corresponds to the magnitude of statistical significance (−log10 of P values).
Supplementary information
Supplementary Information
Supplementary Methods and Supplementary Figure 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gan, YH., Ma, LZ., Zhang, Y. et al. Large-scale proteomic analyses of incident Parkinson’s disease reveal new pathophysiological insights and potential biomarkers. Nat Aging 5, 642–657 (2025). https://doi.org/10.1038/s43587-025-00818-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-025-00818-0