Abstract
Background
Breathing patterns may inform on health. We note that the sites of earliest brain damage in Parkinson’s disease (PD) house the neural pace-makers of respiration. We therefore hypothesized that ongoing long-term temporal dynamics of respiration may be altered in PD.
Methods
We applied a wearable device that precisely logs nasal airflow over time in 28 PD patients (mostly H&Y stage-II) and 33 matched healthy controls. Each participant wore the device for 24 h of otherwise routine daily living.
Results
We observe significantly altered temporal patterns of nasal airflow in PD, where inhalations are longer and less variable than in matched controls (mean PD = −1.22 ± 1.9 (combined respiratory features score), Control = 1.04 ± 2.16, Wilcoxon rank-sum test, z = −4.1, effect size Cliff’s δ = −0.61, 95% confidence interval = −0.79 – (−0.34), P = 4.3 × 10−5). The extent of alteration is such that using only 30 min of recording we detect PD at 87% accuracy (AUC = 0.85, 79% sensitivity (22 of 28), 94% specificity (31 of 33), z = 5.7, p = 3.5 × 10−9), and also predict disease severity (correlation with UPDRS-Total score: r = 0.49; P = 0.008).
Conclusions
We conclude that breathing patterns are altered by H&Y stage-II in the disease cascade, and our methods may be further refined in the future to provide an indication with diagnostic and prognostic value.
Plain Language Summary
In its earliest stages, Parkinson’s disease damages the parts of the brain that control breathing. We built a small device that measures airflow patterns through the nose over time. People with Parkinson’s disease and healthy individuals wore this device for 24 h. We found that nasal inhalations in Parkinson’s patients were longer and less variable than in healthy individuals. This difference was so pronounced that, using only 30 min of recording, we could accurately determine most people who had Parkinson’s disease and how severe their disease was. Future studies will determine whether this tool can contribute to early diagnosis, and it may be useful to monitor disease progression.
Similar content being viewed by others
Introduction
Breathing is the combined outcome of central brain mechanisms1 that act on a host of peripheral skeletal and muscular mechanisms2. The peripheral pulmonary findings in Parkinson’s disease (PD) are assorted, and include altered function in respiratory muscles3,4, and the expected ensuing reduced lung volumes, reduced maximal inspiratory and expiratory flows, and diaphragmatic dyskinesias5,6,7,8,9,10,11,12. Indeed, the ultimate cause of death in PD is often aspiration pneumonia13. In contrast to this extensive investigation on the peripheral mechanisms of breathing in PD, there has been limited investigation on the central mechanisms of breathing in PD14.
In turn, a remarkable overlap between the neural mechanisms of breathing and the neuro-pathophysiology of PD provides good reason to suspect that these central mechanisms may be affected in the disease. The earliest sites of brain pathology in PD are the dorsal motor nuclei of the glossopharyngeal and vagal nerves and the anterior olfactory nucleus15. Indeed, the glossopharyngeal and vagal nerves are critical in the orchestration of respiratory timing16,17,18, and neural activity in the anterior olfactory nucleus is in fact phase-locked with nasal respiration19. Moreover, the ensuing sites of PD-brain-pathology reside in the brain stem, that houses the key elements of neural respiratory timing, primarily the pre-Bötzinger complex1. The pre-Bötzinger complex is difficult to noninvasively pin-point in humans20, but PD-associated deterioration of the overall medulla oblongata as previously assessed with diffuser-tensor imaging was in fact correlated with respiratory alterations in PD21. Moreover, rodent-models of PD, where the pre-Bötzinger complex is easier to locate, imply a clear role for pre-Bötzinger PD pathology in deteriorated breathing22,23. Damage to the central brain drivers of breathing may reflect in the ongoing temporal dynamics even in the absence of effect on overall respiratory volumes. For example, hypoxic damage to the pre-Bötzinger complex can alter respiratory frequency without influencing tidal volume24. Moreover, several lines of evidence imply that nasal rather than oral airflow provides for a better measure of afferent respiratory signals25,26. Given this, we used the wearable Nasal Holter, a device pasted to the nape of the neck, transducing a pressure wave from the nose via a nasal cannula27 (Fig. 1). We applied this device to PD patients (mostly stage II) and controls in order to test the hypothesis that respiratory temporal dynamics are altered by this stage in the disease cascade. We found significantly altered ongoing patterns of respiratory nasal airflow in PD. The differences primarily reflected longer and less variable inhalations than in matched controls. The extent of the differences was such that we could discriminate PD patients from controls at 87% accuracy based on 30 min of nasal airflow recordings alone. Moreover, we could also determine disease severity from these measurements. These results raise the possibility of testing this measure as a diagnostic tool in prodromal individuals in the future.
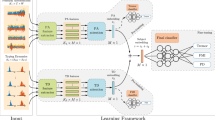
a The Nasal Holter device is pasted onto the nape of a participant’s neck. b The stereo nasal cannula communicating a pressure wave from each nostril separately. Onboard electronics convert the pressure signal into flow. The yellow-red highlights serve to illustrate the general ___location of the pre-Bötzinger complex (a) and the anterior olfactory nucleus (b), two brain areas implicated in early PD and in the control of respiratory timing. By illustration, we measure nasal airflow to gain information on brain function. c, d Examples of the nasal respiratory trace obtained from (c) a healthy control and (d) a PD patient. e Accelerometer data from a single participant demonstrating the data-derived separation between wake and sleep times. Colors reflect spatial axes, X-axis in orange, Y-axis in green and Z-axis in blue.
Methods
Participants
The study protocol and procedures were deemed low-risk, and were approved by TASMC IRB (Helsinki) committee (approval # 0239-20-TLV). All participants provided written informed consent. To estimate the number of participants we conducted a power Analysis using G*Power 3.128 applied to previous results showing reduced sniff inspiratory nasal pressure in PD patients3. This implied that at power of 0.8 we need at least 26 participants per group. Thus, we recruited 28 PD patients (age 66.35 ± 6.6 years (manuscript stats are mean ± s.d. unless stated otherwise); 2 women; 5 left-handed) and 33 age- and gender-matched healthy controls (age, 64.3 ± 7.5 years; 4 women; 5 left-handed). Subjects with history of mental illness, brain surgery (including DBS), head trauma, or any chronic or acute conditions that involve the respiratory tract (nasal polyposis, septum deviation, etc.) were excluded from this study. Recruitment was by offering participation to patients arriving at the TASMC PD clinic, and took place between 2021-2023. PD diagnosis was confirmed both by clinical symptoms using the Movement Disorder Society criteria and by 18 F-DOPA PET/CT brain imaging.
Clinical and demographic characteristics are presented in Table 1. Disease duration ranged from 1 to 29 years (median = 6.5 ± 5.6 years), and severity by MDS-UPDRS was Part I = 11.5 ± 5.6; Part II = 14.35 ± 5.6; Part III = 30.5 ± 11.6; Part IV = 2.3 ± 3.4, Total score = 58.7 ± 18.6; Hoehn and Yahr H&Y stages (median ± s.d.) = 2 ± 0.6. All PD patients were examined during the ON state. None of the patients reported an OFF state during the 24-hour recordings (we note that this is a subjective report and not a clinical evaluation). Two patients had genetic mutations associated with PD (one LRRK2 carrier29, one GBA carrier30). The groups did not differ in cognitive performance (MoCA PD = 24.4 ± 4.1, control = 25.7 ± 2.0, Mann-Whitney U = 388.0, rank-biserial correlation effect size = −0.16, 95% CI = −0.42 – (0.13), P = 0.28). PD patients scored higher on the depression questionnaire (Beck PD = 12.1 ± 8.5, control = 4.9 ± 4.8, U = 726.0, rank-biserial correlation effect size = 0.57, 95% CI = 0.34 – 0.74, P = 1.3 × 10−4) and had poorer olfaction (Sniffin’ Sticks total, PD = 16.35 ± 6.1, control = 29.7 ± 5.5, t(58) = 8.85, Cohen’s d effect size = 2.3, 95% CI = 1.63 – 2.94, P = 2.32 × 10−12).
Measuring nasal airflow
We used a miniaturized version of previously used device27 (Fig. 1, Supplementary Fig. 1). In brief, the Nasal Holter uses highly sensitive pressure sensors (SDP3x, Sensirion, Stäfa, Switzerland) and associated circuitry to convert pressure into flow at 6 Hz. The device measures 6.3 × 1.2 cm, weighs ~22 g, is pasted to the nape of one’s neck, and connected to the nose by nasal cannula (Fig. 1a). The device has onboard 3-degress-of-freedom motion/position sensor allowing determination of sleep times. Data is stored onboard for later download. The device was applied during a clinic/lab visit and removed at the patient’s home 24 h later. The device was applied at the end of the clinic/lab visit, after the participant completed a series of tests (MoCA, Beck, etc.). After the attachment procedure (5–10 min) the participants were asked to stay an additional 10–15 min to confirm the proper functionality of the device, and then left the lab/clinic. During the 24 h afterwards, participants were instructed to go on with their routine daily lives, exactly as they are used to. We opted for 24-hour recordings so as to be able to fully assess the nasal-cycle, which is associated with autonomic arousal27. All the raw data collected in this study is available for download at https://github.com/michalandelman/PD-classification-data.git.
Data preprocessing
Individual airflow (Fig. 1c, d) was first z-scored, assigned to wake or sleep, and divided into 5-minute consecutive blocks. Null blocks (without respiration recording) and blocks that contained naps during the day were removed. We then extracted 25 standard respiration features31 for each block. We applied an unsupervised feature selection process for multi-cluster data32 to rank-order the features. We then selected the top three that are best obtained with the Holter. More specifically, the Holter provides a highly precise temporal trace, thus it is optimized to extract temporal features such as respiratory onsets, offsets, durations, and curve shapes. In turn, because we do not use an air-tight facial mask, it is not optimized for measures such as absolute volume in cubic millimeters, etc. With this in mind, the three selected features are breathing rate variation coefficient (SD of difference between inhale onsets/average difference between inhale onsets), duty cycle inhale (average inhale duration/average inter-breath interval), and duty cycle of inhale pause duration (average duration of pause after inhale/average inter-breath interval). We note that these three features are within the top five features regardless of Holter strengths and weaknesses. To the best of our knowledge, these selected respiratory features have not been depicted in the context of PD. However, reduced variability of respiratory rate has been related to increased mortality in ICU patients33, and may reflect the autonomic dysfunction that often appears early in PD34. The prolonged duty cycle is probably a compensation mechanism to maintain alveolar ventilation during reduced work (load compensation)35, as PD patients suffer from reduced lung volumes and respiratory flows36.
Statistics and Reproducibility
We first tested distribution normality using Kolmogorov-Smirnov tests and QQ plots (Supplementary Fig. 2). For normally distributed data, we tested differences between groups using independent sample t-tests, whereas for abnormally distributed data, we applied non-parametric Wilcoxon rank-sum tests, with Bonferroni correction for multiple comparisons. To classify PD, we applied the Subspace Discriminant classifier, using a leave-one-out cross validation method such that predictions were made on participants who were not in the learning set. For PD severity prediction, we used a linear regression model. To assess severity prediction, we calculated Pearson’s correlation between PD outcome measures (MDS-UPDRS total score) and the result of the linear regression analysis. Analyses were performed using JASP (version 0.17.2.1, JASP team) and MATLAB (version R2023a, MathWorks, Inc.).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Daytime nasal airflow is significantly altered in PD
We initially plotted the respiratory features from all 28 PD patients (Table 1) and 33 controls, over 24 h of recording. We observed significant differences between PD and controls across the entire wake recording period (Fig. 2, Supplementary Fig. 3). To quantify this effect, we calculated a mean wake and mean sleep value for each respiratory feature. In wake, we observed significant differences in Duty Cycle Inhale (mean PD = 0.31 ± 0.06 (ratio), Control = 0.266 ± 0.04, Wilcoxon rank-sum test, z = 3.1, effect size Cliff’s δ = 0.46, 95% Confidence Interval (CI) = 0.18 – 0.68, P = 0.005 (all manuscript P values Bonferroni corrected) (Fig. 2a) and breathing rate variation coefficient (mean PD = 0.24 ± 0.07 (ratio), Control = 0.33 ± 0.11 (ratio), Wilcoxon rank-sum test, z =−3.7, effect size Cliff’s δ = −0.55, CI = −0.75 – (−0.27), P = 0.0006) (Fig. 2b). In other words, PD breathing is characterized by longer and less variable nasal inhalations. We also observed significant differences in duty cycle inhale pause (mean PD = 0.21 ± 0.09 (ratio), Control = 0.27 ± 0.08 (ratio), Wilcoxon rank-sum test, z = −2.86, effect size Cliff’s δ = −0.43, CI = −0.66 – (−0.12), P = 0.01) (Fig. 2c).
a–c Each panel contains line plots of a nasal airflow parameter during wake from starting point of the recording, for PD patients (red) and healthy controls (blue) followed by a box plot for that parameter during wake. For the time-line, solid line is the mean, and shade represents SEM. For the box-plots, Central lines indicate median, Edges of the box indicate 75th and 25th percentiles. Whiskers reflect 1.5 times the interquartile range. a Duty Cycle inhale, P = 0.005. b Breathing rate variation coefficient, P = 0.0006. c Duty cycle inhale pause, P = 0.01. n(control) = 33, n(PD) = 28. The plot is restricted to the duration of the one patient that provided least wake data (390 min). Overall wake recordings ranged from 390 to 1135 min (mean = 868.5 ± 147.7). d The combined score in PD and controls, P = 4.3 × 10−5. e The relation of the combined score to age, P = NS. f The relation of the combined score to disease severity, P = NS. g The relation of the combined score to medication status, P = NS.
To plainly visualize these overall differences, we calculated a combined score by summing the absolute normalized values of the three airflow parameters (i.e., reversing the sign on Duty Cycle Inhale), and indeed observed a pronounced difference between groups (mean PD = −1.22 ± 1.9 (arbitrary units), Control = 1.04 ± 2.16, Wilcoxon rank-sum test, z = −4.1, effect size Cliff’s δ = −0.61, 95% CI = −0.79 – (−0.34), P = 4.3 × 10−5) (Fig. 2d). We further observe that this combined score was unrelated to age (Pearson’s r = −0.03, P = 0.9) (Fig. 2e), disease severity (Spearman’s r = −0.09, P = 0.6) (Fig. 2f) or medication status (no L-Dopa: mean −0.65 ± 1.35 (arbitrary units), with L-Dopa = −1.5 ± 2.1, Wilcoxon rank-sum test, z = 1.18, effect size Cliff’s δ = 0.28, 95% CI = −0.17 – 0.64, P = 0.23) (Fig. 2g). The lack of relation to these factors was apparent also when rather than use the combined score, we looked at each parameter separately (disease severity - all r < 0.18, all P > 0.35, age - all r < 0.28, all P > 0.15, medication status—Wilcoxon rank-sum test, all z < 1.4, all P > 0.46, Supplementary Fig. 4). Finally, using the accelerometer within the device, we could easily parse wake from sleep times (Fig. 1e). Whereas the differences between PD and controls were pronounced during wake, we did not observe similar differences in sleep (Supplementary Fig. 5). On this front we note that in addition to airflow parameters, our separate measurement from each nostril allows us to calculate the nasal cycle, namely a fluctuating asymmetry in flow across nostrils that may indicate on autonomic tone27. We did not observe PD-associated differences in the nasal cycle during wake, but we did observe a small difference during sleep that does not survive the correction for multiple comparisons we apply throughout this manuscript (Supplementary Fig. 6). Finally, given that anxiety is reflected in respiration37 one may raise the possibility that these differences between groups reflected testing anxiety in the PD patients. To address this, we analyzed a separate group of otherwise healthy participants for which we have anxiety ratings38. We observed that the temporal respiratory parameters that were here altered in PD were not related to levels of anxiety, yet other respiratory parameters that were not altered in PD were altered in anxiety. In other words, it is unlikely that anxiety explains the effects we observed (Supplementary Fig. 7).
Although differences were significant across the entire wake recording period, they were particularly pronounced during the initial 30 min of recording. Addressing this is important, because if these results are to serve future development of a diagnostic tool, then obtaining a meaningful result with a shorter recording is meaningful for compliance. If we indeed repeat the above analyses concentrating on the initial 30 min alone, we again observe highly significant differences in Duty Cycle Inhale (mean PD = 0.3 ± 0.07 (ratio), Control = 0.23 ± 0.06 (ratio), Wilcoxon rank-sum test, z = 4.2, effect size Cliff’s δ = 0.63, 95% CI = 0.36 – 0.8, P = 6.9 × 10−5), breathing rate variation coefficient (mean PD = 0.26 ± 0.11 (ratio), Control = 0.38 ± 0.13 (ratio), z = −4.3, effect size Cliff’s δ = −0.64, 95% CI = −0.81 – (−0.37), P = 5.3 × 10−5), and duty cycle inhale pause (mean PD = 0.2 ± 0.1 (ratio), Control = 0.29 ± 0.1 (ratio), z = −3.06, effect size Cliff’s δ = −0.45, 95% CI = −0.68 – (−0.17), P = 0.006) (Fig. 3a–d). We conclude that the temporal dynamics of nasal respiratory airflow are altered in PD, and this is clearly evident within 30 min of observation alone.
a–c Box plots for respiration parameters during the first 30-min of recording during wake, n(control) = 33, n(PD) = 28. a Duty Cycle inhale, P = 6.9 × 10−5. b Breathing rate variation coefficient, P = 5.3 × 10−5. c Duty cycle inhale pause, P = 0.006. Central lines indicate median, Edges of the box indicate 75th and 25th percentiles. Whiskers reflect 1.5 times the interquartile range. d The three features during the first 30 min in healthy (blue) and PD (red) groups. Each circle represents a 5-minute block in a single participant, n(control)=33, n(PD) = 28. e ROC curve for detecting PD from 5 min (green), 30 min (purple), and 6.5 h (orange) using a subspace discriminant classifier (n(control) = 33, n(PD) = 28). f Histogram of classification accuracy for shuffled data with actual classification accuracy marked by red dashed line, P = 3.5×10−9. g. Model accuracy over time.
Nasal airflow alterations are evident by Stage II in the disease cascade
Although most of the patients were Stage II, we had several patients with extended disease duration. To assess whether the effects we observed were carried by these extended duration patients, we recreated the above analysis, restricting the cohort to disease duration equal/under 20 years (n = 27), equal/under 10 years (n = 23), and equal/under 5 years (n = 12). The differences between patients and controls remain highly significant even after removal of all patients with advanced and prolonged disease, such that even in the 5-year group alone we observe significant effects (Duty cycle inhale: mean PD = 0.29 ± 0.08, mean control = 0.23 ± 0.06, z = 2.8, effect size Cliff’s δ = 0.6, 95% CI = 0.1 – 0.8, P = 0.02. Breathing rate variation coefficient: mean PD = 0.26 ± 0.1, mean control = 0.38 ± 0.1, z = −3.5, effect size Cliff’s δ = −0.7, 95% CI = −0.89 – (−0.24), P = 0.001. Duty cycle inhale pause: mean PD = 0.2 ± 0.1, mean control = 0.29 ± 0.1, z = −2.6, effect size Cliff’s δ = −0.5, 95% CI = −0.77 – (−0.1), P = 0.03) (Supplementary Fig. 8).
PD can be detected using nasal airflow alone
Given the pronounced differences between PD and controls, we next asked whether we could classify PD using this data. We applied the Subspace Discriminant Classifier using a leave-one-out cross validation procedure, such that predictions were made on participants who were not in the learning set. Using only the first 5 min from each participant, our model achieved an area under the curve (AUC) of 0.83 (Fig. 3e, green), providing for 74% accuracy, 68% sensitivity (19 of 28) and 79% specificity (26 of 33). Performance improved as 5-minute blocks were added up to 30 min of recording, where we achieved an AUC of 0.85 (Fig. 3e, purple), 87% accuracy, 79% sensitivity (22 of 28) and 94% specificity (31 of 33). Using additional data beyond 30 min did not significantly improve performance, and using all the available data resulted in 0.76 AUC (Fig. 3e, orange, 66% accuracy, 39% sensitivity (11 of 28) and 88% specificity (29 of 33)). Although this result is not statistically significantly different from the result of the first 30 min alone (AUC differences39 = 0.09, χ2 = 2.7, P = 0.1), there was a trend towards improved classifier performance in the first 30 min of recording (3 G). To ask whether the first 30 min were indeed better, we parsed all the data into 30-minute windows, with a sliding window of 5 min, and compared accuracy across these selections. We observe that although all 30-minute windows can be used to significantly classify PD from controls, the first 30 min were better (first 30 min = 87%, all other 30-minute windows = 63 ± 0.05%, z = 4.53, P = 2.9 × 10−6).
To assess the significance of our classification results, we conducted a bootstrap analysis where we randomly shuffled the labels of participants (PD/Control) 1000 times, and reconducted the classification. We observe that the chance probability to obtain the classification we achieved is 1 in 3.5 × 10−9 (bootstrapped z = 5.7, Fig. 3e). Finally, as before, to assess the reliance of this result on disease duration, we recreated the above analysis, restricting the cohort to disease duration ≤20 years (n = 27), ≤10 years (n = 23), and ≤5 years (n = 12). The classifier remains highly effective and significant even within the group of five years or less from diagnosis (n(PD) = 12, accuracy 18 of 24 (75%), sensitivity: 10 of 12 (83%), specificity: 8 of 12 (66%), AUC 0.81) (Supplementary Fig. 9). In contrast to this remarkable classification during wake, we were unable to classify PD during sleep using our method and recordings (Supplementary Fig. 5).
PD severity can be predicted from nasal airflow alone
Given that the parameters used to build our model were unrelated to disease severity, and our model detected PD regardless of disease duration, one would expect that the model values are indeed unrelated to disease severity. Nevertheless, to explore this possibility, we regressed our model scores against the Movement Disorder Society - Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) score for each participant in our original cohort. Based on our findings that the first 30 min are the most valuable for classification purposes, we performed regression analysis for the scores from these first 30 min. We performed a linear regression analysis with leave-one-out cross validation, such that predictions were made on participants who were not in the learning set. Consistent with our ability to detect PD with this model regardless of disease duration, we did not observe a significant correlation between the predicted and the observed total severity score, or any of the sub-scores (all r < ∣0.34∣, all P > 0.1).
One may raise the possibility that PD severity may be predictable from nasal airflow, but not from the parameters that classify PD from non-PD. We observe that two interrelated respiratory features had previously been associated with PD: Percentage of breaths with inhale pause and Percentage of breaths with exhale pause40. To ask whether these two nasal airflow features can predict disease severity, we applied the same analysis scheme as above. We observed a moderate but highly significant correlation between the predicted and the observed total score (Total score: Spearman’s r = 0.49; P = 0.008) (Fig. 4a). Similar correlations emerged between the predicted and the observed three subparts (Part I: Spearman’s r = 0.43, P = 0.02; Part II: Spearman’s r = 0.4, P = 0.03, Part III: Spearman’s r = 0.39, P = 0.04) (Fig. 4b–d), implying that these particular features can predict disease severity. Only correlation with Part IV, that reflects rare therapy complications, was non-significant (Part IV: Spearman’s r = 0.32, P = 0.1), as a majority of patients scored Zero on this part.
Correlation between Severity prediction and actual Movement Disorder Society - Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) a Total score. b Part I. c Part II. d Part III. Each dot represents a participant (n = 28, jittered to prevent overlay). The light red patch represents 95% confidence interval.
Discussion
Ongoing daytime nasal respiratory dynamics were profoundly altered in PD, where inhalations during wake were longer and less variable than in matched controls. This effect remained pronounced even when we limited our analysis to sub-cohorts made of participants who were diagnosed within five years and less, and with and without L-Dopa treatment. Using this measure, only two (male) of 33 controls were wrongly classified as PD. We note that one of these two, male age 53, turned out to have a father with PD, and was himself hyposmic (Sniffin’ Sticks total score = 18.75), hence time will reveal whether this one of only two errors was wrong classification or early detection.
The altered temporal dynamics we observed point to a central brain component underlying disordered breathing in PD. Beyond pointing to the brain rather than to the lungs and associated skeletal-musculature, our results may provide hints as to further pin-pointing the brain site of effect: we note that unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wake41. Here we observed alterations in wake but not sleep. This may imply that the pre-Botzinger complex is in fact not the structure underlying the effects we observed (although the data on sleep-disordered breathing in PD is mixed36,42,43, so this issue remains unresolved.) (Supplementary Fig. 6). Moreover, the difference between the airflow parameters that were informative on disease classification vs. the parameters that were informative on disease severity prediction, may also be informative in this respect. Disease presence was associated with a sharp reduction in variability, and disease progression was associated with the appearance of pauses. On this front we can speculate: Olfaction is a strong driver of nasal airflow variability. Humans sniff in response to odors, even odors they are unaware of44, and these sniffs are a source of variability in the nasal airflow trace. Given the very early olfactory loss associated with PD45,46, its ensuing expected reduced sniffing47 and the relation between olfactory loss and respiratory alteration in other neurological diseases48, we propose that lost olfaction may be the driver of reduced respiratory variability in PD, an effect possibly mediated by the anterior olfactory nucleus. As the anterior olfactory nucleus and the olfactory bulb are amongst the first brain sites affected by PD15, we conjecture that the respiratory changes we observe may be evident early in the disease course. Alternatively, the reduced respiratory variability might reflect the autonomic dysfunction that often occurs in PD34. Taken together, this model places the olfactory loss and its respiratory implications as far more than an incidental observation in early PD, but rather as a possible driving force in the deterioration cascade.
Beyond pointing to a central mechanism underlying disordered breathing in PD, these results may have future clinical implications. There is a profound need for biomarkers in Parkinson’s disease (PD)49,50, and data from daily living rather than from traditional clinical examinations is an exciting new direction in medicine in general, and in PD in particular. Such data can be collected in part using remote “touchless” systems such as radio transmissions to track movement51, including respiratory-related movement43. The latter associated PD with alterations during sleep in contrast to our observation during wake. We do not have an explanation for this difference, and are limited in our ability to explore it, as the report on sleep differences used artificial intelligence (AI) to analyze the data43, so we do not know which particular respiratory parameters were responsible for the effect, and thus cannot revisit them in our sleep data. An alternative to remote “touchless” systems is wearable devices. For example, a wearable tracker of movement and tremor provided strong predictions on disease severity52. One can envision a combined set of wearable sensors including tremor, nasal airflow, and perhaps additional measures, that together may provide for a powerful tool in diagnostics and prognostics of PD. With the thought of developing these findings into such a diagnostic/prognostic tool, we observe that the predictive value of the nasal airflow measure was greatest during the first 30 min of recording (although we again stress that all other 30-minute window during wake still provided for significant classification). We speculate that this window of increased power follows the fact that all participants were engaged in uniform activities during this time window (performing the set of tests applied in lab/clinic before they went home to engage in obviously less-uniform activities, Methods), thus reducing other sources of variance related to individual lifestyle (Supplementary Fig. 10). This implies that future development into a possible diagnostic tool may include associated standardized activities that will be limited in time. On this front, we would also like to point to a possible ultradian rhythm in the respiratory features we extracted, evident in both patients and controls (Supplementary Fig. 3), possibly a reflection of ultradian rhythms in autonomic function53,54,55. This may imply that a future diagnostic application may not only be limited in time (e.g., 30 min), but also conducted at an optimal time of day. The nature of our data, however, prevented us from further exploring this here.
This study had several limitations we would like to acknowledge. First, in this study we did not test a third cohort with a different movement disorder or neurodegenerative condition. Thus, whereas we think we convincingly demonstrate altered temporal dynamics of nasal airflow in PD in comparison to matched healthy controls, we cannot comment on disease specificity, and this awaits separate investigation. Second, we had a very small number of female participants and patients in Stage I, and none in the prodromal stage. Although the results of female participants did not appear different from those in males (Supplementary Fig. 11), and none of the false-positives or false-negatives were female, the current sample size prevents us from saying anything definitive on this front. We note that this small number stems from two sources: a common source is that PD afflicts more males than females56, yet an added study-specific source is that, for reasons we can only speculate on, candidate female participants we approached were more aversed to wearing a nasal cannula for 24 h. Here we note, however, that our current findings of power within only 30 min of recording may generate higher compliance for a more limited duration test in the future. Considering these limitations, we would like to very clearly state that we are not here suggesting that nasal airflow can make for a current diagnostic test or be part of a current clinical evaluation. Current evaluation of PD is highly accurate57 and only extensive additional studies surrounding our method will allow its potential introduction to clinics.
Despite these limitations we end in reiterating the strengths of this study: The extent of alteration in the temporal dynamics of nasal airflow in mostly Stage II PD patients was such that we could classify PD at 87% accuracy using this data alone. This result implies a central brain pace-making component in disordered breathing in PD, and points to possible future development of a tool with diagnostic and prognostic value. We would like to end with a tantalizing speculation: As noted in the introduction, patterns of nasal airflow are closely associated with25,26,58,59,60,61,62,63,64, and in part determine65, patterns of brain activity, with particular influence on cortico-muscular communication66. Moreover, as suggested here above, the olfactory impairment in PD may be a driving force in the respiratory impairment in PD. We note that behavioral training can modify respiratory patterns in PD67,68, and it can improve olfaction in PD69. Thus, we end with the speculative proposition that we think it is worth testing whether a training regimen targeted towards reversing the very particular PD-specific respiratory temporal dynamics we observed here, i.e., to increase variability, may contribute to delaying disease progression.
Data availability
All the raw data collected in this study is available for download at https://github.com/michalandelman/PD-classification-data.git. All the source-data for the figures is available in the supplementary data Microsoft Excel file entitled Supplementary Data 1.
References
Feldman, J. L., Del Negro, C. A. & Gray, P. A. Understanding the rhythm of breathing: So near, yet so far. Annu Rev. Physiol. 75, 423–452 (2013).
Ratnovsky, A., Elad, D. & Halpern, P. Mechanics of respiratory muscles. Respir. Physiol. Neurobiol. 163, 82–89 (2008).
Baille, G. et al. Early occurrence of inspiratory muscle weakness in Parkinson’s disease. PLoS One 13, e0190400 (2018).
Weiner, P. et al. Respiratory muscle performance and the perception of dyspnea in Parkinson’s disease. Can. J. Neurological Sci. 29, 68–72 (2002).
De Pandis, M. F. et al. Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurol. Sci. 23, 69–70 (2002).
Herer, B., Arnulf, I. & Housset, B. Effects of Levodopa on Pulmonary Function in Parkinson’s Disease. Chest 119, 387–393 (2001).
Polatli, M., Akyol, A., Çilda, O. & Bayülkem, K. Pulmonary function tests in Parkinson’s disease. Eur. J. Neurol. 8, 341–345 (2001).
Pal, P. K., Sathyaprabha, T. N., Tuhina, P. & Thennarasu, K. Pattern of subclinical pulmonary dysfunctions in Parkinson’s disease and the effect of levodopa. Mov. Disord. 22, 420–424 (2007).
Sabaté, M., González, I., Ruperez, F. & Rodríguez, M. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J. Neurol. Sci. 138, 14–19 (1996).
Seccombe, L. M. et al. Abnormal ventilatory control in Parkinson’s disease-Further evidence for non-motor dysfunction. Respir. Physiol. Neurobiol. 179, 300–304 (2011).
Mikaelee H., Yazdchi M., Ansarin K., Arami M. Pulmonary function test abnormalities in Parkinson disease. The Internet Journal of Pulmonary Medicine. 2006;8. www.thoracic.org
Izquierdo-Alonso, J. L., Jiménez-Jiménez, F. J., Cabrera-Valdivia, F. & Mansilla-Lesmes, M. Airway Dysfunction in Patients with Parkinson’s Disease. Lung 172, 47–55 (1994).
Won, J. H., Byun, S. J., Oh, B. M., Park, S. J. & Seo, H. G. Risk and mortality of aspiration pneumonia in Parkinson’s disease: a nationwide database study. Sci. Rep. 11, 6597 (2021).
Vijayan, S., Singh, B., Ghosh, S., Stell, R. & Mastaglia, F. L. Brainstem ventilatory dysfunction: A plausible mechanism for dyspnea in Parkinson’s Disease? Mov. Disord. 35, 379–388 (2020).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Tomori, Z., Fung, M. L., Donic, V., Donicova, V. & St John, W. M. Power spectral analysis of respiratory responses to pharyngeal stimulation in cats: comparisons with eupnoea and gasping. J. Physiol. 485, 551–559 (1995).
Sant’ambrogio, G., Tsubone, H. & Sant’ambrogio, F. B. Sensory information from the upper airway: Role in the control of breathing. Respir. Physiol. 102, 1–16 (1995).
Clark, F. J. & von von Euler, C. On the regulation of depth and rate of breathing. J. Physiol. 222, 267–295 (1972).
Kikuta, S. et al. Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc. Natl. Acad. Sci. USA 107, 12363–12368 (2010).
Schwarzacher, S. W., Rüb, U. & Deller, T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134, 24–35 (2011).
Pyatigorskaya, N. et al. Medulla oblongata damage and cardiac autonomic dysfunction in Parkinson disease. Neurology 87, 2540–2545, http://freesurfer.net/ (2016).
Oliveira, L. M., Baertsch, N. A., Moreira, T. S., Ramirez, J. M. & Takakura, A. C. Unraveling the mechanisms underlying irregularities in inspiratory rhythm generation in a mouse model of Parkinson’s disease. J. Neurosci. 41, 4732–4747 (2021).
Fernandes-Junior, S. A., Carvalho, K. S., Moreira, T. S. & Takakura, A. C. Correlation between neuroanatomical and functional respiratory changes observed in an experimental model of Parkinson’s disease. Exp. Physiol. 103, 1377–1389 (2018).
Garcia, A. J., Dashevskiy, T., Khuu, M. A. & Ramirez, J. M. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol. 8, 571 (2017).
Arshamian, A., Iravani, B., Majid, A. & Lundström, J. N. Respiration modulates olfactory memory consolidation in humans. J. Neurosci. 38, 10286–10294 (2018).
Zelano, C. et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 36, 12448–12467 (2016).
Kahana-Zweig, R. et al. Measuring and characterizing the human nasal cycle. PLoS One 11, e0162918 (2016).
Erdfelder, E., FAul, F., Buchner, A. & Lang, A. G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res Methods 41, 1149–1160 (2009).
Maio et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 10, 5429 (2018).
Sidransky, E. & Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 11, 986–998 (2012).
Noto, T., Zhou, G., Schuele, S., Templer, J. & Zelano, C. Automated analysis of breathing waveforms using BreathMetrics: A respiratory signal processing toolbox. Chem. Senses 43, 583–597 (2018).
Cai D., C. Zhang, X. He. Unsupervised Feature Selection for Multi-Cluster Data. In: Proceedings of the 16th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining.; 2010:333-342.
Gutierrez, G. et al. Decreased respiratory rate variability during mechanical ventilation is associated with increased mortality. Intensive Care Med. 39, 1359–1367 (2013).
Chen, Z., Li, G. & Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 134, 104700 (2020).
Napoli N. J., Rodrigues V. R., Davenport P. W. Characterizing and Modeling Breathing Dynamics: Flow Rate, Rhythm, Period, and Frequency. Front Physiol. 12, https://doi.org/10.3389/fphys.2021.772295 (2022).
Mehanna, R. & Jankovic, J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat. Disord. 16, 628–638 (2010).
Giardino, N. D., Friedman, S. D. & Dager, S. R. Anxiety, respiration, and cerebral blood flow: implications for functional brain imaging. Compr. Psychiatry 48, 103–112 (2007).
Spielberg C. DEVELOPMENT OF THE SPANISH EDITION OF THE STATE-TRAIT ANXIETY INVENTORY1. Interamerican Journal of Psychology. Published online 1971:3-4.
Vergara, I. A., Norambuena, T., Ferrada, E., Slater, A. W. & Melo, F. StAR: A simple tool for the statistical comparison of ROC curves. BMC Bioinforma. 9, 1–5 (2008).
Huber, J. E., Darling, M., Francis, E. J. & Zhang, D. Impact of typical aging and Parkinson’s disease on the relationship among breath pausing, syntax, and punctuation. Am. J. Speech Lang. Pathol. 21, 368–379 (2012).
McKay, L. C. & Feldman, J. L. Unilateral ablation of pre-Bötzinger complex disrupts breathing during sleep but not wakefulness. Am. J. Respir. Crit. Care Med. 178, 89–95 (2008).
Stefani, A. & Högl, B. Sleep in Parkinson’s disease. Neuropsychopharmacology 45, 121–128 (2020).
Yang, Y. et al. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med. 28, 2207–2215 (2022).
Arzi A., Rozenkrantz L., Holtzman Y., Secundo L., Sobel N. Sniffing patterns uncover implicit memory for undetected odors. Current Biology. 24, https://doi.org/10.1016/j.cub.2014.02.004 (2014).
Fullard, M. E., Morley, J. F. & Duda, J. E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 33, 515–525 (2017).
Tissingh, G. et al. Loss of olfaction in De Novo and treated Parkinson’s disease: Possible implications for early diagnosis. Mov. Disord. 16, 41–46 (2001).
Sobel, N. et al. An impairment in sniffing contributes to the olfactory impairment in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 98, 4154–4159, https://doi.org/10.1073/pnas.071061598 (2001).
Günther R., et al. Impairment in respiratory function contributes to olfactory impairment in amyotrophic lateral sclerosis. Front Neurol. 9. https://doi.org/10.3389/fneur.2018.00079 (2018).
Sherer, T. B. Biomarkers for Parkinson’s Disease. Sci. Transl. Med. 3, 79ps14–79ps14 (2011).
Chen-Plotkin, A. S. et al. Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 10, 6003 (2018).
Liu, Y. et al. Monitoring gait at home with radio waves in Parkinson’s disease: A marker of severity, progression, and medication response. Sci. Transl. Med. 14, 9669 (2022).
Powers, R. et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci. Transl. Med. 13, 7865 (2021).
Kubin L. Neurobiology of Sleep–Wake Control. In: Pack A. I., ed. Sleep and Its Disorders. Translational Medicine Research. Springer, Dordrecht; 2022. https://doi.org/10.1007/978-94-024-2168-2_2
Cardinali D. P. The Timed Autonomic Nervous System. In: Autonomic Nervous System. Springer, Cham. 19–56. https://doi.org/10.1007/978-3-319-57571-1_2 (2018).
Kleitman, N. Basic Rest-Activity Cycle-22 Years Later. Sleep 5, 311–317, https://academic.oup.com/sleep/article/5/4/311/2753285 (1982).
Zirra, A. et al. Gender Differences in the Prevalence of Parkinson’s Disease. Mov. Disord. Clin. Pract. 10, 86–93 (2023).
Virameteekul, S., Revesz, T., Jaunmuktane, Z., Warner, T. T. & De Pablo-Fernández, E. Clinical Diagnostic Accuracy of Parkinson’s Disease: Where Do We Stand? Mov. Disord. 38, 558–566 (2023).
Kluger, D. S. & Gross, J. Respiration modulates oscillatory neural network activity at rest. PLoS Biol. 19, e3001457 (2021).
Ashhad, S., Kam, K., Del Negro, C. A. & Feldman, J. L. Breathing Rhythm and Pattern and Their Influence on Emotion. Annu Rev. Neurosci. 45, 223–247 (2022).
Ghazvineh, S. et al. Rhythmic air-puff into nasal cavity modulates activity across multiple brain areas: A non-invasive brain stimulation method to reduce ventilator-induced memory impairment. Respir. Physiol. Neurobiol. 287, 103627 (2021).
Salimi, M. et al. Nasal airflow promotes default mode network activity. Respir. Physiol. Neurobiol. 307, 103981 (2023).
Juventin, M. et al. Respiratory influence on brain dynamics: the preponderant role of the nasal pathway and deep slow regime. Pflug. Arch. 475, 23–35 (2023).
Perl, O. et al. Human non-olfactory cognition phase-locked with inhalation. Nat. Hum. Behav. 3, 501–512 (2019).
Del Negro, C. A., Funk, G. D. & Feldman, J. L. Breathing matters. Nat. Rev. Neurosci. 19, 351–367 (2018).
Salimi, M. et al. Nasal Air Puff Promotes Default Mode Network Activity in Mechanically Ventilated Comatose Patients: A Noninvasive Brain Stimulation Approach. Neuromodulation 25, 1351–1363 (2022).
Kluger, D. S. & Gross, J. Depth and phase of respiration modulate cortico-muscular communication. Neuroimage 222, 117272 (2020).
Mohammed Yusuf, S. F., Bhise, A., Nuhmani, S., Alghadir, A. H. & Khan, M. Effects of an incentive spirometer versus a threshold inspiratory muscle trainer on lung functions in Parkinson’s disease patients: a randomized trial. Sci. Rep. 13, 2516 (2023).
Ribeiro, R. et al. Breath-stacking and incentive spirometry in Parkinson’s disease: Randomized crossover clinical trial. Respir. Physiol. Neurobiol. 255, 11–16 (2018).
Haehner, A. et al. Olfactory Training in Patients with Parkinson’s Disease. PLoS One. 8, https://doi.org/10.1371/journal.pone.0061680 (2013).
Acknowledgements
This study was funded by grants from the Sagol Weizmann - MIT Bridge Program (2021/134368), the Minerva Foundation (714146), and a Breakthrough Research Grant from the Israeli Science Foundation (2751/23) awarded to NS. MAG was supported by a doctoral fellowship from the Azrieli Foundation. We would like to thank Dr. Yohai Bar-Sinai and Tamir Scherf for their helpful comments, and Dr. Ofer Perl for help with figure preparation.
Author information
Authors and Affiliations
Contributions
Conceived idea: M.A.G.; Designed experiments: M.A.G., T.G., N.S. Recruited patients: A.E., N.H., T.G. Built devices: A.W., N.S., L.P. Programmed devices: D.H. Conducted experiments: M.A.G. Analyzed data: M.A.G., K.S., T.S., A.R., L.G. Wrote first draft: M.A.G. Edited final draft: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: The Weizmann Institute of Science and the Tel Aviv Sourasky Medical Center are applying for patent authored by co-authors M.A.G., K.S., D.H., A.W., T.S., A.R., L.G., N.H., A.E., T.G., and N.S., for “classifying PD by nasal airflow”. K.S., D.H., A.W., and N.S. have applied for patent on the device used to measure nasal airflow (the Nasal Holter) and together with author L.P. have financial interests in a startup company developing this device. The startup company had no link to this study, and has no link or interests in PD.
Peer review
Peer review information
Communications Medicine thanks Meghashree Sampath and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Andelman-Gur, M., Snitz, K., Honigstein, D. et al. Discriminating Parkinson’s disease patients from healthy controls using nasal respiratory airflow. Commun Med 4, 233 (2024). https://doi.org/10.1038/s43856-024-00660-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00660-2