Abstract
Background
Revascularization is the most widely used treatment for moyamoya disease (MMD) but is associated with relatively high incidence of ischaemic complications in adult patients. At present, the guidelines in various countries do not include effective recommendations regarding postoperative complications, and a simple, practical and reliable scoring system is needed for rapid clinical evaluation and decision-making.
Methods
In this prognostic study, we developed a prediction model based on a single-centre cohort and validated it in a multicentre external prospective cohort. All patients were followed for at least 30 days to confirm whether postoperative acute cerebral infarction occurred.
Results
Among 2992 patients, 1980 patients are included in the derivation cohort, and 1012 patients compose the external validation cohort. Postoperative acute cerebral infarction occurs in 131 patients (6.62%) in the derivation cohort and 91 patients (8.99%) in the external validation cohort. Six risk factors are ultimately included in the development of the scoring system (CAMPIS). In the internal validation cohort, the Matthews correlation coefficient (MCC) and the concordance index (C-index) are 0.690 (0.681–0.698) and 0.956 (0.955–0.956), respectively. In the external validation cohort, the MCC and C-index are 0.762 (0.761–0.764) and 0.972 (0.971–0.973), respectively. In the derivation and validation cohorts, the postoperative infarction rates are 0.96% and 0.53%, 25.95% and 48.68%, and 84.71% and 89.47%, respectively, in the low-risk group, the medium-risk group, and the high-risk group.
Conclusions
CAMPIS is a reliable and practical tool that can be used to facilitate decision-making and avoid potentially harmful interventions, serving as an effective complement to the existing guidelines in the assessment and control of postoperative ischaemic complications.
Plain language summary
The arteries that supply blood to the brain become narrowed in people with moyamoya disease. Surgery to supply blood flow is used to treat moyamoya disease, however people often experience complications after the operation. We develope a model to predict whether people will have postoperative complications. Our scoring system shows high accuracy and so could be used to assess people prior to surgery to enable better management of treatment strategies and post-treatment assessment.
Similar content being viewed by others
Introduction
Moyamoya disease (MMD) is a rare cerebrovascular disease characterised by progressive spontaneous stenosis or occlusion of the distal internal carotid artery (ICA) and the proximal region of its main branches and accompanied by the formation of net-like vessels at the base of the brain1,2. The annual incidence rate is 1.40/100,000 in adults, and the incidence has increased rapidly over time3. Various revascularization procedures have been shown to improve cerebral haemodynamics and decrease the risk of ischaemic attack in the long term4,5. However, acute postoperative ischaemic stroke occurs in 9% of adults6,7,8,9,10,11,12,13,14,15,16,17,18,19,20, which greatly limits surgical protective effect. Therefore, the overall postoperative stroke-free rate is greatly reduced.
At present, there are no simple or effective grading or staging methods for predicting acute postoperative complications in MMD patients. Suzuki staging, a recognised vascular staging method for MMD, cannot represent the severity of the disease, nor can it predict the occurrence of complications after revascularization surgery21, which greatly limits the assessment of surgical risk.
Berlin staging, another popular scoring system, accurately predicts 20% of postoperative infarctions, making it unsuitable for widespread clinical application22. Second, although a cerebrovascular reserve (CVR) < 0.95 has been proven to be a predictive factor for stroke, other Berlin grading criteria have not been statistically tested to prove its validity for predicting the risk of stroke, especially for individuals. In addition, the compensation of the pial branch on DSA can reflect the extent of the CVR23. Stable compensation of the pial branches of the anterior and posterior cerebral circulation is a protective factor for postoperative cerebral perioperative infarction24. Third, although single photon emission computed tomography (SPECT) and positron emission tomography (PET) are excellent for evaluating blood perfusion25,26,27, they were limited in many medical centres due to high cost and the use of radioactive elements.
To provide data support for complementing guidelines and revising surgical indications, we reviewed the possible risk factors and provided large-scale, multicentre, 11-year data to develop a scoring system to predict the individual risk of postoperative ischaemic stroke. We finally developed and validated a clinical prediction model that can well predict postoperative ischaemic stroke complications and highlight the importance of perioperative clinical management.
Methods
Study design
Patients who were diagnosed with MMD and underwent surgical treatment at a single centre between January 1, 2012, and June 30, 2023, were consecutively enroled in this study as the training and internal validation sets. Patients from 8 other neurosurgical clinical centres were allocated to the external validation set to prospectively evaluate the stability, validity, and adaptability of the model. The outcome was defined as acute cerebral infarction within 30 days after surgery. Specific outcome event information is described in the Variable Definitions section. This work is consistent with the STROCSS criteria28. The study was performed according to the guidelines of the Declaration of Helsinki and approved by the research ethics committee of Beijing Tiantan Hospital affiliated to Capital Medical University (KY2022-032-03). All participants provided and signed informed consent for data use.
Inclusion criteria
The diagnosis met the criteria for spontaneous occlusion of the circle of Willis put forwards by the Research Committee29. (2) Age ≥ 16 and ≤70. (3) Discharge diagnosis of MMD (International Classification of Diseases-10: I67.500) (4) Initial direct (superficial temporal artery-middle cerebral artery bypass) or indirect revascularization (encephalo-duro-arterio-synangiosis).
Exclusion criteria
(1) Patients who did not undergo revascularization surgery. (2) Absence of relevant DSA images or postoperative imaging data. (3) Information from the medical records was incomplete. (4) Patients with perioperative stroke due to cardiovascular disease or other factors which were confirmed unrelated to MMD would be excluded. (5) The normal hemisphere of unilateral MMD would be excluded.
Clinical data collection
Before starting the process for data collection, the definitions of the variables were discussed and standardised according to the terminology presented in the published papers or/and reporting standards. Subsequently, the outcome assessors(OA) and neurosurgery residents were trained by neurosurgeons with more than 15 years of clinical cerebrovascular experience. The OA was responsible for collecting the patients’ demographic information and outcome data, and the neurosurgery residents were responsible for collecting the patients’ angiographic characteristics. The OA and neurosurgery residents were all blinded to each other to ensure the collected data were not biased by imaging features or clinical outcomes.
A standard training dataset with 100 cases was used to check the consistency of the data collectors. For those variables or cases where there were differences, final consensus would be reached by modifying confusing definitions or re-training data collectors. Only when the agreement reached at least 90% could the OA or resident independently extract the information.
While recording the data, uncertain cases were either decided in discussions with the cerebrovascular neurosurgeon or marked and discussed in weekly meetings.
Radiologists and neurosurgeons with ≥10 years of experience randomly sampled the data at a ratio of 1:10. The investigators were trained again if their data quality was low. Demographic and outcome data were collected by another neurosurgeon with more than 5 years of experience, and imaging data were reviewed by another neuroradiologist with more than 10 years of experience.
During the study, patients who underwent vascular revascularization were entered into EpiData software (http://www.epidata.dk/). The database included patient-, DSA-, and treatment-specific characteristics, such as sex, age, smoking status, alcohol use, ischaemic stroke history, concomitant diseases, postoperative complications, and follow-up results.
Sample size and missing data
According to previous studies and clinical experience, the estimated percentage of positive events is ~6–9%. According to the principle of events per variable (EVP) ≥ 1030,31, the minimum number of patients needed for the model is 80–100 patients. The minimum total number of patients ranged from 889 to 1667. To allow for a 10% loss of data due to incomplete imaging or medical records, a minimum of 988 to 1853 patients were enroled. All samples with missing data were excluded.
Quality control and perioperative management
Preoperative evaluation and management
Revascularization would be considered only if the patient meets any of the following criteria. (1) Previous cerebral infarction or cerebral haemorrhage, corresponding to the side affected by MMD. The revascularization would be performed at least 3 months after the most recent stroke. (2) TIA in the past 3 months. (3) Seizure, involuntary limb movements related to the side affected by MMD.
Surgical strategies and procedures
Surgical hemisphere selection was based on the side of recent preoperative stroke and the hemisphere corresponding to recent TIA symptoms. For patients with cognitive impairment, dizziness, and headache without stroke and typical TIA symptoms, the side with worse perfusion was selected after CT perfusion examination. If the difference could not be distinguished by the principle above, revascularization would be performed preferentially in the right hemisphere.
Direct bypass surgery is an end-to-side anastomosis of the superficial temporal artery to the cortical branch of the middle cerebral artery (STA-MCA). Indirect revascularization was performed using encephalo-duro-arterio-synangiosis (EDAS). Neurosurgeons from each centres performed indirect revascularization according to the types they were more familiar to. Combined revascularization was defined as simultaneous anastomosis of STA-MCA and EDAS. Combined bypass is still the preferred surgical type. The patency was routinely confirmed with intraoperative indocyanine green (ICG) video angiography or postoperative CT angiography. For the early operations without ICG equipment, Acland vessel strip test was used to verify the patency during the operation. If a suitable donor or recipient vessel was not available, EDAS would be performed.
Perioperative management
(1) Blood pressure would be maintained above the baseline value to preserve cerebral hypoperfusion during perioperative period. (2) Blood pressure should be strictly controlled after operation to avoid cerebral hypoperfusion or hyperperfusion syndrome. (3) The intraoperative blood loss was controlled less than 100 ml. (4)Hyperventilation should be avoided during perioperative period to prevent cerebral ischaemic events in patients with poor cerebrovascular reserve.
The outcome definition
Postoperative acute cerebral infarction was defined as a novel infarction (computed tomography (CT) and/or magnetic resonance (MR) manifestation of cerebral infarction) that developed within 30 days after surgery with/without neurological deficits10,32,33. All patients underwent three routine postoperative CT and MRI examinations within 8 h, 7 days and 30 days. Patients without imaging features of cerebral infarction were ruled out as having no postoperative infarction regardless of whether transient neurological deficits were resolved.
Vascular characteristics in digital subtraction angiography
ICA stenosis in long segments (ICAs): Long-segment stenosis of the ICA.
ACA proximal localised stenosis (ACAs): Blood flow comes from localised stenosis of A1 or proximal A2 to the ipsilateral normal distal ACA, with or without anterior communicating artery (ACoA) opening.
Steal phenomenon (SP): Blood flow originates from localised stenosis of A1 through the ACoA to the contralateral normal distal ACA.
MCA localised stenosis (MCAs): Localised stenosis of the proximal MCA.
Poor bidirectional compensation (PBC): There is no stable ACA backwards pial branch compensation or no stable PCA forwards pial branch compensation; compensatory pial branch vessels do not exceed the midline.
PCA proximal localised stenosis or occlusion (PCAs/PCAo): The P1 or P2 segments of the PCA were stenosed or occluded.
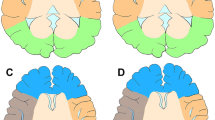
*Note: The score for the steal phenomenon should belong to the contralateral hemisphere. All stenosis was defined as the distal vascular branches were relatively normal but the moderate to severe stenosis (≥50%) in the proximal region. More details can be found in Fig. 1 and Supplementary Fig. 1.
A Internal carotid artery stenosis in long segments (red arrow); B Occlusion and localised stenosis in the proximal posterior cerebral artery (P1/P2) (red arrow); C Localised stenosis in the middle anterior cerebral artery (red arrow); D Localised stenosis (red arrow) in the proximal anterior cerebral artery (ACA); E Blood flow through the stenotic A1 supplies the contralateral ACA (steal phenomenon); F Poor and good bidirectional compensation (There is no stable ACA backwards pial branch compensation or no stable PCA forwards pial branch compensation; compensatory pial branch vessels do not exceed the midline).
Logistic regression machine learning model and nomogram
Potential predictors of postoperative infarction in MMD patients were analysed to construct a nomogram with both statistical and clinical significance, aiming to predict postoperative ischaemic stroke complications.
Keywords such as “moyamoya disease,” “postoperative stroke,” “revascularization,” “ischaemic stroke,” and “risk factor” were used to search the Web of Science and PubMed, and 16 relevant articles were ultimately included (Supplementary Table 1). Based on the DSA data and the basic characteristics of the patients, 21 variables were preliminarily selected for statistical analysis for further screening34.
The data from the single centre were randomly allocated to a training set or a test set. We repeated 5000 simple bootstrap resamples to investigate the stability of the selected variables. Oversampling was applied to reduce class imbalance. The final oversampling rate (0.2) was determined by a statistician with more than 10 years of experience and a clinician with more than 20 years of experience in assessing the best balance of recall and precision in the models.
The following three models were subjected to internal and external validation and compared to determine which model was most suitable for the final clinical scoring system:
Model A (Global model): All variables that were statistically significant in the multivariate analysis were included.
Model B (Selected model): Only DSA-related variables that were statistically significant in the multivariate analysis were included.
Model C: Suzuki stage alone was used as the predictive factor.
A nomogram was created based on the results of multivariate logistic regression analysis. Since the positive samples were oversampled, the final predictive value of infarction was overestimated. Therefore, the final predicted infarction rate was corrected by the following formula to the true infarct prediction value (adjusted probability of postoperative infarction): P represents the unadjusted probability of regression after oversampling, w represents the oversampling rate, and Q represents the adjusted predicted probability.
The following indexes were calculated to evaluate the predictive accuracy of the model: measuring the discrimination by the concordance index (C-index), the calibration by the calibration plot, and the clinical utility by the decision curve analysis (DCA). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), C-index, F1 score, F2 score and Matthews correlation coefficient (MCC) were calculated to assess the internal and external validity of the model. The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated to evaluate which model was more precise.
The C-index (range 0–1) was used to evaluate the predictive ability of the model, with a C-index of 0.50–0.70 indicating low accuracy, between 0.70 and 0.90 indicating medium accuracy, and above 0.90 indicating high accuracy.
The F1 score is the harmonic mean of precision and recall and accounts for both the precision and recall of classification models. It is an index used to measure the accuracy of binary classification (or multitask binary classification) models in statistics. It has a maximum value of 1 and a minimum value of 0, and a higher value indicates a better model.
The MCC ranged from −1 to +1, with +1 indicating perfect prediction, 0 indicating random prediction, and −1 indicating that the prediction was completely inconsistent with the actual observation. Typically, values greater than 0.5 are considered good.
Statistics and reproducibility
The statistical analyses were performed with R (version 4.1.1). Pearson’s χ2 test and Fisher’s exact test were used to compare categorical variables. The Kolmogorov‒Smirnov test was used to test the data for a normal distribution. The Mann–Whitney U test and two-sample t test were used to compare skewed and normally distributed continuous data, respectively. Variables with P < 0.10 in univariate analysis were included in multivariate analysis. Multivariate logistic regression was used to analyse the relationship between ischaemic stroke complications and the variables above. Continuous data are presented as the mean ± standard deviation. The level of significance was set at a P < 0.05. More details could be found in Supplementary Method 1.
The number of predictors was eventually limited to 6–9 to develop a scoring scale. The variance inflation factor (VIF) was used to assess multicollinearity (>3 indicates high collinearity). We also used β coefficients to assign scores as predictors to construct a scoring system that could be easily used in clinical practice.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Participant information
From January 1, 2012, to July 30, 2023, a total of 2992 patients (age ≥ 16 and ≤70) were included in the final analysis. After applying the exclusion criteria, we obtained a total data loss rate of 9.06% (298/3290). Among the included patients, 1980 patients were in the derivation cohort (889 males (44.90%) and 1091 females (55.10%); median age 41.00 (33.09–48.00) years), and 1012 patients were in the external validation cohort (473 men (46.74%) and 539 women (53.26%); median age 45.54 (36.90–52.46) years). Postoperative acute cerebral infarction occurred in 131 patients (6.62%) in the derivation cohort and 91 patients (8.99%) in the validation cohort. In the derivation cohort, 19/131 infarctions occurred only on the contralateral side of the surgery (14.5%), 15/131 infarctions were in both cerebral hemispheres (11.5%), and the remaining 97/131 infarctions were on the ipsilateral side of the surgery (74.0%). In the validation cohort, 24/91 infarctions occurred only on the contralateral side of the surgery (26.4%), 6/91 infarctions were in both cerebral hemispheres (6.6%), and the remaining 61/91 infarctions were on the ipsilateral side of the surgery (67.0%). See Fig. 2 for more information on the flow chat.
Annual incidence rate of postoperative infarction
The annual incidence rate of acute postoperative infarction after initial revascularization in adult MMD patients in the derivation cohort fluctuated between 5.99% and 7.69% (average 6.51%) from 2012 to 2022 (since it was only included in the first half of the 2023 data, the 2023 incidence was not incorporated in the annual assessment). There was no significant difference in the incidence of postoperative ischaemic stroke between 2012 and 2023 (P = 0.996). More details can be found in Supplementary Fig. 2 and Supplementary Table 2.
Variable screening and inclusion criteria
Initial revascularization was performed in 1980 patients with MMD. Of the 3692 sides of the cerebral hemispheres, 3546 had no postoperative infarction, and 146 had acute infarction within 30 days. According to our patient-based univariate analysis, age, hypertension, hyperlipidaemia, diabetes status, alcohol consumption, ischaemic stroke history, and haemorrhagic stroke history were significantly related to each other variables and were therefore included in the subsequent hemisphere-based multivariate analysis. More detailed information on the patients’ characteristics can be found in Table 1 and Supplementary Table 3.
The characteristics with a P value less than 0.10 in the univariate analysis were analysed via multivariate analysis. Finally, age, hypertension, haemorrhagic stroke history, ICA, ACA, SP, PCA, PBC and MCA were included in the final binary logistic regression model. See Supplementary Table 4 and Supplementary Table 3 for more details.
Combined revascularization does not increase the risk of surgery
In addition, in the derivation cohort, 1180 (59.60%) patients underwent combined revascularization, and 800 (40.40%) patients underwent indirect revascularization. The proportion of indirect revascularization in the infarction group was greater than that in the noninfarction group, but there was no significant difference between the two groups (P = 0.141). Similar results were obtained in the external validation cohort, as detailed in Supplementary Table 5.
Time to postoperative infarction
According to the survival curve, 89.31% of the postoperative infarctions were concentrated within 72 h after surgery, and the postoperative infarction rate in patients with ischaemic MMD (8.69%) was significantly greater than that in patients with haemorrhagic MMD (1.97%) (P < 0.001). Regression analysis also revealed that haemorrhagic MMD was a protective factor against infarction after revascularization (β = −0.880). There were no ischaemic strokes from 9 to 30 days after surgery. See Supplementary Fig. 3 for more details.
Logistic regression machine learning model and nomogram
With respect to the single-centre internal validation cohort, the nomogram for Model B had a C-index of 0.956 (0.955–0.956). The risk of postoperative infarction in adult MMD patients was positively correlated with the sum of the scores for the 6 predictive variables in the prediction model (Supplementary Table 6). The sensitivity was 94.98% (94.51–95.49), and the specificity was 86.89% (86.07–87.65). The PPV was 59.23% (57.85–60.50), and the NPV was 98.86% (98.76–98.96). The MCC is 0.690 (0.681–0.698). The clinical decision curve suggested that the prediction model had a high net clinical benefit (Fig. 3).
A Characteristics in the nomogram (Model B) to predict the probability of postoperative infarction in adult MMD patients (*PCA proximal localised stenosis=1, PCA occlusion=2). B Calibration curve of the predictive nomogram for the probability of postoperative infarction in adult MMD patients. C Decision curve analysis (DCA) was used to assess the net clinical benefit of the model
With respect to the multicentre external validation cohort, the C-index of Model B was 0.972 (0.971–0.973). The sensitivity was 90.72% (90.66–90.78), and the specificity was 97.44% (97.41–97.47). The PPV was 66.47% (66.17–66.67), and the NPV was 99.47% (99.47–99.49). The MCC was 0.762 (0.761–0.764). Model B performed significantly better than did Model A, the specific parameters of which are detailed in Supplementary Fig. 4 and Supplementary Table 6.
Model C was hardly successful at predicting postoperative infarction. The sensitivity, specificity and other indicators could not be calculated.
Compared with those of Model B, the NRI = 0.021 (P = 0.080) and IDI = 0.014 (P < 0.001) of Model A were both greater than 0 in the internal validation set, but there was no significant difference between them in the external validation set (NRI = 0.037, P = 0.325; IDI = 0.012, P = 0.181). The likelihood ratio test of the two models showed a statistically significant difference (P < 0.001). Although Model A had better predictive performance than Model B, Model B was simpler and was therefore selected to establish the final clinical scoring system because Model B needed to incorporate fewer variables and because the excellent performance of Model B was not much worse than that of Model A. More details can be found in Supplementary Table 6, Supplementary Fig. 5 and Fig. 3.
Construction of the practical clinical scoring system
The scoring system (CAMPIS, based on an acronym for 6 risk factors, compensation, ACAs, MCAs, PCAo/PCAs, ICAs, and SP), was estimated based on the corresponding β coefficients of Model B.
PBC was assigned 3 points (β = 2.763; 95% CI, 2.396–3.136), ACAs were assigned 4 points (β = 4.430; 95% CI, 4.023–4.852), MCAs were assigned 3 points (β = 3.446; 95% CI, 3.081–3.820), PCAo/PCAs were assigned 2/5 points (β = 2.153; 95% CI, 1.693–2.617; β = 5.374; 95% CI, 4.703–6.129), ICAs were assigned 2 points (β = 2.140; 95% CI, 1.560–2.716), and SP was assigned 5 points (β = 5.081; 95% CI, 4.376–5.843). Analysis of the VIF indicated that there was no significant collinearity between these variables. The total CAMPIS score ranges from 0 to 22. (Fig. 4 and Supplementary Table 7).
As shown in Fig. 4, the predicted probability of postoperative infarction increased with increasing CAMPIS score. According to the probability of cerebral infarction, we further divided the CAMPIS score into 3 groups to determine its clinical usefulness: low risk (0–3), medium risk (4–5), and high risk (≥6). There were significant differences in the incidence of postoperative infarction among the three groups in both the derivation cohort and the validation cohort (P < 0.001).
Discussion
Revascularization surgery is currently the standard treatment for reducing stroke incidence and improving the prognosis in MMD patients. However, this procedure does not benefit all patients, as an average of 9% of patients worldwide experience serious postoperative complications at multiple medical centres (Supplementary Table 8)7,9,10,11,12,13,14,15,16,17,18,19. Even in a large centre specialising in MMD treatment, such as the Stanford Stroke Centre, the 30-day stroke rate after initial revascularization surgery for adult MMD patients is ~6.6% to 7.3%20. We collected data over the past 11 years at a single centre and found that the annual incidence was between 5.99 and 7.69%. This finding suggested that there are some unrecognised internal factors that limit the safety of surgery rather than external adjustable factors. Although previous studies have explored the risk factors for postoperative infarction (Supplementary Table 8), a clinical prediction model to systematically propose the risk factors for infarction and make individualised and accurate predictions is lacking.
This study is the first large cohort study on the prediction of postoperative infarction in MMD patients in the world and is a promising and valuable tool for neurosurgeons in clinical decision-making. The CAMPIS score is helpful for performing individualised evaluations before surgery. The proposal of the CAMPIS score system has the following three points of guiding importance for revascularization:
In the high-risk group, revascularization should be delayed, especially due to the risk of severe vascular stenosis. Close observation should be performed every 6–12 months, and revascularization should be performed after chronic occlusion of narrow vessels.
For patients in the low-risk group, revascularization is relatively safe and can be performed as planned.
For patients in medium-risk group who must undergo surgery within a specified period due to their urgent condition, the risk should be fully assessed, and relatively high blood perfusion should be maintained during the perioperative period. Doctors should pay closer attention to the condition of patients and conduct MRI examinations and drug intervention in a timely manner once corresponding ischaemic symptoms appear.
We selected the ICA, ACA, MCA, and PCA for postoperative acute infarction evaluation. First, acute occlusion of narrow arteries may occur during the perioperative period. Moreover, acute vascular occlusion, unlike chronic vascular occlusion, cannot produce sufficient cerebrovascular compensation in a short time, which easily leads to acute cerebral infarction and fatal results. Cases of postoperative large-area cerebral infarction caused by acute occlusion of different cerebral arteries are summarised in Supplementary Figs. 6, 7 and 8.
Approximately 90% of postoperative ischaemic strokes occurred within 3 days after surgery, and there was no further infarction after 9 days. These findings indicate that surgery, anaesthesia and perioperative management in the first 3 days after surgery have the greatest impact on the incidence of postoperative infarction. Therefore, for patients at medium and high risk, surgeons who are familiar with revascularization should perform surgery to reduce operation time, maintain slightly higher and more stable blood pressure and carbon dioxide pressure during surgery, correct corresponding electrolyte disturbances and maintain an adequate perfusion volume after surgery, which may be better for reducing the risk of perioperative ischaemic stroke.
In terms of type, the risk of ischaemic stroke complications associated with haemorrhagic MMD is much lower than that associated with ischaemic MMD. Previous bleeding is a protective factor against postoperative ischaemic stroke complications. This finding also suggests that for haemorrhagic MMD, even if the CAMPIS score is relatively high, revascularization surgery can be considered appropriate under strict perioperative anaesthesia and blood pressure management.
The Suzuki staging system mainly focuses on stenosis of the main arteries and collaterals35. Several studies have shown that the Suzuki stage is associated with postoperative complications, while other studies and the present study reported contradictory results4,24,33. In our study, Suzuki staging did not pass univariate or multivariate analyses. Even so, we still predicted postoperative acute infarction with Suzuki stage alone as a predictor, while Suzuki stage was not a predictive factor of postoperative acute infarction complications.
Collaterals from the anterior or posterior circulation (bidirectional compensation), mainly the ACA or PCA, are the most important collateral pathway in MMD patients. A well-developed PCA can provide collateral from the posterior choroidal artery/leptomeningeal arteries to provide extra blood for the compromised anterior circulation. Moreover, a well-developed ACA can also provide collateral from the pericallosal artery to provide extra blood for the compromised MCA region and posterior circulation. Under these conditions, the haemodynamic stability of the whole cerebral region largely depends on the PCA and ACA. If the PCA or ACA is involved (for example, severe stenosis), the main collateral vessel will be susceptible to breakage, then resulting in severe ischaemic stroke. In ICA stenosis studies, researchers have found that well-developed collateral circulation is a good prognostic factor in patients with cerebrovascular disease36,37; thus, a similar mechanism may be involved. Therefore, we define this as bidirectional compensation. Bidirectional compensation is a protective factor against postoperative infarction. ACA- or PCA-derived blood flow is provided to protect the appropriate brain area during acute anterior or posterior circulation occlusion, as described in a previous study24,38.
ACA proximal localised stenosis was another main risk factor for postoperative ischaemia complications. A1 is the initial part of the ACA and a component of the circle of Willis. In addition to blood flow to the ipsilateral hemisphere passing through A1, blood flow to the contralateral hemisphere via the ACoA also passes through A1, which provides significant compensation for contralateral A1 stenosis or conclusion. The ACA can also provide collateral blood to the MCA area. Therefore, A1 stenosis has the main impact on the stability of the unilateral or bilateral hemisphere.
Compared to the above findings, the MCA supplies information only to the ipsilateral hemisphere. This can explain why MCA localised stenosis has a relatively small effect on postoperative complications. However, revascularization, especially direct and combined revascularization, may lead to blood flow disturbance, which could cause acute occlusion of the MCA and thus acute cerebral infarction in the middle artery region. According to this theory, when other risk factors are similar, it is safer to perform middle cerebral artery-superficial temporal artery (MCA-STA) bypass in patients with occlusive MCA than in patients with stenosed MCA.
PCA proximal localised stenosis or occlusion is the most important risk factor. Approximately 30% of MMD patients, also known as PCA-involved MMD, have PCA involvement39, indicating a poorer prognosis than MMD without PCA involvement40,41. The PCA provides blood to the posterior hemisphere. In MMD patients, the PCA can also provide a collateral pathway for the anterior circulation. Thus, P1 or P2 stenosis can affect not only posterior but also anterior cerebral blood flow. A narrow PCA, especially with good forwards compensatory flow, once occluded during the perioperative period leads to posterior circulation infarction over a large area. Moreover, PCAo, especially accompanied by poor ACA compensation, tends to cause postoperative infarction.
The steal phenomenon is the second most important risk factor. SP was defined as follows: Given occlusion of A1 on one side, blood flow supplies the distal normal ACA from contralateral stenosis A1 via the ACoA.
The results showed that haemorrhagic MMD had lower postoperative ischemic complications than ischaemic MMD. Haemorrhage is a protective factor for perioperative ischaemic complications18. We speculated that this may be due to the different pathophysiological characteristics of haemorrhage and ischaemia MMD. Cerebral infarction reflects the absence of spontaneous intracranial compensation, while haemorrhage reflects vessel rupture or hemodynamic aneurysm formation due to sufficient intracranial collateral compensation10. Previous studies have also shown that haemorrhagic MMD usually presents with relative normal perfusion42. However, normal perfusion may bring more stable perioperative compensation, which is beneficial to protect patients from ischaemic complications.
Although this is the first complete clinical predictive model for postoperative ischaemic complications of stroke in MMD patients with a large sample size, there are still some limitations that we must declare carefully.
First, in this study, we considered only the patients’ own external risk factors and did not consider blood pressure fluctuations during the perioperative period or other unobserved factors, which would inevitably affect the accuracy of the model. However, prediction models cannot incorporate all the factors that influence outcomes. For example, bypass patency within 30 days after surgery was not validated. We will then evaluate the external factors in subsequent studies.
Second, the study included only patients with MMD who underwent revascularization for the first time. The cerebral blood flow stability in patients who underwent a second revascularization could not be assessed due to changes in the haemodynamics of the operated hemisphere, so such patients were ultimately not included in this model.
Finally, bidirectional compensation via DSA can partially reflect the extent of the CVR, which has also been confirmed in the Berlin staging system. However, due to high cost and need for informed consent from the patients, the CVR could not be calculated to verify the accuracy of either the Berlin staging system or the CAMPIS system. Medical centres which allow the acetazolamide challenge are encouraged to validate the CAMPIS system and make appropriate amendments in the future. In addition, the compensation assessment of the CAMPIS system is relatively simplified. Although the simplified compensation assessment can judge the score more quickly, it also loses part of the information, which affects the potential accuracy.
Conclusions
In this prognostic study, the CAMPIS system, which consists of six central imaging features, was developed and externally validated at multiple centres to predict initial surgical risk in adult patients with MMD. Surgical risk stratification can help surgeons avoid overly aggressive intervention for patients in the high-risk group and prompt consideration of alternative medical decisions for patients in the medium-risk group.
These findings suggest that the CAMPIS system is a reliable and applicable tool for facilitating decision-making and reducing postoperative complications. The CAMPIS system is also an effective complement to the existing guidelines for the assessment and control of revascularization complications and is expected to be extended to other artery occlusion studies, such as the CMOSS.
Data availability
The data that support the findings of this study are available from the authors but restrictions apply to the availability of these data, which were used under license from Beijing Tiantan Hospital affiliated to Capital Medical University (Beijing) for the current study, and so are not publicly available. However, the datasets generated during the current study are available from the corresponding author upon reasonable request.
Code availability
The statistical analyses were performed with R (version 4.1.1). The final oversampling rate is 0.2. Access to the code is unrestricted. The code can be found on: https://doi.org/10.5281/zenodo.1551487043.
References
Suzuki, J. & Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 20, 288–299 (1969).
Kronenburg, A., Braun, K. P., van der Zwan, A. & Klijn, C. J. Recent advances in moyamoya disease: pathophysiology and treatment. Curr. Neurol. Neurosci. Rep. 14, 423 (2014).
Zhang, D. et al. Epidemiology of Moyamoya disease in China: a nationwide hospital-based study. Lancet Reg. Health West Pac. 18, 100331 (2022).
Deng, X. et al. Direct versus indirect bypasses for adult ischemic-type moyamoya disease: a propensity score-matched analysis. J. Neurosurg. 128, 1785–1791 (2018).
Cho, W. S. et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke 45, 3025–3031 (2014).
Kazumata, K. et al. The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-university series and systematic review. J. Neurosurg. 121, 432–440 (2014).
Chen, Y., Gong, X., Yang, Z., Chen, F. & Wang, J. Risk factors and a novel cerebral infarction extent scoring system for postoperative cerebral ischemia in patients with ischemic Moyamoya disease. Sci. Rep. 13, 5726 (2023).
Hyun, S. J., Kim, J. S. & Hong, S. C. Prognostic factors associated with perioperative ischemic complications in adult-onset moyamoya disease. Acta Neurochir. 152, 1181–1188 (2010).
Bao, X. Y. et al. Clinical features, surgical treatment and long-term outcome in adult patients with Moyamoya disease in China. Cerebrovasc. Dis. 34, 305–313 (2012).
Zhao, M. et al. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J. Neurosurg. 130, 531–542 (2018).
Ge, P. et al. Clinical features, surgical treatment, and long-term outcome in elderly patients with Moyamoya disease. World Neurosurg. 100, 459–466 (2017).
Zhao, M. et al. Ischemic stroke in young adults with Moyamoya disease: prognostic factors for stroke recurrence and functional outcome after revascularization. World Neurosurg. 103, 161–167 (2017).
Park, W., Ahn, J. S., Lee, H. S., Park, J. C. & Kwun, B. D. Risk factors for newly developed cerebral infarction after surgical revascularization for adults with Moyamoya disease. World Neurosurg. 92, 65–73 (2016).
Antonucci, M. U. et al. Acute preoperative infarcts and poor cerebrovascular reserve are independent risk factors for severe ischemic complications following direct extracranial-intracranial bypass for Moyamoya disease. Am. J. Neuroradiol. 37, 228–235 (2016).
Kim, T. et al. Stroke prevention by direct revascularization for patients with adult-onset moyamoya disease presenting with ischemia. J Neurosurg. 124, 1788–1793 (2016).
Gross, B. A. & Du, R. Adult moyamoya after revascularization. Acta Neurochir. 155, 247–254 (2013).
Lukshin, V. A. et al. Ischemic complications following surgical treatment of moyamoya disease: risk factors and prevention]. Zh Vopr Neirokhir Im N N Burdenko 85, 26–35 (2021).
Wang, J. et al. Postoperative cerebral infarction after revascularization in patients with moyamoya disease: incidence and risk factors. Front. Neurol. 13, 1053193 (2022).
Zhang, X. et al. Risk factors of transient neurological deficits and perioperative stroke after revascularization in patients with Moyamoya disease. Brain Sci. 12, 1285 (2022).
Teo, M. et al. Short- and long-term outcomes of moyamoya patients post-revascularization. J. Neurosurg. 138, 1374–1384 (2023).
Ge, P. et al. Encephaloduroateriosynangiosis versus conservative treatment for patients with moyamoya disease at late Suzuki stage. J. Clin. Neurosci. 50, 277–280 (2018).
Kashiwazaki, D. et al. Berlin grading system can stratify the onset and predict perioperative complications in adult Moyamoya disease. Neurosurgery 81, 986–991 (2017).
Czabanka, M. et al. Proposal for a new grading of Moyamoya disease in adult patients. Cerebrovasc. Dis. 32, 41–50 (2011).
Liu, Z. W. et al. Collateral circulation in Moyamoya disease: a new grading system. Stroke 50, 2708–2715 (2019).
Morimoto, M., Iwama, T., Hashimoto, N., Kojima, A. & Hayashida, K. Efficacy of direct revascularization in adult Moyamoya disease: haemodynamic evaluation by positron emission tomography. Acta Neurochir. 141, 377–384 (1999).
Kuroda, S., Houkin, K., Kamiyama, H., Abe, H. & Mitsumori, K. Regional cerebral hemodynamics in childhood moyamoya disease. Childs Nerv. Syst. 11, 584–590 (1995).
Ikezaki, K. et al. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J. Neurosurg. 81, 843–850 (1994).
Mathew, G. et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 96, 106165 (2021).
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis).Neurol. Med. Chir. 52, 245–266 (2012).
Harrell, F. E. Jr., Lee, K. L. & Mark, D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15, 361–387 (1996).
Vittinghoff, E. & McCulloch, C. E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 165, 710–718 (2007).
Funaki, T. et al. Unstable moyamoya disease: clinical features and impact on perioperative ischemic complications. J. Neurosurg. 122, 400–407 (2015).
Kim, S. H., Choi, J. U., Yang, K. H., Kim, T. G. & Kim, D. S. Risk factors for postoperative ischemic complications in patients with moyamoya disease. J. Neurosurg. 103, 433–438 (2005).
Heinze, G., Wallisch, C. & Dunkler, D. Variable selection - A review and recommendations for the practicing statistician. Biom J. 60, 431–449 (2018).
Han, Q., Yao, F., Zhang, Z. & Huang, Y. Evaluation of revascularization in different Suzuki stages of ischemic moyamoya disease by whole-brain CT perfusion. Front. Neurol. 12, 683224 (2021).
Kaszczewski, P. et al. Volumetric flow assessment in extracranial arteries in patients with 70-99% internal carotid artery stenosis. Diagnostics 12, 2216 (2022).
Henderson, R. D., Eliasziw, M., Fox, A. J., Rothwell, P. M. & Barnett, H. J. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 31, 128–132 (2000).
Liu, Z. W. et al. Clinical characteristics and leptomeningeal collateral status in pediatric and adult patients with ischemic moyamoya disease. CNS Neurosci. Ther. 26, 14–20 (2020).
Hishikawa, T., Tokunaga, K., Sugiu, K. & Date, I. Assessment of the difference in posterior circulation involvement between pediatric and adult patients with moyamoya disease. J. Neurosurg. 119, 961–965 (2013).
Yamada, I., Murata, Y., Umehara, I., Suzuki, S. & Matsushima, Y. SPECT and MRI evaluations of the posterior circulation in moyamoya disease. J. Nucl. Med. 37, 1613–1617 (1996).
Kimiwada, T., Hayashi, T., Shirane, R. & Tominaga, T. Posterior cerebral artery stenosis and posterior circulation revascularization surgery in pediatric patients with moyamoya disease. J. Neurosurg. Pediatr. 21, 632–638 (2018).
Lu, J. et al. Hemorrhagic transformation in ischemic Moyamoya disease: clinical characteristics, radiological features, and outcomes. Front. Neurol. 11, 517 (2020).
Wang, R. model. https://doi.org/10.5281/zenodo.15514870 (2025).
Acknowledgements
Thank Yehan Xiao for his contribution to this article. Sources of funding. This study was supported by the National Natural Science Foundation of China (82171887) and Peking University International Hospital Research Grant (YN2022ZD04).
Author information
Authors and Affiliations
Contributions
Z.L.: Data curation, writing-original draft, writing-review and editing, methodology. X.H.: Data curation, visualisation, writing-review & editing. S.H.: Writing-original draft, project administration. C.T.: Investigation, project administration. Y.W.: Software, methodology. K.S: Software, formal analysis. X.W.: Investigation. Z.Z.: Investigation. M.L.: Resources. D.M.: Investigation. T.Y.:Data curation. Q.L.: Data curation. Y.J.: Investigation. Y.W.: Investigation. H.S.: Investigation. D.Y.: Investigation. J.L.: Investigation. H.C.: Investigation. J.Z.: Investigation. X.L.: Formal analysis, supervision, methodology, visualisation. Q.G.: Supervision, validation. H.G.: Supervision, validation. Y.Y.: Supervision, validation. J.P.: Supervision, validation. B.L.: Supervision, validation. Y.L.: Supervision, validation. Y.F.: Methodology, software, visualisation. D.Z.: Project administration, supervision. N.M.: Resources, supervision. L.L.: Supervision, writing-review & editing. R.D.: Conceptualisation, supervision, validation, funding acquisition, project administration. R.W.: Conceptualisation, supervision, validation, funding acquisition, project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Hao, X., He, S. et al. Development and validation of a scoring system to evaluate and clinically manage postoperative acute infarction complications in moyamoya disease. Commun Med 5, 215 (2025). https://doi.org/10.1038/s43856-025-00937-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-00937-0