Abstract
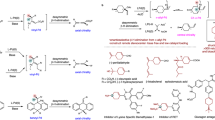
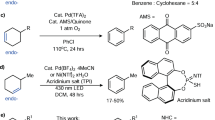
Dehydrogenation of an alkyl group via C–H activation forms a vinyl unit, which can act as a versatile stepping stone for diverse late-stage structural modifications at two adjacent sp3 carbon centres. However, enantioselective dehydrogenation via C–H metalation remains a challenge. Here we describe the realization of palladium-catalysed enantioselective β,γ-dehydrogenation of cycloalkyl amides enabled by chiral oxazoline–pyridone ligands to afford a wide range of highly elaborated carbocycles with exceptional enantioselectivity (>99% e.e.). Notably, the resulting chiral β,γ-unsaturated carbocycles are difficult to access via an inverse electron demand Diels–Alder reaction. Through ligand control, a tandem dehydrogenation and C–H olefination sequence also led to the formation of chiral β-alkylidene-γ-lactams. Remarkably, this catalyst is also compatible with biologically important natural products, including diterpenes and pentacyclic triterpenes, where each enantiomer of our chiral ligand enables site-selective modification at four distinct sites within the E ring.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. The crystallographic data for the structures reported in this study for compounds 2ac, 2ad, 3r and 5c have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under accession numbers 2332771 (2ac), 2300388 (2ad), 2304156 (3r) and 2302270 (5c). These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
Turlik, A., Chen, Y. & Newhouse, T. R. Dehydrogenation adjacent to carbonyls using palladium–allyl intermediates. Synlett 27, 331–336 (2016).
Sharpless, K. B., Lauer, R. F. & Teranishi, A. Y. Electrophilic and nucleophilic organoselenium reagents. New routes to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 95, 6137–6139 (1973).
Diao, T., Pun, D. & Stahl, S. S. Aerobic dehydrogenation of cyclohexanone to cyclohexenone catalyzed by Pd(DMSO)2(TFA)2: evidence for ligand-controlled chemoselectivity. J. Am. Chem. Soc. 135, 8205–8212 (2013).
Chen, M. & Dong, G. Copper-catalyzed desaturation of lactones, lactams, and ketones under pH-neutral conditions. J. Am. Chem. Soc. 141, 14889–14897 (2019).
Teskey, C. J., Adler, P., Gonçalves, C. R. & Maulide, N. Chemoselective α,β‐dehydrogenation of saturated amides. Angew. Chem. Int. Ed. 58, 447–451 (2019).
Chen, Y., Turlik, A. & Newhouse, T. R. Amide α,β-dehydrogenation using allyl–palladium catalysis and a hindered monodentate anilide. J. Am. Chem. Soc. 138, 1166–1169 (2016).
Wang, Z., He, Z., Zhang, L. & Huang, Y. Iridium-catalyzed aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids: activation of both α- and β-C–H bonds through an allyl–iridium intermediate. J. Am. Chem. Soc. 140, 735–740 (2018).
Gnaim, S., Vantourout, J. C., Serpier, F., Echeverria, P.-G. & Baran, P. S. Carbonyl desaturation: where does catalysis stand. ACS. Catal. 11, 883–892 (2021).
Zhu, L., Zhang, L. & Luo, S. Catalytic desymmetrizing dehydrogenation of 4‐substituted cyclohexanones through enamine oxidation. Angew. Chem. Int. Ed. 57, 2253–2258 (2018).
Wang, Z. et al. Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 374, 1281–1285 (2021).
Sheng, T. et al. Synthesis of β,γ-unsaturated aliphatic acids via ligand-enabled dehydrogenation. J. Am. Chem. Soc. 145, 20951–20958 (2023).
Das, J. et al. Access to unsaturated bicyclic lactonesby overriding conventional C(sp3)–H site selectivity. Nat. Chem. 15, 1626–1635 (2023).
Chen, G. et al. Ligand-accelerated enantioselective methylene C(sp3)–H bond activation. Science 353, 1023–1027 (2016).
Shen, P.-X., Hu, L., Shao, Q., Hong, K. & Yu, J.-Q. Pd(II)-catalyzed enantioselective C(sp3)–H arylation of free carboxylic acids. J. Am. Chem. Soc. 140, 6545–6549 (2018).
Hu, L. et al. Pd(II)‐catalyzed enantioselective C(sp3)–H activation/cross‐coupling reactions of free carboxylic acids. Angew. Chem. Int. Ed. 58, 2134–2138 (2019).
Zhang, T. et al. Enantioselective remote methylene C–H (hetero)arylation of cycloalkane carboxylic acids. Science 384, 793–798 (2024).
Jiang, X. & Wang, R. Recent developments in catalytic asymmetric inverse-electron-demand Diels–Alder reaction. Chem. Rev. 113, 5515–5546 (2013).
Corey, E. J. Catalytic enantioselective Diels–Alder reactions: methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. 41, 1650–1667 (2002).
Kagan, H. B. & Riant, O. Catalytic asymmetric Diels Alder reactions. Chem. Rev. 92, 1007–1019 (1992).
Mackay, E. G. & Sherburn, M. S. The Diels–Alder reaction in steroid synthesis. Synthesis 47, 1–21 (2014).
Sheng, T. et al. One-step synthesis of β-alkylidene-γ-lactones via ligand-enabled β,γ-dehydrogenation of aliphatic acids. J. Am. Chem. Soc. 144, 12924–12933 (2022).
Kang, G., Strassfeld, D. A., Sheng, T., Chen, C.-Y. & Yu, J.-Q. Transannular C–H functionalization of cycloalkane carboxylic acids. Nature 618, 519–525 (2023).
Ammazzalorso, A., De Filippis, B., Giampietro, L. & Amoroso, R. N‐acylsulfonamides: synthetic routes and biological potential in medicinal chemistry. Chem. Biol. Drug Des. 90, 1094–1105 (2017).
Ballatore, C., Huryn, D. M. & Smith, A. B. III Carboxylic acid (bio)isosteres in drug design. ChemMedChem 8, 385–395 (2013).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Stansfield, I. et al. Development of carboxylic acid replacements in indole-N-acetamide inhibitors of hepatitis C virus NS5B polymerase. Bioorg. Med. Chem. Lett. 17, 5143–5149 (2007).
Wu, K. et al. Palladium(II)‐catalyzed C–H activation with bifunctional ligands: from curiosity to industrialization. Angew. Chem. Int. Ed. 63, e202400509 (2024).
Lucas, E. L. et al. Palladium-catalyzed enantioselective β-C(sp3)–H activation reactions of aliphatic acids: a retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res. 55, 537–550 (2022).
He, J., Shao, Q., Wu, Q. & Yu, J.-Q. Pd(II)-catalyzed enantioselective C(sp3)–H borylation. J. Am. Chem. Soc. 139, 3344–3347 (2017).
Zhuang, Z. & Yu, J.-Q. Pd(II)-catalyzed enantioselective γ-C(sp3)–H functionalizations of free cyclopropylmethylamines. J. Am. Chem. Soc. 142, 12015–12019 (2020).
Wu, Q.-F. et al. Formation of α-chiral centers by asymmetric β-C(sp3)–H arylation, alkenylation, and alkynylation. Science 355, 499–503 (2017).
Mathur, S., Bulchandani, N., Parihar, S. & Shekhawat, G. S. Critical review on steviol glycosides: pharmacological, toxicological and therapeutic aspects of high potency zero caloric sweetener. Int. J. Pharmacol. 13, 916–928 (2017).
Zhuang, Z. et al. Ligand‐enabled β‐C(sp3)–H lactamization of tosyl‐protected aliphatic amides using a practical oxidant. Angew. Chem. Int. Ed. 134, e202207354 (2022).
Mehta, P. D., Sengar, N. P. S. & Pathak, A. K. 2-Azetidinone—a new profile of various pharmacological activities. Eur. J. Med. Chem. 45, 5541–5560 (2010).
Decuyper, L. et al. Antibacterial and β-lactamase inhibitory activity of monocyclic β-lactams. Med. Res. Rev. 38, 426–503 (2018).
Pierrat, O. A. et al. Monocyclic β-lactams are selective, mechanism-based inhibitors of rhomboid intramembrane proteases. ACS Chem. Biol. 6, 325–335 (2011).
Acknowledgements
We acknowledge The Scripps Research Institute, the NIH (National Institute of General Medical Sciences grant R01GM084019) for financial support. J. Chen, B. Sanchez, J. Lee and Q. N. Wong from the Scripps Automated Synthesis Center are acknowledged for purification guidance. We acknowledge M. Gembicky, J. Bailey, E. Samolova and the UCSD Crystallography Facility for X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. conceived the concept. T.S. discovered and developed the dehydrogenation reaction. T.S. developed the substrate scope, T.Z. developed the chiral oxazoline–pyridone ligands. T.S., Z.Z. and J.-Q.Y. wrote the paper. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, experimental procedures, additional reaction optimization, and characterization data.
Supplementary Data 1
Crystallographic data for compound 2ac, CCDC 2332771.
Supplementary Data 2
Crystallographic data for compound 2ad, CCDC 2300388.
Supplementary Data 3
Crystallographic data for compound 3r, CCDC 2304156.
Supplementary Data 4
Crystallographic data for compound 5c, CCDC 2302270.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, T., Zhang, T., Zhuang, Z. et al. Synthesis of chiral carbocycles via enantioselective β,γ-dehydrogenation. Nat. Synth 3, 1550–1559 (2024). https://doi.org/10.1038/s44160-024-00628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00628-z