Abstract
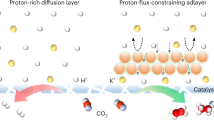
The use of acidic electrolytes in CO2 reduction avoids costly carbonate loss. However, the energy efficiency of acid-fed electrolysers has been limited by high hydrogen production and operating potentials. We find that these stem from the lack of alkali cations at the catalyst surface, limiting CO2 and CO adsorption. In acid-fed membrane electrode assembly systems, the incorporation of these cations is challenging as there is no flowing catholyte. Here an interfacial cation matrix (ICM)–catalyst heterojunction is designed that directly attaches to the catalyst layer. The negatively charged nature of the ICM enriches the alkali cation concentration near the cathode surface, trapping generated hydroxide ions. This increases the local electric field and pH, increasing multi-carbon production. Integrating the ICM strategy with a tailored copper–silver catalyst enables selective ethanol production through a proton-spillover mechanism. We report a 45% CO2-to-ethanol Faradaic efficiency at 200 mA cm−2, carbon efficiency of 63%, full-cell ethanol energy efficiency of 15% (3-fold improvement over the best previous acidic CO2 reduction value) and energy cost of 260 GJ per tonne ethanol, the lowest among reported ethanol-producing CO2 electrolysers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study, including computational specifics, and other experimental and microscopic analyses, can be found in the Article and its Supplementary Information.
References
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
Gao, D., Arán-Ais, R. M., Jeon, H. S. & Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019).
Nguyen, T. N. et al. Catalyst regeneration via chemical oxidation enables long-term electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 144, 13254–13265 (2022).
Zhang, S., Fan, Q., Xia, R. & Meyer, T. J. CO2 reduction: from homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 53, 255–264 (2020).
Ethanol Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2017–2022. Transparency Market Research (2018). https://www.transparencymarketresearch.com/ethanol-market.html.
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Wang, P. et al. Boosting electrocatalytic CO2-to-ethanol production via asymmetric C–C coupling. Nat. Commun. 13, 1–11 (2022).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Adnan, M. A. et al. Directly-deposited ultrathin solid polymer electrolyte for enhanced CO2 electrolysis. Adv. Energy Mater. https://doi.org/10.1002/aenm.202203158 (2023).
Shang, L. et al. Efficient CO2 electroreduction to ethanol by Cu3Sn catalyst. Small Methods 6, 2101334 (2022).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2019).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Kim, C. et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat. Energy 6, 1026–1034 (2021).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 10–12 (2020).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Xu, Y. et al. A microchanneled solid electrolyte for carbon-efficient CO2 electrolysis. Joule 6, 1333–1343 (2022).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Fan, M. et al. Cationic-group-functionalized electrocatalysts enable stable acidic CO2 electrolysis. Nat. Catal. 6, 763–772 (2023).
Zhao, Y. et al. Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment. Nat. Synth. 2, 403–412 (2023).
Bohra, D., Chaudhry, J. H., Burdyny, T., Pidko, E. A. & Smith, W. A. Modeling the electrical double layer to understand the reaction environment in a CO2 electrocatalytic system. Energy Environ. Sci. 12, 3380–3389 (2019).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Yang, B. et al. Accelerating CO2 electroreduction to multicarbon products via synergistic electric–thermal field on copper nanoneedles. J. Am. Chem. Soc. 144, 3039–3049 (2022).
Yang, K. et al. Cation-driven increases of CO2 utilization in a bipolar membrane electrode assembly for CO2 electrolysis. ACS Energy Lett. 6, 4291–4298 (2021).
Pan, B. et al. Close to 90% single-pass conversion efficiency for CO2 electroreduction in an acid-fed membrane electrode assembly. ACS Energy Lett. 7, 4224–4231 (2022).
Garg, S. et al. How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers. Energy Environ. Sci. 16, 1631–1643 (2023).
Konwar, L. J., Mäki-Arvela, P. & Mikkola, J. P. SO3H-containing functional carbon materials: synthesis, structure, and acid catalysis. Chem. Rev. 119, 11576–11630 (2019).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Shi, S., Liu, Z., Lin, Q., Chen, X. & Kusoglu, A. Role of ionic interactions in the deformation and fracture behavior of perfluorosulfonic-acid membranes. Soft Matter 16, 1653–1667 (2020).
Shi, S., Weber, A. Z. & Kusoglu, A. Structure–transport relationship of perfluorosulfonic-acid membranes in different cationic forms. Electrochim. Acta 220, 517–528 (2016).
Jervis, R. et al. The importance of using alkaline ionomer binders for screening electrocatalysts in alkaline electrolyte. J. Electrochem. Soc. 164, F1551–F1555 (2017).
Lees, E. W. et al. Linking gas diffusion electrode composition to CO2 reduction in a flow cell. J. Mater. Chem. A 8, 19493–19501 (2020).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Ooka, H., Figueiredo, M. C. & Koper, M. T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307–9313 (2017).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Weng, L.-C., Bell, A. T. & Weber, A. Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018).
Li, G. et al. Electrode engineering for electrochemical CO2 reduction. Energy Fuels 36, 4234–4249 (2022).
Li, M. et al. The role of electrode wettability in electrochemical reduction of carbon dioxide. J. Mater. Chem. A 9, 19369–19409 (2021).
Ozden, A. et al. Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption. Nat. Energy 8, 179–190 (2023).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Xie, K. et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products. Nat. Commun. 13, 3609 (2022).
Zheng, Y. et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Chang, X., Zhao, Y. & Xu, B. pH dependence of Cu surface speciation in the electrochemical CO reduction reaction. ACS Catal. 10, 13737–13747 (2020).
Hou, J., Chang, X., Li, J., Xu, B. & Lu, Q. Correlating CO coverage and co electroreduction on cu via high-pressure in situ spectroscopic and reactivity investigations. J. Am. Chem. Soc. 144, 22202–22211 (2022).
Chang, X. et al. C–C coupling is unlikely to be the rate-determining step in the formation of C2+ products in the copper-catalyzed electrochemical reduction of CO. Angew. Chem. Int. Ed. 61, e202111167 (2022).
Li, J. et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 12, 1–11 (2021).
Santatiwongchai, J., Faungnawakij, K. & Hirunsit, P. Comprehensive mechanism of CO2 electroreduction toward ethylene and ethanol: the solvent effect from explicit water–Cu(100) interface models. ACS Catal. 11, 9688–9701 (2021).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Miao, R. K. et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule 5, 2742–2753 (2021).
Li, F., Zhou, C. & Klinkova, A. Simulating electric field and current density in nanostructured electrocatalysts. Phys. Chem. Chem. Phy. https://doi.org/10.1039/d2cp02846h (2022).
Lark, T. J. et al. Environmental outcomes of the US Renewable Fuel Standard. Proc. Natl Acad. Sci. USA 119, e2101084119 (2022).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Wang, X. et al. Ag+-doped InSe nanosheets for membrane electrode assembly electrolyzer toward large-current electroreduction of CO2 to ethanol. Angew. Chem. Int. Ed. 62, e202313646 (2023).
Acknowledgements
We acknowledge funding for this work from the Government of Canada’s New Frontiers in Research Fund (NFRF), CANSTOREnergy project NFRFT-2022-00197, NRC and the University of Toronto Collaboration Centre Program in Green Energy Materials (CC-GEM, GEM-PRJ-01), Ontario Research Foundation Research Excellence Program and the CIFAR Bio-Inspired Solar Energy programme. D.S. acknowledges the support received from the Canada Research Chairs programme. A.S.Z. thanks NSERC for a Postdoctoral Fellowship. T.A. acknowledges graduate scholarships and fellowships from Hatch, NSERC and the University of Toronto. We also acknowledge Compute Canada for providing computing resources. We appreciate the Ontario Centre for the Characterization of Advanced Materials (OCCAM) at the University of Toronto for conducting microscopy characterizations. We thank M. Chehelamirani for his assistance in preparing the graphical abstract.
Author information
Authors and Affiliations
Contributions
D.S. and E.H.S. supervised the project. A.S.Z. conceived the idea and performed the electrochemical experiments and analyses. A.S.Z. and T.A. co-wrote the manuscript. F.L. performed the DFT calculations. F.L. and T.A. carried out the FEM simulation. R.D. assisted with the DFT calculation and manuscript writing. E.S., F.A., C.P.O., R.K.M. and D.Y. helped with electrochemical experiments. J.K. assisted with TEM imaging. A.O. and Y.Z. assisted with the catalyst preparation. P.P., D.K. and S.P. carried out the XAS measurements. M.Z. assisted with the schematics. L.F. carried out the X-ray diffraction measurement. C.M.G., J.P.E. and A.H.I. assisted with the research advice, discussions and editing. All authors contributed to the manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Remco Hartkamp, Yanguang Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Discussion and Tables 1–8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shayesteh Zeraati, A., Li, F., Alkayyali, T. et al. Carbon- and energy-efficient ethanol electrosynthesis via interfacial cation enrichment. Nat. Synth 4, 75–83 (2025). https://doi.org/10.1038/s44160-024-00662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00662-x
This article is cited by
-
Electrocatalytic CO2 hydrogenation to C2+ alcohols catalysed by Pr–Cu oxide heterointerfaces
Nature Synthesis (2025)
-
Scaled CO Electroreduction to Alcohols
Nature Communications (2025)