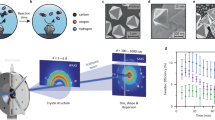
Abstract
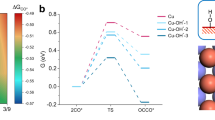
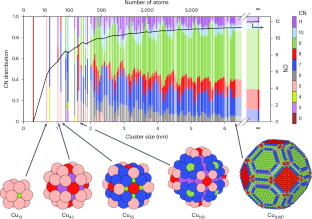
The electrochemical conversion of CO2 into acetaldehyde offers a sustainable and green alternative to the Wacker process. However, current electrocatalysts cannot effectively compete with heterogeneous processes owing to their limited selectivity towards acetaldehyde, resulting in low energy efficiencies. Here we report a theory-guided synthesis of a series of Cu-cluster catalysts (~1.6 nm) immobilized on various heteroatom-doped carbonaceous supports, produced via spark ablation of Cu electrodes (2.6 μg h−1 production rate, 6 Wh energy consumption). These catalysts achieve acetaldehyde selectivity of up to 92% at only 600 mV from the equilibrium potential. In addition, the catalysts exhibit exceptional catalytic stability during a rigorous 30 h stress test involving three repeated start–stop cycles. In situ X-ray absorption spectroscopy reveals that the initial oxide clusters were completely reduced under cathodic potential and maintained their metallic nature even after exposure to air, explaining the stable performance of the catalyst. First-principles simulations further elucidate a possible mechanism of CO2 conversion to acetaldehyde.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the article and its Supplementary Information. Source data are provided with this paper.
Code availability
DFT-simulated atomic structures and scripts necessary for reproducing the simulated results have been made freely available at https://nano.ku.dk/english/research/theoretical-electrocatalysis/katladb/co2-to-acetaldehyde/.
References
Barton, J. L. Electrification of the chemical industry. Science 368, 1181–1182 (2020).
Health Assessment Document for Acetaldehyde: Review Draft (U.S. Environmental Protection Agency, 1987).
Smidt, J. et al. Katalytische Umsetzungen von Olefinen an Platinmetall-Verbindungen Das Consortium-Verfahren zur Herstellung von Acetaldehyd. Angew. Chem. 71, 176–182 (1959).
Jira, R. Acetaldehyde from ethylene—a retrospective on the discovery of the Wacker. process. Angew. Chem. Int. Ed. 48, 9034–9037 (2009).
Smidt, J. et al. Olefins with palladium chloride catalysts. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.196200801 (1962).
Imbao, J., van Bokhoven, J. A., Clark, A. & Nachtegaal, M. Elucidating the mechanism of heterogeneous Wacker oxidation over Pd-Cu/zeolite Y by transient XAS. Nat. Commun. 11, 1118 (2020).
Fan, L. et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. https://doi.org/10.1126/sciadv.aay3111 (2020).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Hori, Y., Murata, A., Takahashi, R. & Suzuki, S. Electroreduction of carbon monoxide to methane and ethylene at a copper electrode in aqueous solutions at ambient temperature and pressure. J. Am. Chem. Soc. 109, 5022–5023 (1987).
Hori, Y., Murata, A. & Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 85, 2309–2326 (1989).
Hori, Y., Takahashi, I., Koga, O. & Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. A 199, 39–47 (2003).
Hori, Y. in Modern Aspects of Electrochemistry Vol. 42 (eds Vayenas, C. G. et al.) 89–189 (Springer, 2008).
Takahashi, I., Koga, O., Hoshi, N. & Hori, Y. Electrochemical reduction of CO2 at copper single crystal Cu(S)-[n(111)×(111)] and Cu(S)-[n(110)×(100)] electrodes. J. Electroanal. Chem. 533, 135–143 (2002).
Piqué, O., Low, Q. H., Handoko, A. D., Yeo, B. S. & Calle-Vallejo, F. Selectivity map for the late stages of CO and CO2 reduction to C2 species on copper electrodes. Angew. Chem. Int. Ed. 60, 10784–10790 (2021).
Schouten, K. J. P., Qin, Z., Pérez Gallent, E. & Koper, M. T. M. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J. Am. Chem. Soc. 134, 9864–9867 (2012).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Chen, Z. et al. Grain-boundary-rich copper for efficient solar-driven electrochemical CO2 reduction to ethylene and ethanol. J. Am. Chem. Soc. 142, 6878–6883 (2020).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Zhu, C. et al. Product-specific active site motifs of Cu for electrochemical CO2 reduction. Chem 7, 406–420 (2021).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Batchelor, T. A. A. et al. High-entropy alloys as a discovery platform for electrocatalysis. Joule 3, 834–845 (2019).
Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47–50 (2017).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Koolen, C. D. et al. Low-temperature non-equilibrium synthesis of anisotropic multimetallic nanosurface alloys for electrochemical CO2 reduction. Nat. Synth. https://doi.org/10.1038/s44160-023-00387-3 (2023).
Koolen, C. D., Luo, W. & Züttel, A. From single crystal to single atom catalysts: structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. https://doi.org/10.1021/acscatal.2c03842 (2022).
Karapinar, D. et al. Electroreduction of CO2 on single-site copper–nitrogen-doped carbon material: selective formation of ethanol and reversible restructuration of the metal sites. Angew. Chem. Int. Ed. 58, 15098–15103 (2019).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Su, X. et al. Complementary operando spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2–CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 13, 1322 (2022).
Bagger, A., Ju, W., Varela, A. S., Strasser, P. & Rossmeisl, J. Electrochemical CO2 reduction: classifying Cu facets. ACS Catal. 9, 7894–7899 (2019).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Tran, R. et al. Surface energies of elemental crystals. Sci. Data 3, 160080 (2016).
Schmidt-Ott, A. Spark Ablation: Building Blocks for Nanotechnology (Jenny Stanford Publishing, 2020).
Reinmann, R. & Akram, M. Temporal investigation of a fast spark discharge in chemically inert gases. J. Phys. D 30, 1125 (1997).
Schwyn, S., Garwin, E. & Schmidt-Ott, A. Aerosol generation by spark discharge. J. Aerosol Sci. 19, 639–642 (1988).
Maisser, A., Barmpounis, K., Attoui, M. B., Biskos, G. & Schmidt-Ott, A. Atomic cluster generation with an atmospheric pressure spark discharge generator. Aerosol Sci. Technol. 49, 886–894 (2015).
Lee, D. C., Yang, H. N., Park, S. H. & Kim, W. J. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 452, 20–28 (2014).
Yang, F. et al. Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew. Chem. Int. Ed. 57, 12303–12307 (2018).
Chen, D.-R. et al. Design and evaluation of a nanometer aerosol differential mobility analyzer (Nano-DMA). J. Aerosol Sci. 29, 497–509 (1998).
Liu, Y. et al. Mapping XANES spectra on structural descriptors of copper oxide clusters using supervised machine learning. J. Chem. Phys. 151, 164201 (2019).
Nan, B. et al. Copper oxide clusters modified by bismuth single atoms to catalyze CO oxidation. Appl. Catal. Gen. 636, 118578 (2022).
Yang, H. et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 141, 12717–12723 (2019).
Guan, A. et al. Boosting CO2 electroreduction to CH4 via tuning neighboring single-copper sites. ACS Energy Lett. 5, 1044–1053 (2020).
Zhao, K. et al. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon. Nat. Commun. 11, 2455 (2020).
Bunău, O. & Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 21, 345501 (2009).
Hursán, D. et al. Morphological attributes govern carbon dioxide reduction on N-doped carbon electrodes. Joule 3, 1719–1733 (2019).
Hursán, D. et al. CO2 conversion on N-doped carbon catalysts via thermo- and electrocatalysis: role of C–NOx moieties. ACS Catal. 12, 10127–10140 (2022).
Pham, T. H. M. et al. Enhanced electrocatalytic CO2 reduction to C2+ products by adjusting the local reaction environment with polymer binders. Adv. Energy Mater. 12, 2103663 (2022).
Birdja, Y. Y. & Koper, M. T. M. The importance of Cannizzaro-type reactions during electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 139, 2030–2034 (2017).
Qiao, Y., Hochfilzer, D., Kibsgaard, J., Chorkendorff, I. & Seger, B. Real-time detection of acetaldehyde in electrochemical CO reduction on Cu single crystals. ACS Energy Lett. 9, 880–887 (2024).
Meng, D.-L. et al. Highly selective tandem electroreduction of CO2 to ethylene over atomically isolated nickel–nitrogen site/copper nanoparticle catalysts. Angew. Chem. Int. Ed. 60, 25485–25492 (2021).
Li, S. et al. Boosting CO2 electrochemical reduction with atomically precise surface modification on gold nanoclusters. Angew. Chem. Int. Ed. 60, 6351–6356 (2021).
Povia, M. et al. Combining SAXS and XAS to study the operando degradation of carbon-supported Pt-nanoparticle fuel cell catalysts. ACS Catal. 8, 7000–7015 (2018).
Diercks, J. S. et al. Spectroscopy vs. electrochemistry: catalyst layer thickness effects on operando/in situ measurements. Angew. Chem. Int. Ed. 62, e202216633 (2023).
Diklić, N. et al. Potential pitfalls in the operando XAS study of oxygen evolution electrocatalysts. ACS Energy Lett 7, 1735–1740 (2022).
Binninger, T. et al. Electrochemical flow-cell setup for in situ X-ray investigations: I. Cell for SAXS and XAS at synchrotron facilities. J. Electrochem. Soc. 163, H906–H912 (2016).
Alfke, J. L. et al. BCC–Cu nanoparticles: from a transient to a stable allotrope by tuning size and reaction conditions. Phys. Chem. Chem. Phys. 24, 24429–24438 (2022).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron. Rad. 12, 537–541 (2005).
Frenkel, A. Solving the 3D structure of metal nanoparticles. Z. Krist. Cryst. Mater. 222, 605–611 (2007).
Marinkovic, N. S., Sasaki, K. & Adzic, R. R. Determination of single- and multi-component nanoparticle sizes by X-ray absorption spectroscopy. J. Electrochem. Soc. 165, J3222 (2018).
Yamazoe, S. et al. Hierarchy of bond stiffnesses within icosahedral-based gold clusters protected by thiolates. Nat. Commun. 7, 10414 (2016).
Apai, G., Hamilton, J. F., Stohr, J. & Thompson, A. Extended X-ray—absorption fine structure of small Cu and Ni clusters: binding-energy and bond-length changes with cluster size. Phys. Rev. Lett. 43, 165–169 (1979).
Cook, A. W., Jones, Z. R., Wu, G., Scott, S. L. & Hayton, T. W. An organometallic Cu20 nanocluster: synthesis, characterization, immobilization on silica, and ‘click’ chemistry. J. Am. Chem. Soc. 140, 394–400 (2018).
Mortensen, J. J., Hansen, L. B. & Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 71, 035109 (2005).
Enkovaara, J. et al. Electronic structure calculations with GPAW: a real-space implementation of the projector augmented-wave method. J. Phys. Condens. Matter 22, 253202 (2010).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Merkys, A. et al. Graph isomorphism-based algorithm for cross-checking chemical and crystallographic descriptions. J. Cheminform. 15, 25 (2023).
Vaitkus, A., Merkys, A. & Gražulis, S. Validation of the crystallography open database using the crystallographic information framework. J. Appl. Cryst. 54, 661–672 (2021).
Quirós, M., Gražulis, S., Girdzijauskaitė, S., Merkys, A. & Vaitkus, A. Using SMILES strings for the description of chemical connectivity in the Crystallography Open Database. J. Cheminform. 10, 23 (2018).
Merkys, A. et al. COD::CIF::Parser: an error-correcting CIF parser for the Perl language. J. Appl. Crystallogr. 49, 292–301 (2016).
Gražulis, S., Merkys, A., Vaitkus, A. & Okulič-Kazarinas, M. Computing stoichiometric molecular composition from crystal structures. J. Appl. Cryst. 48, 85–91 (2015).
Gražulis, S. et al. Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 40, D420–D427 (2012).
Downs, R. T. & Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 88, 247–250 (2003).
Aebi-Müller, R. E. et al. Code of conduct for scientific integrity. Zenodo https://doi.org/10.5281/zenodo.4707560 (2021).
Feng, J., Biskos, G. & Schmidt-Ott, A. Toward industrial scale synthesis of ultrapure singlet nanoparticles with controllable sizes in a continuous gas-phase process. Sci. Rep. 5, 15788 (2015).
Feng, J. et al. General approach to the evolution of singlet nanoparticles from a rapidly quenched point source. J. Phys. Chem. C 120, 621–630 (2016).
Preger, C., Overgaard, N. C., Messing, M. E. & Magnusson, M. H. Predicting the deposition spot radius and the nanoparticle concentration distribution in an electrostatic precipitator. Aerosol Sci. Technol. 54, 718–728 (2020).
Megyeri, D., Kohut, A. & Geretovszky, Z. Effect of flow geometry on the nanoparticle output of a spark discharge generator. J. Aerosol Sci. 154, 105758 (2021).
Feng, J., Ramlawi, N., Biskos, G. & Schmidt-Ott, A. Internally mixed nanoparticles from oscillatory spark ablation between electrodes of different materials. Aerosol Sci. Technol. 52, 505–514 (2018).
Feng, J. et al. Unconventional alloys confined in nanoparticles: building blocks for new matter. Matter 3, 1646–1663 (2020).
Tabrizi, N. S., Xu, Q., van der Pers, N. M. & Schmidt-Ott, A. Generation of mixed metallic nanoparticles from immiscible metals by spark discharge. J. Nanopart. Res. 12, 247–259 (2010).
Voloshko, A. & Itina, T. E. in Nanoparticles Technology (ed. Aliofkhazraei, M.) Ch. 1 (IntechOpen, 2015).
Feng, J. Scalable Spark Ablation Synthesis of Nanoparticles: Fundamental Considerations and Application in Textile Nanofinishing. PhD Thesis, Delft Univ. Technology (2016).
Acknowledgements
This research was supported by Swiss National Science Foundation (Ambizione Project PZ00P2_179989, W.L.). The in situ XAS cell used in this work was developed in the framework of the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement number 955650 (O.V.S. and J.H.). We acknowledge the financial support from China Scholarship Council (grant number 201506060156, M.L). We acknowledge support from the Danish National Research Foundation Center for High Entropy Alloy Catalysis (CHEAC) DNRF-149 (J.K.P.). We acknowledge the financial support by the Swiss National Foundation (project 184817, A.A.) and the Energy Systems Integration (ESI) Platform at the Paul Scherrer Institute. We also thank C. Ludwig of the Bioenergy and Catalysis Laboratory (LBK), Energy and Environment Research Division (ENE), Paul Scherrer Institute (PSI) and the School of Architecture, Civil, and Environmental Engineering (ENAC IIE GR-LUD), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland for the lively discussions. L. Menin and N. Gasilova of the Mass Spectrometry and Elemental Analysis Platform (MSEAP), Institute of Chemical Sciences and Engineering (ISIC), Basic Science Faculty (SB), École Polytechnique Fédérale de Lausanne (EPFL) Valais/Wallis, Energypolis, Sion, Switzerland, are acknowledged for their facilitation of the inductively coupled plasma mass spectrometry measurements. B. Boshuizen of Faculty of Applied Sciences Technical University of Delft, Delft, the Netherlands, is acknowledged for his XPS measurement of Cu(–Ag) oxide catalysts after production. A. Bornet of the Nuclear Magnetic Resonance Platform, Institute of Chemical Sciences and Engineering (ISIC), Basic Science Faculty (SB), École polytechnique fédérale de Lausanne (EPFL), Switzerland, is acknowledged for his assistance with H-NMR experiments. Y. Ko, Y. Wang and L. Zhong of the Laboratory of Materials for Renewable Energy (LMER), Institute of Chemical Sciences and Engineering (ISIC), Basic Science Faculty (SB), École polytechnique fédérale de Lausanne (EPFL) Valais/Wallis, Energypolis, Sion, Switzerland, are acknowledged for their assistance with the GO synthesis protocol, their assistance with the Brunauer-Emmett-Teller (BET) measurements and the design of Fig. 2 and contribution to XPS measurements, respectively. We acknowledge A. H. Clark of the Paul Scherrer Institute, CH-5232 Villigen PSI, Switzerland, for his support during the in situ synchrotron experiment. Finally, we acknowledge J. Rossmeisl of the Center for High Entropy Alloy Catalysis, Department of Chemistry, University of Copenhagen, Copenhagen, Denmark, for initial discussions on the design of the DFT simulations. Graphical abstract adapted with permission from ref. 28, American Chemical Society.
Author information
Authors and Affiliations
Contributions
C.D.K. and J.K.P. conceptualized the project. C.D.K., W.L. and A.Z. supervised the project. C.D.K. and B.Z. developed the cluster production and associated characterization with contributions from T.V.P., W.V. and A.S.-O. C.D.K. developed the catalyst synthesis, characterization and related data processing. C.D.K. performed and interpretated the electrochemical tests with contributions from J.Z. W.L. and M.L. performed the XPS analysis and data treatment with contributions from Y.K. C.D.K., M.W., A.A., J.Z., J.H. and O.V.S. designed the in situ XAS experiments. M.W. and J.H. provided the spectroelectrochemical cell. C.D.K., M.W., A.A., Z.A. and Y.K. performed the in situ XAS experiment. O.V.S. performed the ex situ XAS experiments. C.D.K. performed the data treatment of XAS experiments with contributions from M.W. and O.V.S. J.K.P performed all DFT simulations. C.D.K., J.K.P. and W.L. co-wrote the paper. All the authors discussed the results and revised the paper. Questions regarding the synthesis, catalysis and characterization should be directed to C.D.K. All questions related to the DFT simulation should be directed to J.K.P.
Corresponding authors
Ethics declarations
Competing interests
This technology is part of a patent application (PCT/EP2024/058885). C.D.K. has become an advisor of VSParticle B.V. as a direct result of this collaboration. T.V.P. and A.S.-O. are founders of VSParticle B.V. B.Z. and W.V. are former employees of VSParticle B.V. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Yan Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Description of the spark ablation and electrostatic deposition experimental set-up, operation, and electrical components.
(a) Faraday cup used to collect either negatively or positively charged particles from the aerosol. The measured current (via an electrometer) allows to determine the number of ions hitting the cup per unit of time giving a measure of the ablation rate (cluster production rate)77,78. (b) Deposition chamber allowing for a filter deposition in which the entire aerosol flow is passed through a substrate as well as an electrostatic deposition method in which a bias is applied to a substrate and as such only particles of opposite polarity are adhered to it79. (c) Aerosol exhaust. (d) Positive electrode (grounded). (e) Spark chamber. (f) Carrier gas (Ar) flow inlet. Direction of flow is from (E) to (B). (g) Negative electrode. (h) Pin-to-hole configuration of the electrode set-up of the spark ablator showing two Cu electrodes80. Exchanging the pin or negative electrode for Ag allows for the production of bimetallic clusters81,82,83,84. (i) Picture of the spark in operation. (j) Resistance-inductance-capacitance (RLC) electrical circuit, in which I denote the power supply. C denote(s) the capacitor, L denotes the inductor needed to store potential energy via the magnetic field needed for the oscillatory nature of the spark85. The spark is indicated by the damped exponential with a ~100 ns time constant of the oscillation of the spark between the grounded and negative electrode.
Supplementary information
Supplementary Information
Supplementary Figs. 1–40, Notes 1–9, Tables 1–14 and References 1–26.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koolen, C.D., Pedersen, J.K., Zijlstra, B. et al. Scalable synthesis of Cu-cluster catalysts via spark ablation for the electrochemical conversion of CO2 to acetaldehyde. Nat. Synth 4, 336–346 (2025). https://doi.org/10.1038/s44160-024-00705-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00705-3