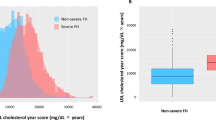
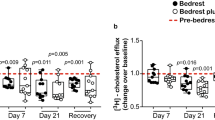
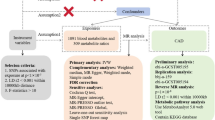
Abstract
The burden of cardiovascular disease is rising in the Asia-Pacific region, in contrast to falling cardiovascular disease mortality rates in Europe and North America. Here we perform quantification of 883 metabolites by untargeted mass spectroscopy in 8,124 Asian adults and investigate their relationships with carotid intima media thickness, a marker of atherosclerosis. Plasma concentrations of 3beta-hydroxy-5-cholestenoate (3BH5C), a cholesterol metabolite, were inversely associated with carotid intima media thickness, and Mendelian randomization studies supported a causal relationship between 3BH5C and coronary artery disease. The observed effect size was 5- to 6-fold higher in Asians than Europeans. Colocalization analyses indicated the presence of a shared causal variant between 3BH5C plasma levels and messenger RNA and protein expression of ferredoxin-1 (FDX1), a protein that is essential for sterol and bile acid synthesis. We validated FDX1 as a regulator of 3BH5C synthesis in hepatocytes and macrophages and demonstrated its role in cholesterol efflux in macrophages and aortic smooth muscle cells, using knockout and overexpression models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Researchers may apply for access to individual-level data for the HELIOS study through the study Data Access Committee (email, [email protected]). All summary data have been made available in the supplementary tables. Full GWAS summary statistics of metabolites are available through the GWAS catalog (https://www.ebi.ac.uk/gwas/). GWAS summary statistics for van der Harst et al.22 (accession codes GCST005194–005195), Yeung et al.17 (accession codes GCST90100572–90100582) and Chen et al.20 (accession codes GCST90199621–90201020) are available through the GWAS catalog (https://www.ebi.ac.uk/gwas/), for Nikpay et al.23 at the CARDIoGRAMplusC4D Consortium website (http://www.cardiogramplusc4d.org/data-downloads/), for Koyama et al.18 at the National Bioscience Database Center (https://biosciencedbc.jp/en; ID hum0014), and for Yin et al.21 at the METSIM Metabolomics PheWeb (https://pheweb.org/metsim-metab/pheno/C100006370).
Code availability
Genetic analysis was performed using GCTA v.1.93 (https://yanglab.westlake.edu.cn/software/gcta/#Overview) and PLINK v.1.90 and v.2.0 (https://www.cog-genomics.org/plink/). FUMA annotation was performed using the webtool v.1.4.1 (https://fuma.ctglab.nl/). Fine-mapping was performed using R package susieR (v.0.12.27) (https://cran.r-project.org/web/packages/susieR/vignettes/finemapping_summary_statistics.html). MR was performed using GSMR in GCTA v.1.93 (https://yanglab.westlake.edu.cn/software/gcta/#MendelianRandomisation) and R package TwoSampleMR (v.0.5.6) (https://mrcieu.github.io/TwoSampleMR/articles/introduction.html). Colocalization analysis was performed using SMR v.1.3.1 for Linux (https://yanglab.westlake.edu.cn/software/smr/#Overview) and R package coloc (v.5.2.1) (https://cran.r-project.org/web/packages/coloc/vignettes/a01_intro.html). Regional plots were generated using LocusZoom (https://my.locuszoom.org/). Other analyses and plotting were performed using the following R packages: data.table (v.1.14.2), dplyr (v.1.0.9), forestplot (v.3.1.1), ggplot2 (v.3.4.2), ggrepel (v.0.9.1), MatrixEQTL (v.2.3), metafor (v.4.6-0), ppcor (v.1.1), randomForest (v.4.7-1.1), RcppEigen (v.0.3.4.0.0), stringr (v.1.4.0), tibble (v.3.1.7) and tidyr (v.1.2.0). R v.4.2.1 or v.4.2.2 was used for all data analysis in R. Analyses and plotting scripts are available via GitHub at https://github.com/nsadhu/metabolomics_atherosclerosis.
References
Nayor, M., Brown, K. J. & Vasan, R. S. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ. Res. 128, 287–303 (2021).
Vaduganathan, M., Mensah, G. A., Turco, J. V., Fuster, V. & Roth, G. A. The global burden of cardiovascular diseases and risk: a compass for future health. J. Am. Coll. Cardiol. 80, 2361–2371 (2022).
Collaborators, G. Co. D. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet https://doi.org/10.1016/S0140-6736(24)00367-2 (2024).
Forouhi, N. G. & Sattar, N. CVD risk factors and ethnicity–a homogeneous relationship? Atheroscler. Suppl. 7, 11–19 (2006).
Patel, A. P., Wang, M., Kartoun, U., Ng, K. & Khera, A. V. Quantifying and understanding the higher risk of atherosclerotic cardiovascular disease among south Asian individuals: results from the UK Biobank prospective cohort study. Circulation 144, 410–422 (2021).
Zhang, Y. et al. Cardiovascular risk assessment tools in Asia. J. Clin. Hypertens. 24, 369–377 (2022).
Zhao, D. Epidemiological features of cardiovascular disease in Asia. JACC Asia 1, 1–13 (2021).
Wishart, D. S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug. Discov. 15, 473–484 (2016).
Nicholson, J. K., Lindon, J. C. & Holmes, E. Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29, 1181–1189 (1999).
Gowda, G. A. & Djukovic, D. Overview of mass spectrometry-based metabolomics: opportunities and challenges. Methods Mol. Biol. 1198, 3–12 (2014).
Wishart, D. S. et al. NMR and metabolomics-A roadmap for the future. Metabolites https://doi.org/10.3390/metabo12080678 (2022).
Chambless, L. E. et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am. J. Epidemiol. 146, 483–494 (1997).
Yu, B. et al. Genome-wide association study of a heart failure related metabolomic profile among African Americans in the Atherosclerosis Risk in Communities (ARIC) study. Genet. Epidemiol. 37, 840–845 (2013).
Zhang, J. et al. Association of metabolites on ischemic stroke subtypes: a 2-sample Mendelian randomization study. Front. Neurol. 15, 1417357 (2024).
Zhu, J., Xia, X., Jiang, H., Wang, C. & Jin, Y. Association between human blood metabolome and the risk of coronary heart disease: Mendelian randomization study. Preprint at medRxiv https://doi.org/10.1101/2024.01.31.24302109 (2024).
Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun. 9, 224 (2018).
Yeung, M. W. et al. Twenty-five novel loci for carotid intima-media thickness: a genome-wide association study in >45 000 individuals and meta-analysis of >100 000 individuals. Arterioscler. Thromb. Vasc. Biol. 42, 484–501 (2022).
Koyama, S. et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat. Genet. 52, 1169–1177 (2020).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Chen, Y. et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 55, 44–53 (2023).
Yin, X. et al. Genome-wide association studies of metabolites in Finnish men identify disease-relevant loci. Nat. Commun. 13, 1644 (2022).
van der Harst, P. & Verweij, N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 122, 433–443 (2018).
Nikpay, M. et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130 (2015).
Hughes, K., Lun, K. C. & Yeo, P. P. Cardiovascular diseases in Chinese, Malays, and Indians in Singapore. I. Differences in mortality. J. Epidemiol. Community Health 44, 24–28 (1990).
Liu, J. J. et al. Ethnic disparities in risk of cardiovascular disease, end-stage renal disease and all-cause mortality: a prospective study among Asian people with type 2 diabetes. Diabet. Med. 33, 332–339 (2016).
Yuan, S. et al. Plasma phospholipid fatty acids, FADS1 and risk of 15 cardiovascular diseases: a Mendelian randomisation study. Nutrients https://doi.org/10.3390/nu11123001 (2019).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Matsunaga, H. et al. Transethnic meta-analysis of genome-wide association studies identifies three new loci and characterizes population-specific differences for coronary artery disease. Circ. Genom. Precis. Med. 13, e002670 (2020).
Julkunen, H. et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat. Commun. 14, 604 (2023).
Karjalainen, M. K. et al. Genome-wide characterization of circulating metabolic biomarkers. Nature 628, 130–138 (2024).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622, 329–338 (2023).
Miller, W. L. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146, 2544–2550 (2005).
Autio, M. I. et al. Computationally defined and in vitro validated putative genomic safe harbour loci for transgene expression in human cells. eLife https://doi.org/10.7554/eLife.79592 (2024).
Mohibi, S. et al. Ferredoxin 1 is essential for embryonic development and lipid homeostasis. eLife https://doi.org/10.7554/eLife.91656 (2024).
Sheftel, A. D. et al. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl Acad. Sci. USA 107, 11775–11780 (2010).
Adyshev, D. M. et al. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L240–L255 (2013).
Liu, L. et al. Zc3h12c inhibits vascular inflammation by repressing NF-κB activation and pro-inflammatory gene expression in endothelial cells. Biochem. J. 451, 55–60 (2013).
Jiang, J. et al. A novel macrophage subpopulation conveys increased genetic risk of coronary artery disease. Circ. Res. 135, 6–25 (2024).
Pandak, W. M. & Kakiyama, G. The acidic pathway of bile acid synthesis: not just an alternative pathway. Liver Res. 3, 88–98 (2019).
Chong Nguyen, C. et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci. Rep. 11, 22661 (2021).
Li, W. et al. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis 292, 193–200 (2020).
Ross, S. et al. Effect of bile acid sequestrants on the risk of cardiovascular events: a Mendelian randomization analysis. Circ. Cardiovasc. Genet. 8, 618–627 (2015).
Annema, W. & Tietge, U. J. Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies. Nutr. Metab. 9, 25 (2012).
Leitersdorf, E. et al. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J. Clin. Invest. 91, 2488–2496 (1993).
Verrips, A. et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain 123, 908–919 (2000).
Lee, M. H. et al. Fine-mapping, mutation analyses, and structural mapping of cerebrotendinous xanthomatosis in US pedigrees. J. Lipid Res. 42, 159–169 (2001).
Nie, S., Chen, G., Cao, X. & Zhang, Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 9, 179 (2014).
Cui, Y. et al. A novel cuproptosis-related diagnostic gene signature and differential expression validation in atherosclerosis. Mol. Biomed. 4, 21 (2023).
Yang, S. et al. Copper homeostasis and cuproptosis in atherosclerosis: metabolism, mechanisms and potential therapeutic strategies. Cell Death Discov. 10, 25 (2024).
Bingham, T. C. et al. Cholesterol 27-hydroxylase but not apolipoprotein apoE contributes to A2A adenosine receptor stimulated reverse cholesterol transport. Inflammation 35, 49–57 (2012).
Hendrikx, T. et al. Hematopoietic overexpression of Cyp27a1 reduces hepatic inflammation independently of 27-hydroxycholesterol levels in Ldlr(-/-) mice. J. Hepatol. 62, 430–436 (2015).
Zhang, Y. et al. Ferredoxin reductase and p53 are necessary for lipid homeostasis and tumor suppression through the ABCA1-SREBP pathway. Oncogene 41, 1718–1726 (2022).
Committee, A. D. A. P. P. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care 45, S17–S38 (2021).
Diagnosis and Management of T2 Diabetes (HEARTS-D). Report No. WHO/UCN/NCD/20.1, 35 (World Health Organization, 2020).
Unger, T. et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension 75, 1334–1357 (2020).
Stein, J. H. et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 21, 93–111 (2008).
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M. & Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 81, 6656–6667 (2009).
Wong, E. et al. The Singapore national precision medicine strategy. Nat. Genet. 55, 178–186 (2023).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Kwong, A. M. et al. Robust, flexible, and scalable tests for Hardy–Weinberg equilibrium across diverse ancestries. Genetics https://doi.org/10.1093/genetics/iyab044 (2021).
Stegle, O., Parts, L., Piipari, M., Winn, J. & Durbin, R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 7, 500–507 (2012).
Boughton, A. P. et al. LocusZoom.js: interactive and embeddable visualization of genetic association study results. Bioinformatics 37, 3017–3018 (2021).
Yu, H. et al. GPR146 deficiency protects against hypercholesterolemia and atherosclerosis. Cell 179, 1276–1288.e1214 (2019).
Netsrithong, R. et al. Multilineage differentiation potential of hematoendothelial progenitors derived from human induced pluripotent stem cells. Stem Cell Res. Ther. 11, 481 (2020).
Cao, X. et al. Differentiation and functional comparison of monocytes and macrophages from hiPSCs with peripheral blood derivatives. Stem Cell Rep. 12, 1282–1297 (2019).
Dong, R., Zhang, B. & Zhang, X. Liver organoids: an in vitro 3D model for liver cancer study. Cell Biosci. 12, 152 (2022).
Ramli, M. N. B. et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology 159, 1471–1486 e1412 (2020).
Acknowledgements
We are very grateful for the outstanding support of past and present members of the HELIOS study steering committee, operational study team, and administrative staff for driving the study and assisting in the data collection. We also express our gratitude to the Nightingale Health Biobank Collaborative Group for providing GWAS summary statistics of NMR data from the UKBB Study. This work was supported by intramural funding from Nanyang Technological University, Lee Kong Chian School of Medicine, and the National Healthcare Group. J.C.C. is supported by the Singapore Ministry of Health and National Medical Research Council STaR funding scheme (NMRC/StaR/0028/2017), Large Collaborative Grant funding (MOH-000271), Phase II National Precision Medicine Programme (Research Platform and Data Enablers) (NMRC/PRECISE/2020), the Singapore Agency for Science Technology and Research Industry Alignment Fund - Pre-positioning Programme (IAF-PP) National Precision Medicine Program Phase 1A (A Population Level Genomic Infrastructure) (H17/01/a0/007), and the IAF-PP Asian Skin Microbiome Programme (H18/01/a0/016). R.S.Y.F. is supported by grants from the Singapore Ministry of Health’s National Medical Research Council under the Clinician Scientist-Individual Research Grant (MOH-001480-00), Open Fund - Large Collaborative Grant (MOH-001226-00), Ministry of Education (MOH-000333-00), and the Biomedical Research Council, Agency for Science, Technology and Research. C.J.M.L. is supported by grants from the Singapore Ministry of Health’s National Medical Research Council under the Young Investigator Research Grant (MOH-001712-01). Haojie Yu is supported by grants from the Singapore Ministry of Health’s National Medical Research Council Open Fund - Individual Research Grant (MOH-000896-00) and the Ministry of Education (MOE-000322-00). C.C. is supported by a grant from the Singapore Ministry of Education Academic Research Fund (MOE-T2EP30122-0018). N.S. and J.C.C. had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. Computational work for this study was partially performed on resources of the National Supercomputing Centre, Singapore (https://www.nscc.sg).
Author information
Authors and Affiliations
Contributions
J.C.C., P.E., E.R., J.N., E.S.L., J.L., J.B. and T.H.M. conceived and designed the HELIOS study. R.D. and T.H.M. collected phenotype data. K.E.W., P.A.S., G.A.M. and R.S. carried out metabolite quantification. N.S. and P.R.J. performed the data analyses. C.J.M.L., L.S.P., Y.M., M.A.-J., T.T.T., V.G.K., Y.S., Y.L., Hanry Yu, V.L., Y.Y., Haojie Yu, C.L.D., R.S.Y.F., K.Y.T. and C.C. designed and carried out the experimental studies. D.L., M. Lam, M. Loh, H.K.N., T.H.M., D.T., X.W., X.L.G., N.B., E.W. and P.T. provided critical feedback on the manuscript and interpretation of results. N.S., J.C.C., P.R.J., C.J.M.L., Haojie Yu, L.S.P., K.Y.T., T.H.M. and P.A.S. wrote the manuscript. All authors reviewed and contributed to the revision of the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.E.W. and G.A.M. are employees of Metabolon, and P.A.S. and R.S. were employees of Metabolon. The authors do not hold stocks in Metabolon and declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Distribution of mean cIMT in the study population (N = 8,124).
Extended Data Fig. 2
Distribution of quantified metabolites across different metabolite categories.
Extended Data Fig. 3 MR estimates from inverse variance weighted leave-one-out sensitivity analysis of 3BH5C (exposure) on CAD (outcome).
Data are presented as beta estimates +/- 95% confidence interval.
Extended Data Fig. 4 Genome-wide association analysis of metabolite 3BH5C GWAS in the HELIOS cohort (N = 1,876).
(a) Manhattan plot of 3BH5C GWAS where x-axis is displaying position of genetic variants on chromosome (hg38), and y-axis is displaying the strength of association of genetic variants with mean-cIMT. Horizontal red line indicates a GWAS threshold of P = 5×10-8. The top variant is rs2051466 (P = 7.9x10-43). (b) Quantile-Quantile plot of 3BH5C GWAS (λGC = 1.002) where x- and y-axes indicate the expected P-values under a null distribution and the observed P-values, respectively.
Extended Data Fig. 5 Phenome-wide association of rs10488763 in the Biobank Japan Project.
Volcano plot displaying the association of rs10488763 effect allele T with 259 traits listed in the Biobank Japan PheWeb (https://pheweb.jp/). Vertical grey line indicates no effect. Associations highlighted in green are significant at a P-value threshold of P = 2x10-4 after correcting for 259 tests.
Extended Data Fig. 6 Correlation of metabolite 3BH5C with traditional vascular risk factors.
Bar plot displaying Pearson correlation coefficient estimates adjusted for age, sex, and ethnicity. Bars are coloured by risk factor categories that include (1) BMI: Body Mass Index [red], (2) SBP: Systolic Blood Pressure [green], (3) DBP: Diastolic Blood Pressure [green], (4) TC: Total Cholesterol [blue], (5) HDL-C: High Density Lipoprotein Cholesterol [blue], (6)LDL-C: Low Density Lipoprotein Cholesterol [blue], (7) TG: Triglyceride[blue], (8) FGlucose, fasting plasma glucose [purple], (9) HbA1C: Glycated hemoglobin [purple]. For all correlations, Bonferroni-corrected P-value < 0.05.
Extended Data Fig. 7
Illustration of the acidic pathway of cholesterol metabolism.
Extended Data Fig. 8 Supporting data for experimental studies.
(a) FDX1 protein expression in FDX1 knock-out (FDX1 KO) and FDX1 over-expression (FDX1 OE) human hepatoma Huh7 cells, FDX1 KO mouse hepatocyte-derived AML12 cells, and FDX1 KO human monocyte-derived THP1 cells, compared to scrambled controls (SC). (b) FDX1 protein expression in FDX1 WT and KO, followed by over-expression (OE) and rescue, show robust and stable transgene expression following macrophage differentiation. (c) Sequencing validation with two independent FDX1 knock-out (KO) human Embryonic Stem Cell (hESC) clones using two independent sgRNAs targeting Exon 1 splice junction, and a 16-bp deletion on Exon 1 respectively. (d) Schematic illustration of the human FDX1 transgene payload, over-expression (OE) and rescue by knocking into the safe-harbour Pansio-1 locus in H1-hESCs Pansio-1 Safe Harbour line. (e) Representative flow cytometry profile of FDX1 WT and KO cells in the presence and absence of 200 ng/ul HDL (inducer of cholesterol efflux), with its corresponding median fluorescence intensity (MFI). (f) Quantitative RT-PCR of FDX1 expression in FDX1 knock-down (KD) human aortic smooth muscle cells compared to scrambled controls (SC).
Supplementary information
Supplementary Tables 1–15
Excel file with 15 worksheets, one for each table.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8a
Unprocessed blots.
Source Data Extended Data Fig. 8b
Unprocessed blots.
Source Data Extended Data Fig. 8f
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadhu, N., Dalan, R., Jain, P.R. et al. Metabolome-wide association identifies ferredoxin-1 (FDX1) as a determinant of cholesterol metabolism and cardiovascular risk in Asian populations. Nat Cardiovasc Res 4, 567–583 (2025). https://doi.org/10.1038/s44161-025-00638-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-025-00638-w
This article is cited by
-
Functional genomics identifies a protective role for FDX1 in atherosclerosis
Nature Cardiovascular Research (2025)