Abstract
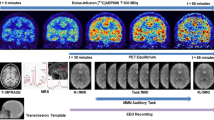
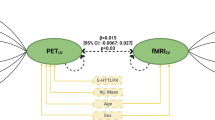
The limited efficacy of antidepressants for major depressive disorder (MDD) underscores the urgent need to explore mechanisms behind treatment heterogeneity and identify new antidepressant targets. This study explores the role of metabotropic glutamate receptor 5 (mGluR5) in MDD, examining mGluR5 availability changes pre- and post-treatment, and their link to clinical outcomes. We studied 25 patients with MDD and 21 healthy controls, with 13 undergoing eight-week vortioxetine treatment (10 mg per day). mGluR5 availability was measured at baseline and follow-up using [18F]3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile positron emission tomography ([18F]FPEB PET) scans, and patients were categorized on the basis of their response. Results showed lower mGluR5 availability in patients with MDD versus the control group at baseline. Post-treatment, the group with MDD exhibited significant increases in mGluR5 availability in the dorsolateral prefrontal cortex and ventromedial prefrontal cortex (N = 13, Cohen’s d = 0.83 and 1.01). The percentage increase in mGluR5 availability correlated with the percentage reduction in scores on the Hamilton rating scale for depression. These findings underscore mGluR5’s key role in MDD pathophysiology and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
79,00 € per year
only 6,58 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
These are individual participant data from clinical trials, which cannot be publicly shared due to ethical and consent restrictions. Data access requests can be directed to the corresponding author ([email protected]). Requests will be reviewed on a case-by-case basis, and access may require submission of a research proposal and approval by an ethics committee. Responses to requests will be provided within 30 days.
Code availability
The code for the analysis performed in this study is available at https://github.com/AoqianDeng/mGluR5-Availability.
References
Warden, D., Rush, A. J., Trivedi, M. H., Fava, M. & Wisniewski, S. R. The STAR*D project results: a comprehensive review of findings. Curr. Psychiatry Rep. 9, 449–459 (2007).
Dean, R. L. et al. Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane Database Syst. Rev. 9, Cd011612 (2021).
Chu, Z. & Hablitz, J. J. Quisqualate induces an inward current via mGluR activation in neocortical pyramidal neurons. Brain Res. 879, 88–92 (2000).
Shigemoto, R. et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522 (1997).
Takumi, Y., Matsubara, A., Rinvik, E. & Ottersen, O. P. The arrangement of glutamate receptors in excitatory synapses. Ann. N. Y. Acad. Sci. 868, 474–482 (1999).
Niciu, M. J., Ionescu, D. F., Richards, E. M. & Zarate, C. A. Jr. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J. Neural Transm. 121, 907–924 (2014).
Samojedny, S., Czechowska, E., Pańczyszyn-Trzewik, P. & Sowa-Kućma, M. Postsynaptic proteins at excitatory synapses in the brain-relationship with depressive disorders. Int. J. Mol. Sci. 23, 11423 (2022).
Shin, S. et al. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat. Neurosci. 18, 1017–1024 (2015).
Kim, J., Kang, S., Choi, T. Y., Chang, K. A. & Koo, J. W. Metabotropic glutamate receptor 5 in amygdala target neurons regulates susceptibility to chronic social stress. Biol. Psychiatry 92, 104–115 (2022).
Terbeck, S., Akkus, F., Chesterman, L. P. & Hasler, G. The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human positron emission tomography (PET) studies. Front. Neurosci. 9, 86 (2015).
Bhattacharyya, S. Inside story of group I metabotropic glutamate receptors (mGluRs). Int. J. Biochem. Cell Biol. 77, 205–212 (2016).
Dickson, E. J., Falkenburger, B. H. & Hille, B. Quantitative properties and receptor reserve of the IP(3) and calcium branch of G(q)-coupled receptor signaling. J. Gen. Physiol. 141, 521–535 (2013).
Nicoletti, F. et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 60, 1017–1041 (2011).
Iyo, A. H. et al. Chronic corticosterone administration down-regulates metabotropic glutamate receptor 5 protein expression in the rat hippocampus. Neuroscience. 169, 1567–1574 (2010).
Kovačević, T., Skelin, I., Minuzzi, L., Rosa-Neto, P. & Diksic, M. Reduced metabotropic glutamate receptor 5 in the Flinders sensitive line of rats, an animal model of depression: an autoradiographic study. Brain Res. Bull. 87, 406–412 (2012).
Lee, K. W. et al. Alteration by p11 of mGluR5 localization regulates depression-like behaviors. Mol. Psychiatry 20, 1546–1556 (2015).
Esterlis, I., Holmes, S. E., Sharma, P., Krystal, J. H. & DeLorenzo, C. Metabotropic glutamatergic receptor 5 and stress disorders: knowledge gained from receptor imaging studies. Biol. Psychiatry 84, 95–105 (2018).
Howard, D. M. et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 9, 1470 (2018).
Deschwanden, A. et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [11C]ABP688 PET and postmortem study. Am J Psychiatry 168, 727–734 (2011).
Kim, J. H. et al. In vivo metabotropic glutamate receptor 5 availability-associated functional connectivity alterations in drug-naïve young adults with major depression. Eur. Neuropsychopharmacol. 29, 278–290 (2019).
Esterlis, I. et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol. Psychiatry 23, 824–832 (2018).
DeLorenzo, C. et al. Characterization of brain mGluR5 binding in a pilot study of late-life major depressive disorder using positron emission tomography and [11C]ABP688. Transl Psychiatry. 5, e693 (2015).
Davis, M. T. et al. In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proc. Natl Acad. Sci. USA 116, 11490–11495 (2019).
Holmes, S. E. et al. Differences in quantification of the metabotropic glutamate receptor 5 across bipolar disorder and major depressive disorder. Biol. Psychiatry 93, 1099–1107 (2022).
Wang, X. & Cheng, Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 158, S65–S71 (2020).
Sanchez, C., Asin, K. E. & Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol. Ther. 145, 43–57 (2015).
Pehrson, A. L. & Sanchez, C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 19, 121–133 (2014).
Spurny, B. et al. Effects of SSRI treatment on GABA and glutamate levels in an associative relearning paradigm. Neuroimage. 232, 117913 (2021).
Oh, Y. S. et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 152, 831–843 (2013).
Warner-Schmidt, J. L. et al. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol. Psychiatry 68, 528–535 (2010).
Svenningsson, P., Kim, Y., Warner-Schmidt, J., Oh, Y. S. & Greengard, P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci. 14, 673–680 (2013).
Egeland, M., Warner-Schmidt, J., Greengard, P. & Svenningsson, P. Neurogenic effects of fluoxetine are attenuated in p11(S100A10) knockout mice. Biol. Psychiatry 67, 1048–1056 (2010).
Sanacora, G., Treccani, G. & Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 62, 63–77 (2012).
Belleau, E. L., Treadway, M. T. & Pizzagalli, D. A. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol. Psychiatry 85, 443–453 (2019).
Choi, K. W. et al. Comparison of shape alterations of the thalamus and caudate nucleus between drug-naïve major depressive disorder patients and healthy controls. J. Affect Disord. 264, 279–285 (2020).
Hart, X. M., Spangemacher, M., Defert, J., Uchida, H. & Gründer, G. Update lessons from PET imaging part II: a systematic critical review on therapeutic plasma concentrations of antidepressants. Ther Drug Monit. 46, 155–169 (2024).
Müller-Gärtner, H. W. et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J. Cereb. Blood Flow Metab. 12, 571–583 (1992).
American Psychiatric Association, DSM-5 Task Force Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th edn (APA, 2013).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
McIntyre, R. S. et al. Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. Can. Med. Assoc. J. 173, 1327–1334 (2005).
Hamill, T. G. et al. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 56, 205–216 (2005).
Park, E. et al. Test–retest reproducibility of the metabotropic glutamate receptor 5 ligand [18F]FPEB with bolus plus constant infusion in humans. Eur. J. Nucl. Med. Mol. Imaging 42, 1530–1541 (2015).
Mecca, A. P. et al. PET imaging of mGluR5 in Alzheimer’s disease. Alzheimers Res. Ther. 12, 15 (2020).
Sullivan, J. M. et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J. Cereb. Blood Flow Metab. 33, 532–541 (2013).
Mecca, A. P. et al. Effect of age on brain metabotropic glutamate receptor subtype 5 measured with [18F]FPEB PET. Neuroimage. 238, 118217 (2021).
Abdallah, C. G. et al. Metabotropic glutamate receptor 5 and glutamate involvement in major depressive disorder: a multimodal imaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2, 449–456 (2017).
Mark, J. et al. FSL. Neuroimage 62, 782–790 (2012).
Penny, W., Friston, K., Ashburner, J., Kiebel, S. & Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images (Elsevier, 2007).
Xu, X. et al. mGluR5-mediated eCB signaling in the nucleus accumbens controls vulnerability to depressive-like behaviors and pain after chronic social defeat stress. Mol. Neurobiol. 58, 4944–4958 (2021).
IBM SPSS Statistics v.28.0 (IBM, 2021); https://www.ibm.com/products/spss-statistics
ggplot2 v.3.4.1 (ggplot2, 2023); http://ggplot2.tidyverse.org
BrainNet Viewer v.1.7 (NITRC, 2019); http://www.nitrc.org/projects/bnv/
Acknowledgements
This work was supported by the STI2030-Major Projects (grant no. 2021ZD0202000 to Y.Z.), the National Natural Science Foundation of China projects (grant no. 81671353 to Y.Z.), a major grant of the Key Research and Development Program of Hunan Province of China (grant nos. 2019SK2252 and 2022SK2035 to Y.Z.), and the Fundamental Research Funds for the Central Universities of Central South University (grant no. 2022ZZTS0856 to Y.Z.). We are grateful to C. Sun from the Shaanxi Brain Modulation and Scientific Research Center (Xi’an, Shaanxi, P. R. China), and S. Qi and Z. Huang from Xi’an Jiaotong University, for their assistance with image processing.
Author information
Authors and Affiliations
Contributions
B.L. was responsible for clinical study design, patient recruitment and follow-up coordination. A.D. contributed to study design, clinical data interpretation and drafting the manuscript. C.D. handled PET imaging data acquisition, preprocessing and quality control, and participated in the PET experimental design. W.C., Q.Z., W.O. and M.M. conducted patient recruitment, supported clinical assessments and optimized the study workflow. L.Z., F.H. and X.X. provided technical guidance on PET imaging methodology and assisted in data interpretation. J.L. and X.W. supervised clinical operations and workflow optimization. Y.W. and X.M. provided critical guidance on PET imaging study design and technical methodologies. H.H. offered expert advice on PET tracer applications and neuroimaging data interpretation. Y.J. and Y.Z. conceptualized the project, secured funding and supervised all aspects of the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Gregor Gryglewski, Xenia Hart and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Figs. 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Deng, A., Dong, C. et al. Enhanced mGluR5 availability marks the antidepressant efficacy in major depressive disorder: an [18F]FPEB PET study. Nat. Mental Health 3, 298–305 (2025). https://doi.org/10.1038/s44220-025-00386-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-025-00386-7