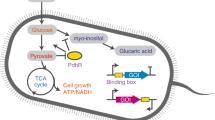
Abstract
The rate of glucose import directly affects the maximum possible flux of central carbon metabolism. However, few tools can directly monitor the cellular glucose uptake rate. Here we report the development of a set of programmable bifunctional glucose uptake rate biosensors (GURBs) for real-time monitoring of glucose uptake rate, which enable the dynamic activation and inhibition of glucose uptake and central metabolism in Escherichia coli. These genetic circuits are used to monitor the glucose uptake rates of strains under different culture conditions. Also, feedback-loop control systems are designed to make cells rely on the glucose uptake rate to tune the target metabolic modules, resulting in a substantial increase of the titers of l-tryptophan, riboflavin and d-lactic acid. The glucose-uptake-rate-responsive genetic circuits developed in this study will serve as an effective tool for the dynamic control of glucose uptake and central metabolism.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the Article and its Supplementary Information. Specific data P values are included in the source data files. Additional details on the datasets and protocols that support the findings of this study will be made available by the corresponding author upon request. Data are provided at https://doi.org/10.6084/m9.figshare.24790872 (ref. 71). Source data are provided with this paper.
References
Chae, T. U. et al. Recent advances in systems metabolic engineering tools and strategies. Curr. Opin. Biotechnol. 47, 67–82 (2017).
Krivoruchko, A., Siewers, V. & Nielsen, J. Opportunities for yeast metabolic engineering: lessons from synthetic biology. Biotechnol. J. 6, 262–276 (2011).
Liu, Y., Shin, H. D., Li, J. & Liu, L. Toward metabolic engineering in the context of system biology and synthetic biology: advances and prospects. Appl. Microbiol. Biotechnol. 99, 1109–1118 (2015).
Kotte, O., Zaugg, J. B. & Heinemann, M. Bacterial adaptation through distributed sensing of metabolic fluxes. Mol. Syst. Biol. 6, 619–627 (2010).
Fang, L. et al. Genome-scale target identification in Escherichia coli for high-titer production of free fatty acids. Nat. Commun. 12, 4976 (2021).
Yeom, S. J. et al. Single-cell-based screening and engineering of d-amino acid amidohydrolases using artificial amidophenol substrates and microbial biosensors. J. Agric. Food Chem. 70, 1203–1211 (2022).
Dekker, L. & Polizzi, K. M. Sense and sensitivity in bioprocessing-detecting cellular metabolites with biosensors. Curr. Opin. Chem. Biol. 40, 31–36 (2017).
Rogers, J. K., Taylor, N. D. & Church, G. M. Biosensor-based engineering of biosynthetic pathways. Curr. Opin. Biotechnol. 42, 84–91 (2016).
Shi, S., Ang, E. L. & Zhao, H. In vivo biosensors: mechanisms, development and applications. J. Ind. Microbiol. Biotechnol. 45, 491–516 (2018).
Zhang, J., Jensen, M. K. & Keasling, J. D. Development of biosensors and their application in metabolic engineering. Curr. Opin. Chem. Biol. 28, 1–8 (2015).
Liu, D., Evans, T. & Zhang, F. Applications and advances of metabolite biosensors for metabolic engineering. Metab. Eng. 31, 35–43 (2015).
Binder, S. et al. A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol. 13, R40 (2012).
Liu, D. et al. Construction, model-based analysis, and characterization of a promoter library for fine-tuned gene expression in Bacillus subtilis. ACS Synth. Biol. 7, 1785–1797 (2018).
Jiao, S., Yu, H. & Shen, Z. Core element characterization of Rhodococcus promoters and development of a promoter-RBS mini-pool with different activity levels for efficient gene expression. N. Biotechnol. 44, 41–49 (2018).
Guiziou, S. et al. A part toolbox to tune genetic expression in Bacillus subtilis. Nucleic Acids Res. 44, 7495–7508 (2016).
Li, H. et al. Monitoring in vivo metabolic flux with a designed whole-cell metabolite biosensor of shikimic acid. Biosens. Bioelectron. 98, 457–465 (2017).
Wang, W. et al. E. coli biosensor based on modular GFP and luxI/luxR cyclic amplification circuit for sensitive detection of lysine. Anal. Bioanal. Chem. 414, 8299–8307 (2022).
Yeom, S. J. et al. A synthetic microbial biosensor for high-throughput screening of lactam biocatalysts. Nat. Commun. 9, 5053 (2018).
Kang, Z. Q. et al. An l-2-hydroxyglutarate biosensor based on specific transcriptional regulator LhgR. Nat. Commun. 12, 3619 (2021).
Xiao, D. et al. A d-2-hydroxyglutarate biosensor based on specific transcriptional regulator DhdR. Nat. Commun. 12, 7108 (2021).
Canbay, E. et al. A microbial biosensor based on Lactobacillus delbruecki sp. bacterial cells for simultaneous determination of lactic and pyruvic acid. Food Chem. 169, 197–202 (2015).
Pereira, A. C. et al. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sensors Actuat. B Chem. 124, 269–276 (2007).
Suman, S. et al. Development of a lactate biosensor based on conducting copolymer bound lactate oxidase. Sensors Actuat. B Chem. 107, 768–772 (2005).
Gomes, S. P. et al. Application of lactate amperometric sol-gel biosensor to sequential injection determination of l-lactate. J. Pharm. Biomed. Anal. 43, 1376–1381 (2007).
Zhao, Y., Takahashi-Yamashiro, K., Shen, Y. & Campbell, R. E. Quantification of intracellular citrate concentrations with genetically encoded biosensors. Methods Mol. Biol. 2564, 247–258 (2023).
Ewald, J. C. et al. Engineering genetically encoded nanosensors for real-time in vivo measurements of citrate concentrations. PLoS One 6, e28245 (2011).
Zhao, Y., Shen, Y., Wen, Y. & Campbell, R. E. High-performance intensiometric direct- and inverse-response genetically encoded biosensors for citrate. ACS Cent. Sci. 6, 1441–1450 (2020).
Akyilmaz, E. & Dinckaya, E. An amperometric microbial biosensor development based on Candida tropicalis yeast cells for sensitive determination of ethanol. Biosens. Bioelectron. 20, 1263–1269 (2005).
Kunjapur, A. M. & Prather, K. L. J. Development of a vanillate biosensor for the vanillin biosynthesis pathway in E. coli. ACS Synth. Biol. 8, 1958–1967 (2019).
Feng, J. et al. A general strategy to construct small molecule biosensors in eukaryotes. elife 4, e10606 (2015).
Bianchi-Smiraglia, A. et al. Internally ratiometric fluorescent sensors for evaluation of intracellular GTP levels and distribution. Nat. Methods 14, 1003–1009 (2017).
Kochanowski, K. et al. Functioning of a metabolic flux sensor in Escherichia coli. Proc. Natl. Acad. Sci. USA 110, 1130–1135 (2013).
Lehning, C. E., Siedler, S., Ellabaan, M. M. H. & Sommer, M. O. A. Assessing glycolytic flux alterations resulting from genetic perturbations in E. coli using a biosensor. Metab. Eng. 42, 194–202 (2017).
Xu, X. et al. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 16, 1261–1268 (2020).
San Martin, A. et al. Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate. PLoS ONE 9, e85780 (2014).
Zhang, F., Carothers, J. M. & Keasling, J. D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 30, 354–359 (2012).
Peters, G. et al. Development of N-acetylneuraminic acid responsive biosensors based on the transcriptional regulator NanR. Biotechnol. Bioeng. 115, 1855–1865 (2018).
Plumbridge, J. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5, 187–193 (2002).
Tchieu, J. H., Norris, V., Edwards, J. S. & Saier, M. H. Jr. The complete phosphotransferase system in Escherichia coli. J. Mol. Microbiol. Biotechnol. 3, 329–346 (2001).
Jiang, S. et al. Reconstructing a recycling and nonauxotroph biosynthetic pathway in Escherichia coli toward highly efficient production of l-citrulline. Metab. Eng. 68, 220–231 (2021).
Moriya, H. & Johnston, M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101, 1572–1577 (2004).
Brink, D. P., Borgstrom, C., Tueros, F. G. & Gorwa-Grauslund, M. F. Real-time monitoring of the sugar sensing in Saccharomyces cerevisiae indicates endogenous mechanisms for xylose signaling. Microb. Cell Fact. 15, 183 (2016).
Tan, S. I. & Ng, I. S. CRISPRi-mediated nimply logic gate for fine-tuning the whole-cell sensing toward simple urine glucose detection. ACS Synth. Biol. 10, 412–421 (2021).
Hosono, K., Kakuda, H. & Ichihara, S. Decreasing accumulation of acetate in a rich medium by Escherichia coli on introduction of genes on a multicopy plasmid. Biosci. Biotechnol. Biochem. 59, 256–261 (1995).
Pradhanang, V. & Ghimire, S. Hirsutism: a rare presentation of an adult granulosa cell tumor of ovary. Nepal Med. Coll. J. 7, 152–154 (2005).
Plumbridge, J. DNA binding sites for the Mlc and NagC proteins: regulation of nagE, encoding the N-acetylglucosamine-specific transporter in Escherichia coli. Nucleic Acids Res. 29, 506–514 (2001).
Nam, T. W. et al. Analyses of Mlc-IIBGlc interaction and a plausible molecular mechanism of Mlc inactivation by membrane sequestration. Proc. Natl. Acad. Sci. USA 105, 3751–3756 (2008).
Nam, T. W. et al. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 20, 491–498 (2001).
Lee, S. J., Boos, W., Bouche, J. P. & Plumbridge, J. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 19, 5353–5361 (2000).
Luker, K. E. et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc. Natl. Acad. Sci. USA 101, 12288–12293 (2004).
Lim, J. H. & Jung, G. Y. A simple method to control glycolytic flux for the design of an optimal cell factory. Biotechnol. Biofuels 10, 160 (2017).
Tanaka, Y., Kimata, K. & Aiba, H. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 19, 5344–5352 (2000).
Morita, T., Maki, K. & Aiba, H. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 19, 2176–2186 (2005).
Vanderpool, C. K. & Gottesman, S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol. Microbiol. 54, 1076–1089 (2004).
Lutz, R. & Bujard, H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25, 1203–1210 (1997).
Kikuchi, A. M., Tanabe, A. & Iwahori, Y. A systematic review of the effect of l-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 18, 316–333 (2021).
Shen, T. et al. Improved production of tryptophan in genetically engineered Escherichia coli with TktA and PpsA overexpression. J. Biomed. Biotechnol. 2012, 605219 (2012).
Liu, Q. et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 10, 4976 (2019).
Flores, N. et al. Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat. Biotechnol. 14, 620–623 (1996).
Bornemann, S. Flavoenzymes that catalyse reactions with no net redox change. Nat. Prod. Rep. 19, 761–772 (2002).
Stahmann, K. P., Revuelta, J. L. & Seulberger, H. Three biotechnical processes using Ashbya gossypii, Candida famata or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol. 53, 509–516 (2000).
Lin, Z. et al. Metabolic engineering of Escherichia coli for the production of riboflavin. Microb. Cell Fact. 13, 104 (2014).
Wang, Z. et al. Enhancement of riboflavin production with Bacillus subtilis by expression and site-directed mutagenesis of zwf and gnd gene from Corynebacterium glutamicum. Bioresour. Technol. 102, 3934–3940 (2011).
Zhou, S. et al. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69, 399–407 (2003).
Ylinen, A. et al. Production of d-lactic acid containing polyhydroxyalkanoate polymers in yeast Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 48, kuab028 (2021).
Ma, Y. et al. Recombinant Escherichia coli for producing d-lactate and use thereof. US patent US9944957B2 (2018).
Wang, Q. et al. Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions. Biotechnol. Lett. 28, 89–93 (2006).
Lara, A. R. et al. Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures. Biotechnol. Bioeng. 99, 893–901 (2008).
Death, A. & Ferenci, T. Between feast and famine: endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J. Bacteriol. 176, 5101–5107 (1994).
Ferenci, T. Adaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol. Rev. 18, 301–317 (1996).
Ding, D. Q., Zhu, Y. R., Bai, D. Y., Wan, T. X., Lee, S. Y. & Zhang, D. W. Monitoring and dynamically controlling glucose uptake rate and central metabolism. figshare https://doi.org/10.6084/m9.figshare.24790872 (2025).
Acknowledgements
S.Y.L. served as the chairman of the scientific advisory board of the Tianjin Institute of Industrial Biotechnology. D.D. was supported by the National Key R&D Program of China (2021YFC2100900), National Nature Science Foundation of China (32100062), the Postdoctoral Science Foundation of China (2021M703438) and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-029). D.Z. was supported by the National Science Fund for Distinguished Young Scholars (22325807), the National Nature Science Foundation of China (22178372) and the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-055). S.Y.L. was supported by the Development of Platform Technologies of Microbial Cell Factories for the Next-Generation Biorefineries Project (2022M3J5A1056117) of the National Research Foundation supported by the Korean Ministry of Science and ICT. We thank T. Chen for plasmid p20C-Bsrib and X. Zhang for strain Lac04. We thank J. Shen for SPR technology assistance, Z. Zhang for MS contributions and L. Qi for flow cytometry expertise.
Author information
Authors and Affiliations
Contributions
D.D., SY.L. and D.Z. initiated the project and designed the experiments. D.D., Y.Z., D.B. and T.W. performed the experiments. D.D., Y.Z., SY.L. and D.Z. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Wilfred Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Supplementary Tables 1–4 and Source data file of Fig. 3 in the main text.
Supplementary Data 1
Source data for Supplementary Fig. 4.
Supplementary Data 2
Source data for Supplementary Fig. 5.
Supplementary Data 3
Source data for Supplementary Fig. 7.
Supplementary Data 4
Source data for Supplementary Fig. 8.
Supplementary Data 5
Source data for Supplementary Fig. 9.
Supplementary Data 6
Source data for Supplementary Fig. 10.
Supplementary Data 7
Source data for Supplementary Fig. 11.
Supplementary Data 8
Source data for Supplementary Fig. 12.
Supplementary Data 9
Source data for Supplementary Fig. 13.
Supplementary Data 10
Source data for Supplementary Fig. 14.
Supplementary Data 11
Source data for Supplementary Fig. 15.
Supplementary Data 12
Source data for Supplementary Fig. 16.
Supplementary Data 13
Source data for Supplementary Fig. 17.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, D., Zhu, Y., Bai, D. et al. Monitoring and dynamically controlling glucose uptake rate and central metabolism. Nat Chem Eng 2, 50–62 (2025). https://doi.org/10.1038/s44286-024-00163-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-024-00163-w