Abstract
Coronaviruses (CoVs) have caused three global outbreaks: severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) in 2003, Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, and SARS-CoV-2 in 2019, with significant mortality and morbidity. The impact of coronavirus disease 2019 (COVID-19) raised serious concerns about the global preparedness for a pandemic. Furthermore, the changing antigenic landscape of SARS-CoV-2 led to new variants with increased transmissibility and immune evasion. Thus, the development of broad-spectrum vaccines against current and future emerging variants of CoVs will be an essential tool in pandemic preparedness. Distinct phylogenetic features within CoVs complicate and limit the process of generating a pan-CoV vaccine capable of targeting the entire Coronaviridae family. In this review, we aim to provide a detailed overview of the features of CoVs, their phylogeny, current vaccines against various CoVs, the efforts in developing broad-spectrum coronavirus vaccines, and the future.
Similar content being viewed by others
Introduction
Vaccines are safe and effective biological formulations synthesized to protect the population from infectious diseases by preventing, limiting, or eradicating the infection and spread of a specific pathogen by inducing specific immune responses1. The design and development of an effective vaccine against infectious diseases requires considerable knowledge about the causative agent, including the nature of the infection, phenotypic characteristics, and infection-associated pathogenesis. The safety, broad-spectrum efficacy, ease of administration, induction of long-term immunity, minimal cost of production, and extended shelf life through improved thermostability are other factors of paramount importance in developing an ideal vaccine2. The medical, social, and economic burden caused by COVID-19 and the evolution and emergence of new variants has highlighted the urgent need for next-generation vaccines with increased breadth of protection, durability, and ability to block infection and transmission.
CoVs belong to the Coronaviridae family and are known to cause outbreaks on endemic and pandemic scales. These viruses are genetically diverse and distinct as demonstrated by phylogenetic analysis and their evolutionary origin. To date, seven human CoVs (HCoVs) have been identified, namely HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV-1, MERS-CoV, and SARS-CoV-23,4,5,6,7,8. Studies focused on the origin of these viruses have shown that bats, rodents, and domestic animals serve as the main reservoir species9. Concerns regarding the possibility of future spillovers with unknown CoVs and the emergence of SARS-CoV-2 variants of concern (VOCs) with unique mutations necessitate generating broad-spectrum vaccines. In addition, waning immunity and breakthrough infections within the population strongly advocate for developing improved vaccines with increased immunogenicity and durability. The first-generation vaccines designed to prevent disease with the ancestral SARS-CoV-2 were proven to be less effective against VOCs. For instance, multiple studies have shown that neutralizing antibody responses induced by the first-generation COVID-19 vaccines were limited against most of the Omicron variants10,11,12,13,14,15,16. Waning immunity against SARS-CoV-2 in the population necessitates the need for frequent boosters17,18,19.
Vaccine design and the delivery route are essential aspects to consider while developing a vaccine. SARS-CoV-2 vaccine design mainly focuses on two antigen targets: the spike (S) protein and the receptor binding ___domain (RBD) in the S protein. Both targets result in the induction of neutralizing antibody responses20,21. The S protein amino acid sequence similarity between SARS-CoV-1 and SARS-CoV-2 is 76%, and between SARS-CoV-2 and SARS-related CoVs (SARSr-CoVs) is ~80%22. Likewise, the RBDs of SARS-CoV-1 and ancestral SARS-CoV-2 have an amino acid sequence similarity of 73.5%23. Hence, in the design of pan-CoV vaccines, it is wise to consider S protein and RBDs from different CoVs.
Available COVID-19 vaccines are administered intramuscularly (IM) and provide robust systemic antibody responses. Since CoVs are respiratory pathogens and the primary route of virus entry is through the upper respiratory tract, inducing a local mucosal immune response could be an effective way to protect against CoV infection and transmission. Studies focusing on mucosal vaccines have shown induction of mucosal, cellular, and humoral immune responses24. Transmission still occurs from IM-vaccinated individuals25,26,27, and combining mucosal and IM vaccination routes could reduce or completely prevent virus transmission28,29,30.
Coronaviruses—an overview
Coronaviruses are enveloped, positive-sense, single-stranded RNA viruses belonging to the order Nidovirales and the family Coronaviridae. The family Coronaviridae is further divided into three subfamilies: Letovirinae, Pitovirinae, and Orthocoronavirinae, the latter of which is the focus of this review. The subfamily Orthocoronavirinae is subdivided into four genera: Alpha, Beta, Gamma, and Deltacoronaviruses. SARS-CoV-1, SARS-CoV-2, and MERS-CoV belong to the Betacoronavirus genera31.
The general structure of the virus is spherical, with crown-like protrusions present on the outer surface of the virus, which are formed by the S protein, required for the attachment, fusion, and entry of the virus into the host cell. Besides S, CoVs are made of additional structural proteins: the membrane protein (M), the envelope protein (E), and the nucleocapsid protein (N). Both M and E proteins play major roles in viral assembly32,33. HCoV-OC43 and HCoV-HKU1 contain an additional structural protein called the hemagglutinin-esterase protein (HE)34. These proteins, together with the lipid bilayer, form the viral envelope seen surrounding the helical capsid formed by N. The large genome of CoVs, typically 22 to 36 kb, is packaged within the capsid32,35.
Coronaviruses can cause a myriad of diseases both in animal and human populations. Animal CoVs target various animals, including cows, pigs, dogs, cats, mice, and chickens, and can cause gastroenteritis, encephalitis, and bronchitis. For instance, murine hepatitis virus (MHV) causes respiratory, enteric, hepatic, and neurologic infections in mice36,37. Although there are multiple animal CoVs, we focus on HCoVs in this review. HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1 cause mild respiratory tract infections mainly in immunocompromised individuals, which are self-limiting4,5,6,38. However, infection with SARS-CoV-1, MERS-CoV, and SARS-CoV-2 can affect multiple organs, and patients can develop acute respiratory distress syndrome (ARDS), extrapulmonary manifestations, and multiple organ dysfunction syndrome (MODS)39.
The presentation of COVID-19 can either be asymptomatic or symptomatic, ranging from mild to moderate to severe40,41,42,43,44,45. Lung damage is directly proportional to the virus replication and the titer, immune cell infiltration, and elevated levels of various proinflammatory cytokines and chemokines46,47,48,49,50. The immunological response, including the induction of a cytokine storm, can lead to severe lung damage and increased mortality47,51. Several COVID-19 survivors have prolonged health complications associated with COVID-19, often referred to as long COVID52.
The phylogeny and evolution of coronaviruses
Acquisition of mutations and recombination within the virus genome are major driving forces that create genetic diversity, eventually aiding in increased virus survival, pathogenicity, transmissibility, and immune evasion53. CoVs have an internal proofreading mechanism: the 3′ exonuclease (nsp14 for SARS-CoV-2), which limits the mutation rate of CoVs54,55. It has been estimated that the mutation rate of CoVs is 10−6 per base per infection cycle56 and 10-3 per site per year57. Recombination events in conjunction with purifying selection result in the generation of new CoV variants58,59.
The comparative genomic analysis of MERS-CoV, SARS-CoV-1, and SARS-CoV-2 shows that SARS-CoV-2 is distinct and holds a different position on the phylogenetic tree (Fig. 1). Whole-genome sequence alignments of SARS-CoV-2 with other CoVs have revealed that SARS-CoV-2 is most related to certain SARS-related-CoVs including bat CoVs (96%) and pangolin CoVs (86%-92%)60. For example, the whole genome sequence identity of SARS-CoV-2 with BatCoV RaTG13 is 96%, with SARS-CoV-1 79.6%, and with MERS-CoV 50%61,62.
Full-length genomes were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/), and alignments were built with MAFFT (FFT-NS-1 algorithm181. Maximum likelihood phylogenetic tree was constructed using IG-TREE2182 with the best model for distance estimates identified with the ModelFinder function183 as the one with the lowest Bayesian information criterion (BIC). Branch support was assessed using both ultrafast bootstrap approximation (ufBoot, 1000 replicates)184 and SH-like approximate likelihood ratio test (SH-aLRT). The tree was visualized in FigTree (http://tree.bio.ed.ac.uk/software/figtree/), and midpoint rooted for purposes of clarity. Only bootstrap values greater than 70% are shown. Bars indicate nucleotide substitutions per site.
The S protein comprises three segments: a large ectodomain, a single-pass transmembrane anchor, and a short intracellular tail. The ectodomain consists of a receptor-binding subunit S1 and a membrane-fusion subunit S2. The S is a clove-shaped trimer with three S1 heads and a trimeric S2 stalk63,64,65.
The D614G mutation was the first notable substitution in the S protein of SARS-CoV-2 and quickly spread worldwide66. Hereafter, multiple VOCs, including Alpha, Beta, Gamma, Delta, and Omicron, emerged independently across the globe67. One of the variants, Omicron (BA.1), which first appeared in Nov 2021 in Botswana, was antigenically distinct from previous VOCs and became dominant within a short span of time68. Following Omicron BA.1, several subvariants have appeared in different regions all around the globe69,70,71. Recombination events resulted in the appearance of additional VOCs72. During evolution, Omicron lineages exhibited antigenic shifts, resulting in poor neutralization by antibodies induced by first-generation vaccines and pre-Omicron infection15,73.
In the initial stages of the pandemic, the evolution of SARS-CoV-2 appeared to select for VOCs with higher transmissibility and, in some cases, higher severity74,75,76,77. Mutations resulting in antigenic changes leading to immune escape were recognized during the later stages of the pandemic78. Now, it is postulated that the evolution of SARS-CoV-2 is shifting towards adapting to different tissue tropisms as well as immune evasion79. For example, Omicron BA.1 has evolved a preference for efficient replication in the nasopharynx, a better vantage point for entering aerosols80,81. These changes may have a notable impact on the development of a successful vaccine that can prevent virus infection and transmission.
Coronavirus vaccine development: the past and the present
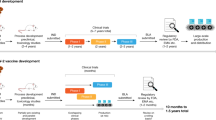
HCoVs have been circulating in the population for decades, and no licensed CoV vaccine was available until December 2020 as a direct consequence of the COVID-19 pandemic. Before the pandemic, a few vaccine candidates against MERS-CoV, MVA-MERS-S, GLS-5300 DNA, and ChAdOx1-MERS were tested in phase I clinical trials82,83,84,85,86. Nevertheless, none were entered into phase II or III trials and approved for human use by the US FDA (United States Food and Drug Administration).
It has been 20 years since the scientific community has identified the potential of using S protein as a vaccine candidate against CoVs87,88,89. Multiple studies have demonstrated that S-specific antibodies can neutralize SARS-CoV-1 or MERS-CoV and protect animals90,91,92. Different variations on the S protein, including full-length, S1 ___domain only, or RBD, can be used to develop effective vaccines against SARS-CoV-220,93. Moreover, a multitude of vaccines and vaccine delivery platforms have been developed, including mRNA, DNA, protein subunits, whole virus in inactivated form, live-attenuated virus, viral vectors, and lipid nanoparticles94,95,96,97,98. Most of the first-generation vaccines against SARS-CoV-2 are based on the ancestral Wuhan virus strain and use a prefusion stabilized S. Two mutations in the S2 subunit between the central helix (CH) and heptad repeat 1 (HR1), K986P and V987P, stabilize the prefusion confirmation and were critical for the effectiveness of COVID-19 vaccines99. Substitution of these residues was initially described for SARS-CoV-1 and MERS-CoV100,101. Subsequent studies showed that the S could be further stabilized by substituting six prolines, resulting in improved neutralizing antibody responses and efficacy102,103.
Eleven vaccines are approved for human use (Table 1) and more than 5 billion people are vaccinated with at least one dose of vaccine104. During the initial COVID-19 waves, multiple vaccines were shown to provide partial protection from infection, severe disease, and death105,106,107. mRNA-1273 and BNT162b2 vaccines, both mRNA vaccines, showed more than 90% efficacy in preventing symptomatic infection and disease severity108,109. AZD1222, a replication-incompetent adenovirus vaccine, prevented disease severity to 100%110. The efficacy of preventing symptomatic infection by a single dose of Ad26.CO.2 was 52.4%, and disease severity was 74.6%108. The mean efficiency of inactivated SARS-CoV-2 vaccines, including Covaxin, Covilo and CoronaVac, in preventing severe disease was 61.80%, 73.78% and 70.96%, respectively111. The emergence of VOCs completely altered this picture. Omicron VOCs have at least 32 mutations in the S protein and can partially escape the neutralizing antibody response elicited by Wuhan strain-based vaccines15. For instance, omicron subvariant BA.2.75 appeared early 2022, a descendent from BA.2, had several distinct mutations in its S protein, including five substitutions in the N-terminal ___domain (NTD), K147E, W152R, F157L, I210V, and G257S, and four substitutions in the RBD, D339H, G446S, N460K, and R493Q. BA.2.75 exhibited higher resistance to vaccine- and infection-induced serum neutralizing activity than BA.2, and the resistance was largely attributed to the K147E and N460K mutations112. Similarly, major genetic drift within the Omicron genome has resulted in the emergence of a highly mutated variant BA.2.86 in 2023 and replaced the circulating XBB and EG.5.1 variants. L455S, F456L, R346T, and D339H mutations within the S of BA.2.86 caused the emergence of a newer variant JN1 with reduced ACE2 binding and increased immune evasion113,114,115. These newer variants exhibited reduced neutralizing capacity within the convalescent and vaccinated individuals116,117.
Breakthrough infections within vaccinated individuals by the Omicron variants substantiated that first-generation COVID-19 vaccines are inadequate in preventing transmission and the requirement of alternate strategies118. Despite the increase in antibody levels after breakthrough infection, neutralization titers against Omicron variants are significantly lower than the earlier variants119,120,121,122,123. To counteract the immunological imprinting effect of SARS-CoV-2, repeated Omicron boosting is required124. All these facts highlight the pressing need to develop next-generation vaccines that can broadly protect against novel variants and multiple related viruses from the same family.
It is important to emphasize that even though the neutralizing antibody response was reduced, all vaccine candidates continued to protect against severe disease caused by most of these VOCs. Protection could be via non-neutralizing antibodies125,126,127 or the induction of CD4+ and CD8 + T cells, for which epitopes are unaffected by S mutations128,129,130,131,132. S-specific CD4 + T cell memory responses induced by natural infection or mRNA vaccination are conserved against different VOCs, including Alpha, Beta, Gamma, and Omicron133. Despite partial escape of humoral immunity induced by SARS-CoV-2 infection or BNT162b2 vaccination by Alpha and Beta VOCs, S-specific CD4 + T-cell activation is not significantly affected by the mutations in these variants134. A similar study showed that SARS-CoV-2-specific memory CD4+ and CD8 + T cell recognition is not disrupted by the VOCs, including Alpha, Beta, and Gamma in COVID-19 convalescents and in recipients of the mRNA-1273 or BNT162b2 COVID-19 vaccines135. A study conducted in the BALB/c mouse model showed that T cell-based SARS-CoV-2 spike protein vaccine could provide significant protection against SARS-CoV-2 and Omicron BA.5 variant infection without inducing any specific antibodies. Moreover, the depletion of CD4+ or CD8 + T cells led to a significant loss of protection, indicating the role of T cells in limiting infection136. Patients with X-linked agammaglobulinemia without B cells can develop pneumonia and still recover from SARS-CoV-2 infection137. Computational tools have been developed to predict immunogenic and conserved T-cell epitopes, thus augmenting the precise selection of SARS-CoV-2 vaccine targets138. Thus, incorporating more T-cell epitopes while developing a vaccine would be able to provide more breadth and durability.
The future of CoV vaccines
Organizations like the Coalition for Epidemic Preparedness Innovations (CEPI) and the National Institute of Allergy and Infectious Diseases (NIAID) have taken the initiative to provide funding for the development of advanced vaccines. These vaccines aim to offer protection against all current and future variants of CoVs by stimulating durable, broad-spectrum immunity. Researchers are updating available vaccines or considering an entirely new approach to achieve this goal.
Since developing a vaccine that protects against all CoVs is likely difficult to achieve due to its genetic variability, as discussed above, a tiered approach likely has a higher chance of success, beginning with COVID-19 vaccines, which can protect against all VOCs, to pan-sarbecovirus vaccines, to pan-betacoronavirus vaccines, to universal or pan-coronavirus vaccines139. Both Pfizer and Moderna have developed bivalent vaccines targeting ancestral SARS-CoV-2 and Omicron. Compared to the monovalent booster, these second-generation vaccines have moderately higher or similar effectiveness in inducing neutralizing antibody responses against Omicron variants in vaccinated infection-naïve cohorts140,141,142. However, immune imprinting and the antigenic variation within Omicron limit the induction of neutralizing antibody responses against newer variants143,144,145,146,147. A recent study carried out on serum samples obtained from participants who had received one or two monovalent boosters or the mRNA bivalent booster indicated that the neutralizing antibody responses are lower against BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB as that of the ancestral strain WA1 in all three cohorts148.
Other groups have focused on the development of broadly neutralizing sarbecoviruses vaccines by including multiple different antigens. Mosaic-8b, a multivalent spike receptor-binding ___domain nanoparticle vaccine displaying RBDs from SARS-CoV-2 and seven animal sarbecoviruses, was found to induce broadly neutralizing antibody responses and protected K18-hACE2 mice and non-human primates from SARS-CoV-1 and SARS-CoV-2 challenge, even though SARS-CoV-1 was not included in the vaccine. In contrast, nanoparticles with SARS-CoV-2 RBDs protected only against SARS-CoV-2 challenge. Compared to homotypic RBD nanoparticles expressing only SARS-CoV-2, immunization with mosaic-8b resulted in more robust binding and neutralizing antibody responses against SARS-CoV-1, SARS-CoV-2, and SARS-CoV-2 VOCs149. Another multivalent spike receptor-binding ___domain nanoparticle vaccine, GBP511, contains RBDs from SARS-CoV-2, SARS-CoV-1, and the bat CoVs WIV1 and RaTG13. This vaccine reduced SARS-CoV-1-MA15 virus replication in lung tissue of vaccinated mice. Furthermore, it elicited broad neutralizing antibody responses against multiple sarbecoviruses after a single immunization150. Likewise, a chimeric spike mRNA vaccine containing RBD, NTD, and S2 domains of various SARS-related CoVs could protect aged mice against SARS-CoV-1, SARS-CoV-2, SARS-CoV-2 Beta, bat CoVs RsSHC014, and a heterologous WIV-1 challenge151. Spike Ferritin Nanoparticle (SpFN) COVID-19 vaccine comprises S from multiple CoVs linked to the surface of a multifaceted ferritin nanoparticle and utilizes Army Liposome Formulation containing QS-21 (ALFQ) as an adjuvant. Upon vaccination, broadly neutralizing antibody responses against major SARS-CoV-2 VOCs and SARS-CoV-1 were induced, resulting in protection against ancestral SARS-CoV-2 in NHPs152,153. Finally, RBD-sortase-A-conjugated ferritin nanoparticle (RBD-scNP) vaccine protects both NHPs and mice from multiple CoV challenges154,155. RBD-scNP contains recombinant SARS-CoV-2 RBD with a C-terminal sortase A donor sequence and self-assembling Helicobacter pylori ferritin with an N-terminal sortase A acceptor sequence.
Antigen design may be important in inducing a broadly protective immune response. DIOSynVax is developing an mRNA vaccine, T2_17, based on the common viral structures on all sarbeco viruses reducing the need for synthesizing, processing, and manufacturing multiple components within one vaccine. A membrane-anchored form (T2_17_TM) mRNA immunogen was shown to be immunogenic against SARS-CoV-1, SARS-CoV-2, WIV16, and RaTG13 in BALB/c mice, guinea pigs, and outbred rabbits. Challenge studies on K18-hACE-2 mice primed with AZD1222 vaccine and boosted with either AZD1222 or T2_17 as an MVA-vectored immunogen showed induction of neutralizing antibody responses against pseudoviruses of SARS-CoV-1, SARS-CoV-2 and the Delta VOC156.
Several studies indicated that intranasally (IN) administered vaccines could induce comparable systemic humoral and cellular immunity as IM vaccination and protect the upper and lower respiratory tract of animals against SARS-CoV-2 infection (Fig. 2)24,157,158,159,160,161,162. Moreover, reduced nasal shedding of the virus was observed upon IN challenge, suggesting that mucosal vaccines could be better at reducing SARS-CoV-2 transmission163,164. Dimeric IgA in the nasopharynx has higher neutralizing capability than monomeric IgA and IgG165. A recent study conducted to evaluate the effectiveness of intranasal administration of sCPD9-ΔFCS, a replication-competent yet fully attenuated virus vaccine, showed induction of robust IgA in nasal washes, which neutralized Omicron BA.1 and BA.5, and limited virus transmission in hamsters models165,166.
Various delivery systems currently used to administer vaccines are shown on the far left. Vaccine delivery routes and devices are depicted in the middle, and the resulting immune pathway after intranasal inoculation is shown on the right. Intranasal delivery of vaccine is achieved by using an inhaler, nebulizer, or spray method. Vaccination via the mucosal route can mount an immune response both in the upper (URT) and lower (LRT) respiratory tract (indicated by green arrows). Microfold cells present on the nasal mucosa actively transport antigens to the dendritic cells (DCs) and macrophages in the subepithelial space. Additionally, DCs situated in the lamina propria or interspersed among epithelial cells sample the mucosal environment using extensions. Activated DCs and macrophages migrate to regional draining lymph nodes or tertiary lymphoid follicles and present antigens to T and B cells. Resulting effector T cells may traffic to the respiratory tract as tissue-resident memory T cells (TRM). Activated B cells either differentiate into low-affinity IgG or IgA-producing plasma cells, which traffic to the respiratory tract, or move to the germinal center, undergo class-switching and somatic hypermutation, and differentiate into long-lived plasma or memory B cells, secreting high-affinity immunoglobulins. These memory B cells may traffic to the respiratory tract. Here, IgA is mainly produced in its polymeric form (pIgA), predominantly dimeric, and transported across the epithelium of the respiratory tract by polymeric Immunoglobulin receptor (pIgR). The pIgR-pIgA complex is cleaved at the apical surface of the epithelium. Thereby, IgA gains part of the pIgR named the secretory component and is released as secretory IgA (sIgA). The secretory component increases the stability of sIgA. Intramuscular immunization can mount robust systemic and LRT immune responses (indicated by red arrows). Circulating IgG antibody levels are generally higher upon vaccination via the intramuscular route in comparison with the intranasal route. NALT Nasal-associated lymphoid tissue. Created with BioRender.com.
More than 20 mucosal vaccine candidates are in development and clinical trials, and two are approved for human use. iNCOVACC (BBV154) is an intranasal SARS-CoV-2 vaccine developed by Bharath Biotech, which was approved in India in early 2023 as a booster dose for those above 18 years of age167. iNCOVACC is a recombinant replication-deficient adenovirus-vectored vaccine encoding a pre-fusion stabilized SARS-CoV-2 S formulated as nasal drops to allow IN delivery. A preclinical study on mice expressing the hACE2 receptor indicated that a single IN dose of iNCOVACC induced high levels of neutralizing antibodies in serum and anti-SARS-CoV-2 IgG and IgA levels in serum and BAL fluid. Challenge studies confirmed complete protection against SARS-CoV-2 infection in the upper and lower respiratory tracts159. Further comparative clinical trial data on healthy adults showed that salivary IgA levels were higher in those who received two doses of iNCOVACC IN, 28 days apart, compared to the licensed intramuscular vaccine, Covaxin. Both vaccines induced significant serum-neutralizing antibody responses against ancestral SARS-CoV-2 and Omicron BA.5167.
A recombinant adenovirus type-5 vectored COVID-19 vaccine (Ad5-nCoV) has been authorized for use as a prime and booster dose in China168. This vaccine is administered via oral inhalation using a nebulizer to deliver the vaccine as aerosol particles into the lungs. Ad5-nCoV is highly immunogenic in clinical trials and efficacious in preventing severe COVID-19169,170,171,172.
IN administration of AZD1222, currently approved as an IM vaccine, can reduce viral shedding and protect hamsters and non-human primates from SARS-CoV-2 challenge163. A phase I clinical trial to investigate the mucosal and systemic immune responses elicited by intranasal AZD1222 as a booster was not encouraging, possibly due to the low number of participants and lack of proper control groups, and more studies have to be conducted to draw final conclusions173.
Two additional nasal vaccine candidates, NDV-HXP-S and CoviLiv, have completed phase I clinical trials174. NDV-HXP-S, a recombinant Newcastle disease virus-based vaccine, which expresses HexaPro S, has shown enhanced neutralizing and binding antibodies from serum samples obtained postvaccination in comparison with samples from mRNA BNT162b2 vaccinees175. A live-attenuated COVID-19 candidate, CoviLiv by Codagenix, expresses all SARS-CoV-2 proteins. IN administration of this vaccine in unvaccinated/uninfected volunteers induced mucosal immunity in a phase I clinical trial. Moreover, participants who received two doses of CoviLiv showed induction of both humoral and cellular immune responses176.
Most mucosal vaccine data comes from preclinical studies. IN vaccination with an N1-methyl-pseudouridine–modified mRNA-LNP results in decreased viral loads in the respiratory tract and reduced lung pathology in Syrian hamsters after the SARS-CoV-2 challenge compared to IM controls177. Vaccination of IFNAR1 − /− mice and hamsters using IN trivalent measles-mumps-SARS-CoV-2 spike protein vaccine induced robust neutralizing antibody responses, mucosal IgA, and systemic and lung resident T cell immune responses and resulted in protection against three different variants of SARS-CoV-2178. An alternate vaccine-boosting strategy, a primary IM vaccination with an mRNA vaccine (“prime”), and an intranasal dose of unadjuvanted SARS-CoV-2 S (“spike”) showed robust cellular and humoral mucosal immune responses in K18-hACE2 mice and protected the animals against SARS-CoV-2 infection. The same strategy reduced viral shedding and prevented transmission in the Syrian hamster model compared to naïve animals24. Finally, MigVax-101 is an orally administered disintegrating freeze-dried tablet vaccine that aims to provide mucosal and humoral immune responses and offers advantages, including ease of administration, low cost, and easy storage. MigVax-101 is a multi-antigen vaccine that contains the RBD and two domains of the N from SARS-CoV-2 and heat-labile enterotoxin B (LTB) as a potent mucosal adjuvant. Immunization studies performed in BALB/c mice and Sprague Dawley rats using MigVax-101 as a homologous oral vaccination of three doses and a heterologous subcutaneous prime and oral booster regimen indicated potent humoral, mucosal, and cell-mediated immune responses compared with the control animals179.
Future challenges
The continuous evolution of SARS-CoV-2 has posed several challenges and raised serious concerns about the effectiveness of currently available vaccines regarding virus infection and transmission. We posit that the development of broad vaccines with a mucosal immune activation component would be ideal for coronaviruses, along with other respiratory viruses.
Several challenges need to be addressed for both the development and clinical testing of vaccines. For example, the human population now exhibits extensive heterologous immunity, and it is not well understood how this affects subsequent vaccination. Furthermore, efficacy testing of novel vaccines in Phase III clinical trials is hindered by this existing immunity, as there will be no placebo group to compare infection rates to. Identifying correlates of protection is necessary to determine a vaccine’s effectiveness against SARS-CoV-2. Another intriguing question is how one can test the efficacy of a vaccine against an as-of-yet-unknown SARS-CoV-3, and likely fully hinges on understanding the correlates of protection.
As detailed above, mucosal vaccination is a promising and logical approach to induce immunity in the respiratory tract. However, more research is required to understand the specific correlates of protection in the respiratory tract, as these are currently unknown. Furthermore, due to previous issues with adjuvants, extra attention should be paid to the safety of mucosal vaccine candidates180. Factors to consider on the way to a successful mucosal vaccine include safe antigens and adjuvants, which vaccine platforms are best at inducing mucosal correlates of protection, what animal models can be used to test efficacy and safety, and what delivery devices are optimal. Another hurdle in developing a nasal vaccine is to address nasal clearing of the vaccine by mucus and cilia. Both mucus and cilia act as a protective barrier in the nasal mucosa and adversely affect antigen absorption. One way to address this issue would be by using virus-like particles and replication incompetent viral vectors that can infect the nasal epithelium or by using substances like gel that can remain in the nasal mucosa for a longer period to ensure proper antigen absorption.
In this review, we have highlighted the pressing need for pan-CoV vaccines against existing and novel emerging CoVs. Developing new tools and finding new vaccination strategies will be an effective way to achieve broad protection against existing and emerging coronaviruses. Multiple broad-spectrum vaccines are currently in various stages of development. Choosing alternative routes for vaccine delivery, along with proper immunogen design, are key approaches to move toward the development of broad-spectrum vaccines. Generating a vaccine against conserved epitopes within CoVs could provide a broader range of protection. The comparative effectiveness of IM and IN vaccination routes is debatable; however, designing and administering vaccines using both routes is an efficient way forward. Combining both routes can theoretically protect individuals from disease severity and virus transmission. Since the population has already received at least one dose of the IM vaccine, a potential IN booster in the form of a self-administrable nasal spray or inhaler would be an intriguing option. More must be addressed before moving into the next step of vaccination in humans.
The COVID-19 pandemic showcased the rigor and potential of the scientific community in developing, testing, and deploying multiple vaccine candidates within an unprecedented timeframe, achieving milestones in the history of vaccines. The COVID-19 pandemic also reminds us of the importance of global pandemic preparedness and the need to invest in research and development. Although COVID-19 is no longer considered a serious threat, the development of innovative vaccine formulations and delivery strategies resulting in improved immunogenicity, safety, and efficacy could make a significant impact on future pandemics.
References
Ghattas, M., Dwivedi, G., Lavertu, M. & Alameh, M. G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines (Basel) 9, https://doi.org/10.3390/vaccines9121490 (2021).
Beverley, P. C. Immunology of vaccination. Br. Med. Bull. 62, 15–28 (2002).
Tyrrell, D. A. & Bynoe, M. L. Cultivation Of A Novel Type Of Common-Cold Virus In Organ Cultures. Br. Med. J. 1, 1467–1470 (1965).
Hamre, D. & Procknow, J. J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 121, 190–193 (1966).
van der Hoek, L. et al. Identification of a new human coronavirus. Nat. Med. 10, 368–373 (2004).
Woo, P. C. et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79, 884–895 (2005).
Zhong, N. S. et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 362, 1353–1358 (2003).
Zaki, A. M., Van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. & Fouchier, R. A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012).
Ye, Z. W. et al. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 16, 1686–1697 (2020).
Xie, X. et al. Neutralization of SARS-CoV-2 Omicron sublineages by 4 doses of the original mRNA vaccine. Cell Rep. 41, 111729 (2022).
Liu, Y. et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 384, 1466–1468 (2021).
Evans, J. P. et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 14, eabn8057 (2022).
Kurhade, C. et al. Neutralization of Omicron sublineages and Deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection. Emerg. Microbes Infect. 11, 1828–1832 (2022).
Andrews, N. et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657–663 (2022).
Lau, J. J. et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat. Med. 29, 348–357 (2023).
Bar-On, Y. M. et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 385, 1393–1400 (2021).
Burckhardt, R. M., Dennehy, J. J., Poon, L. L. M., Saif, L. J. & Enquist, L. W. Are COVID-19 Vaccine Boosters Needed? The Science behind Boosters. J. Virol. 96, e0197321 (2022).
Corbett, K. S. et al. Protection against SARS-CoV-2 Beta variant in mRNA-1273 vaccine–boosted nonhuman primates. Science 374, 1343–1353 (2021).
Tai, W. et al. Characterization of the receptor-binding ___domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 17, 613–620 (2020).
Premkumar, L. et al. The receptor binding ___domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 5, https://doi.org/10.1126/sciimmunol.abc8413 (2020).
Chan, J. F. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9, 221–236 (2020).
Sharifkashani, S. et al. Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: Potential therapeutic targeting. Eur. J. Pharmacol. 884, 173455 (2020).
Mao, T. et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 378, eabo2523 (2022).
Port, J. R. et al. Infection- or AZD1222 vaccine-mediated immunity reduces SARS-CoV-2 transmission but increases Omicron competitiveness in hamsters. Nat. Commun. 14, 6592 (2023).
Brown, C. M. et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly Rep. 70, 1059–1062 (2021).
Singanayagam, A. et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 22, 183–195 (2022).
Li, X. et al. Combining intramuscular and intranasal homologous prime-boost with a chimpanzee adenovirus-based COVID-19 vaccine elicits potent humoral and cellular immune responses in mice. Emerg. Microbes Infect. 11, 1890–1899 (2022).
Sui, Y. et al. Adjuvanted subunit intranasal vaccine prevents SARS-CoV-2 onward transmission in hamsters. bioRxiv, 2024.2005.2013.593816, https://doi.org/10.1101/2024.05.13.593816 (2024).
McMahan, K. et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 626, 385–391 (2024).
Woo, P. C. Y. et al. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J Gen Virol 104, https://doi.org/10.1099/jgv.0.001843 (2023).
Lai, M. M. & Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 48, 1–100 (1997).
Neuman, B. W. et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 174, 11–22 (2011).
de Groot, R. J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 23, 59–72 (2006).
Woo, P. C. Y., Huang, Y., Lau, S. K. P. & Yuen, K. Y. Coronavirus genomics and bioinformatics analysis. Viruses 2, 1804–1820 (2010).
Haring, J. & Perlman, S. Mouse hepatitis virus. Curr. Opin. Microbiol 4, 462–466 (2001).
Stohlman, S., Bergmann, C. & Perlman, S. (John Wiley & Sons, Ltd. New York, 1998).
Bradburne, A. F., Bynoe, M. L. & Tyrrell, D. A. Effects of a “new” human respiratory virus in volunteers. Br. Med J. 3, 767–769 (1967).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020).
He, J., Guo, Y., Mao, R. & Zhang, J. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta‐analysis. J. Med. Virol. 93, 820–830 (2021).
Ki, M. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiology and health 42 (2020).
Nishiura, H. et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 94, 154–155 (2020).
Gao, Z. et al. A systematic review of asymptomatic infections with COVID-19. J. Microbiol., Immunol. Infect. 54, 12–16 (2021).
Sayampanathan, A. A. et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet 397, 93–94 (2021).
Khan, S. et al. COVID-19: Clinical aspects and therapeutics responses. Saudi Pharm. J. 28, 1004–1008 (2020).
Da Silva, E. et al. An adverse outcome pathway for lung surfactant function inhibition leading to decreased lung function. Curr. Res Toxicol. 2, 225–236 (2021).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Channappanavar, R. & Perlman, S. In Seminars in immunopathology. 529-539 (Springer).
Peiris, J. S. et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361, 1767–1772 (2003).
Wong, C. K. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136, 95–103 (2004).
Kwok, K. O. et al. Epidemiology, clinical spectrum, viral kinetics and impact of COVID-19 in the Asia-Pacific region. Respirology 26, 322–333 (2021).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Villarreal, L. P. In Encyclopedia of Virology (Third Edition) (eds Brian W. J. Mahy & Marc H. V. Van Regenmortel) 174-184 (Academic Press, 2008).
Robson, F. et al. Coronavirus RNA proofreading: molecular basis and therapeutic targeting. Mol. cell 79, 710–727 (2020).
Ogando, N. S. et al. The enzymatic activity of the nsp14 exoribonuclease is critical for replication of MERS-CoV and SARS-CoV-2. J. Virol. 94, https://doi.org/10.1128/jvi.01246-01220 (2020).
Amicone, M. et al. Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. EvolB Med. Public Health 10, 142–155 (2022).
Chaw, S.-M. et al. The origin and underlying driving forces of the SARS-CoV-2 outbreak. J. Biomed. Sci. 27, 73 (2020).
Li, X. et al. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Sci. Adv. 6, https://doi.org/10.1126/sciadv.abb9153 (2020).
Zhu, Z., Meng, K. & Meng, G. Genomic recombination events may reveal the evolution of coronavirus and the origin of SARS-CoV-2. Sci. Rep. 10, 21617 (2020).
Ruiz-Aravena, M. et al. Author Correction: Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol 20, 315 (2022).
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Kirchdoerfer, R. N. et al. Pre-fusion structure of a human coronavirus spike protein. Nature 531, 118–121 (2016).
Walls, A. C. et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature 531, 114–117 (2016).
Beniac, D. R., Andonov, A., Grudeski, E. & Booth, T. F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 13, 751–752 (2006).
Plante, J. A. et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 592, 116–121 (2021).
Duong, D. Alpha Beta, Delta, Gamma: What’s important to know about SARS-CoV-2 variants of concern? Cmaj 193, E1059–e1060 (2021).
Vitiello, A., Ferrara, F., Auti, A. M., Di Domenico, M. & Boccellino, M. Advances in the Omicron variant development. J. Intern Med 292, 81–90 (2022).
Evans, J. P. et al. Neutralization of SARS-CoV-2 Omicron sub-lineages BA. 1, BA. 1.1, and BA. 2. Cell host microbe 30, 1093–1102.e1093 (2022).
Mahase, E. Covid-19: What do we know about omicron sublineages. BMJ 376, o358 (2022).
Tallei, T. E. et al. Update on the omicron sub-variants BA.4 and BA.5. Rev. Med Virol. 33, e2391 (2023).
Chatterjee, S., Bhattacharya, M., Nag, S., Dhama, K. & Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 15, https://doi.org/10.3390/v15010167 (2023).
Ai, J. et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 11, 337–343 (2022).
López-Cortés, G. I. et al. Neutral evolution test of the spike protein of SARS-CoV-2 and its implications in the binding to ACE2. Sci. Rep. 11, 18847 (2021).
Magazine, N. et al. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 14, https://doi.org/10.3390/v14030640 (2022).
Davies, N. G. et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B. 1.1. 7. Nature 593, 270–274 (2021).
Mohsin, M. & Mahmud, S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine 101, e29165 (2022).
Markov, P. V. et al. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 21, 361–379 (2023).
Wickenhagen, A. et al. Evolution of Omicron lineage towards increased fitness in the upper respiratory tract in the absence of severe lung pathology. bioRxiv, 2024.2006.2013.598902, https://doi.org/10.1101/2024.06.13.598902 (2024).
Wu, C. T. et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 186, 112–130 e120 (2023).
Hui, K. P. Y. et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 603, 715–720 (2022).
Lan, J. et al. Recombinant Receptor Binding Domain Protein Induces Partial Protective Immunity in Rhesus Macaques Against Middle East Respiratory Syndrome Coronavirus Challenge. EBioMedicine 2, 1438–1446 (2015).
Modjarrad, K. et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 19, 1013–1022 (2019).
Weskamm, L. M. et al. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep. Med 3, 100685 (2022).
Kandeel, M. et al. Safety and immunogenicity of the ChAdOx1, MVA-MERS-S, and GLS-5300 DNA MERS-CoV vaccines. Int Immunopharmacol. 118, 109998 (2023).
Alharbi, N. K. et al. Immunogenicity of High-Dose MVA-Based MERS Vaccine Candidate in Mice and Camels. Vaccines (Basel) 10, https://doi.org/10.3390/vaccines10081330 (2022).
Du, L. et al. The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat. Rev. Microbiol 7, 226–236 (2009).
He, Y. et al. Receptor-binding ___domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. BiophysB Res. Commun. 324, 773–781 (2004).
Weingartl, H. et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 78, 12672–12676 (2004).
Qiu, M. et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 7, 882–889 (2005).
Tang, X. C. et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc. Natl Acad. Sci. USA 111, E2018–E2026 (2014).
Li, Y. et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding ___domain of the spike protein. Cell Res 25, 1237–1249 (2015).
Burton, D. R. & Walker, L. M. Rational Vaccine Design in the Time of COVID-19. Cell Host Microbe 27, 695–698 (2020).
Amanat, F. & Krammer, F. SARS-CoV-2 vaccines: status report. Immunity 52, 583–589 (2020).
Liu, Y. et al. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions. Nat. Commun. 13, 4337 (2022).
Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020).
Folegatti, P. M. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Tan, T. J. C. et al. High-throughput identification of prefusion-stabilizing mutations in SARS-CoV-2 spike. Nat. Commun. 14, 2003 (2023).
Pallesen, J. et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl Acad. Sci. 114, E7348–E7357 (2017).
Kirchdoerfer, R. N. et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 8, 15701 (2018).
Lu, M. J. et al. SARS-CoV-2 prefusion spike protein stabilized by six rather than two prolines is more potent for inducing antibodies that neutralize viral variants of concern. P Natl Acad. Sci. USA 119, e2110105119 (2022).
Hsieh, C.-L. et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 369, 1501–1505 (2020).
WHO Coronavirus (COVID-19) Dashboard, Accessed June 13, 2024, https://covid19.who.int/.
Yan, V. K. C. et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study. Emerg. Microbes Infect. 11, 2304–2314 (2022).
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 384, 403–416 (2020).
El Sahly, H. M. et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 385, 1774–1785 (2021).
Sadoff, J. et al. Final analysis of efficacy and safety of single-dose Ad26. COV2. S. N. Engl. J. Med. 386, 847–860 (2022).
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
Falsey, A. R. et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 385, 2348–2360 (2021).
Meo, S. A., ElToukhy, R. A., Meo, A. S. & Klonoff, D. C. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19. Vaccines Sinopharm CoronaVac. Covaxin: Observat. Study Vaccines 11, 826 (2023).
Wang, Q. et al. Antigenic characterization of the SARS-CoV-2 Omicron subvariant BA.2.75. Cell Host Microbe 30, 1512–1517.e1514 (2022).
Jeworowski, L. M. et al. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro Surveill 29, https://doi.org/10.2807/1560-7917.Es.2024.29.2.2300740 (2024).
Li, P. et al. Characteristics of JN.1-derived SARS-CoV-2 subvariants SLip, FLiRT, and KP.2 in neutralization escape, infectivity and membrane fusion. bioRxiv https://doi.org/10.1101/2024.05.20.595020 (2024)
Yang, S. et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 24, e70–e72 (2024).
Carreño, J. M. et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 602, 682–688 (2022).
Uraki, R. et al. Humoral immune evasion of the omicron subvariants BQ. 1.1 and XBB. Lancet Infect. Dis. 23, 30–32 (2023).
Tan, S. T. et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat. Med 29, 358–365 (2023).
Reynolds, C. J. et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 377, eabq1841 (2022).
Baerends, E. A. M. et al. Omicron variant-specific serological imprinting following BA.1 or BA.4/5 bivalent vaccination and previous SARS-CoV-2 infection: A cohort study. Clin Infect Dis https://doi.org/10.1093/cid/ciad402 (2023).
Seaman, M. S. et al. Vaccine Breakthrough Infection with the SARS-CoV-2 Delta or Omicron (BA.1) Variant Leads to Distinct Profiles of Neutralizing Antibody Responses. medRxiv https://doi.org/10.1101/2022.03.02.22271731 (2022).
Garcia-Beltran, W. F. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185, 457–466. e454 (2022).
Hoffmann, M. et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 185, 447–456.e411 (2022).
Yisimayi, A. et al. Repeated Omicron infection alleviates SARS-CoV-2 immune imprinting. bioRxiv, 2023.2005.2001.538516, https://doi.org/10.1101/2023.05.01.538516 (2023).
Monge, S. et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect. Dis. 22, 1313–1320 (2022).
Collie, S., Champion, J., Moultrie, H., Bekker, L.-G. & Gray, G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 386, 494–496 (2022).
Gray, G. et al. Effectiveness of Ad26. COV2. S and BNT162b2 vaccines against Omicron variant in South Africa. N. Engl. J. Med. 386, 2243–2245 (2022).
Keeton, R. et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 603, 488–492 (2022).
GeurtsvanKessel, C. H. et al. Divergent SARS-CoV-2 Omicron–reactive T and B cell responses in COVID-19 vaccine recipients. Sci. immunol. 7, eabo2202 (2022).
Gao, Y. et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 28, 472–476 (2022).
Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021).
Geers, D. et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci. Immunol. 6, eabj1750 (2021).
Mazzoni, A. et al. SARS-CoV-2 Spike-Specific CD4 + T Cell Response Is Conserved Against Variants of Concern, Including Omicron. Front Immunol. 13, 801431 (2022).
Geers, D. et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 6, https://doi.org/10.1126/sciimmunol.abj1750 (2021).
Tarke, A. et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8( + ) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2, 100355 (2021).
Shi, J. et al. A T cell-based SARS-CoV-2 spike protein vaccine provides protection without antibodies. JCI Insight 9, https://doi.org/10.1172/jci.insight.155789 (2024).
Soresina, A. et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr. Allergy Immunol. 31, 565–569 (2020).
Poran, A. et al. Sequence-based prediction of SARS-CoV-2 vaccine targets using a mass spectrometry-based bioinformatics predictor identifies immunogenic T cell epitopes. Genome Med. 12, 1–15 (2020).
Moore, K. A. et al. A research and development (R&D) roadmap for broadly protective coronavirus vaccines: A pandemic preparedness strategy. Vaccine 41, 2101–2112 (2023).
Chalkias, S. et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 387, 1279–1291 (2022).
Davis-Gardner, M. E. et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 388, 183–185 (2022).
Springer, D. N. et al. Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants. npj Vaccines 8, 110 (2023).
Offit, P. A. Bivalent Covid-19 vaccines—a cautionary tale. N. Engl. J. Med. 388, 481–483 (2023).
Tan, C. W., Valkenburg, S. A., Poon, L. L. M. & Wang, L. F. Broad-spectrum pan-genus and pan-family virus vaccines. Cell Host Microbe 31, 902–916 (2023).
Park, Y.-J. et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 378, 619–627 (2022).
Cao, Y. et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 614, 521–529 (2023).
Kaku, C. I. et al. Evolution of antibody immunity following Omicron BA. 1 breakthrough infection. Nat. Commun. 14, 2751 (2023).
Davis-Gardner, M. E. et al. Neutralization against BA. 2.75. 2, BQ. 1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 388, 183–185 (2023).
Cohen, A. A. et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 377, eabq0839 (2022).
Walls, A. C. et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding ___domain nanoparticle vaccines. Cell 184, 5432–5447.e5416 (2021).
Martinez, D. R. et al. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science 373, 991–998 (2021).
Joyce, M. G. et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci. Transl. Med. 14, eabi5735 (2022).
King, H. A. D. et al. Efficacy and breadth of adjuvanted SARS-CoV-2 receptor-binding ___domain nanoparticle vaccine in macaques. Proc. Natl Acad. Sci. 118, e2106433118 (2021).
Li, D. et al. Breadth of SARS-CoV-2 neutralization and protection induced by a nanoparticle vaccine. Nat. Commun. 13, 6309 (2022).
Saunders, K. O. et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature 594, 553–559 (2021).
Vishwanath, S. et al. A computationally designed antigen eliciting broad humoral responses against SARS-CoV-2 and related sarbecoviruses. Nat. Biomed. Engineer. https://doi.org/10.1038/s41551-023-01094-2 (2023).
Bricker, T. L. et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 36 (2021).
Wu, S. et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 11, 4081 (2020).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e113 (2020).
Hassan, A. O. et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 36, 109452 (2021).
Ku, M.-W. et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell host microbe 29, 236–249.e236 (2021).
Sun, W. et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 12, 6197 (2021).
van Doremalen, N. et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Science translational medicine 13, eabh0755 (2021).
Marsh, G. A. et al. ChAdOx1 nCoV-19 (AZD1222) vaccine candidate significantly reduces SARS-CoV-2 shedding in ferrets. npj Vaccines 6, 67 (2021).
Wang, Z. et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 13, https://doi.org/10.1126/scitranslmed.abf1555 (2021).
Adler, J. M. et al. An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission. Nat. Commun. 15, 995 (2024).
Singh, C. et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin®). npj Vaccines 8, 125 (2023).
China and India approve nasal COVID vaccines — are they a game changer? Nature 609, 450, https://www.nature.com/articles/d41586-022-02851-0 (2022).
Wang, S. Y., Liu, W. Q., Li, Y. Q., Li, J. X. & Zhu, F. C. A China-developed adenovirus vector-based COVID-19 vaccine: review of the development and application of Ad5-nCov. Expert Rev. Vaccines 22, 704–713 (2023).
Zhu, F.-C. et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395, 1845–1854 (2020).
Zhu, F. C. et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396, 479–488 (2020).
Halperin, S. A. et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 399, 237–248 (2022).
Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. EBioMedicine 85, 104298 (2022).
Pitisuttithum, P. et al. Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Interim results of a randomised, placebo-controlled, phase 1 trial. Eclinicalmedicine 45, 101323 (2022).
Carreño, J. M. et al. An inactivated NDV-HXP-S COVID-19 vaccine elicits a higher proportion of neutralizing antibodies in humans than mRNA vaccination. Sci. Transl. Med. 15, eabo2847 (2023).
Kaufmann, J. K. et al. in Open Forum Infectious Diseases. ofad500. 2469 (Oxford University Press US).
Baldeon Vaca, G. et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci. Adv. 9, eadh1655 (2023).
Xu, J. et al. A next-generation intranasal trivalent MMS vaccine induces durable and broad protection against SARS-CoV-2 variants of concern. Proc. Natl Acad. Sci. 120, e2220403120 (2023).
Pitcovski, J. et al. Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. Vaccine 40, 1098–1107 (2022).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Minh, B. Q. et al. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Acknowledgements
We thank Dr. Joydeep Nag, Florida Research and Innovation Centre, Cleveland Clinic Lerner Research Institute and Dr. Arunkumar R. C. for providing critical comments and valuable suggestions while writing.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
R.K.M. and C.K.Y. wrote the main manuscript text. R.K.M. and C.K.Y. prepared the figures and table. V.J.M. and N.v.D. edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koolaparambil Mukesh, R., Yinda, C.K., Munster, V.J. et al. Beyond COVID-19: the promise of next-generation coronavirus vaccines. npj Viruses 2, 39 (2024). https://doi.org/10.1038/s44298-024-00043-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44298-024-00043-3