Abstract
Studying the subtle and intricate three-dimensional structure of the human cochlea embedded in the temporal bone requires structure-preserving imaging approaches with adaptable field of view and resolution. Synchrotron X-ray phase-contrast tomography at the novel beamline BM18 (EBS, ESRF) offers the unique capability to achieve histological resolution at the scale of the entire organ, based on high lateral coherence, long propagation distances, and optimized spectral range. At the same time advances in laboratory μ-CT instrumentation and protocols also open up new opportunities for 3D micro-anatomy and histopathology, including 3D reconstruction of nerve tissue when suitable staining protocols are used. Here we report on post mortem 3D imaging of human temporal bones and excised human cochleae, both unstained and stained to visualize the auditory nerve. Further, we highlight the use of this imaging modality for development of novel cochlear implant technology.
Similar content being viewed by others
Introduction
Disabling hearing loss affects 5% of the world’s population. Access to the micro-anatomy of the ear is crucial for understanding sensorineural hearing loss and for the development of cochlear implants (CIs) for hearing restoration. The subtle and intricate three-dimensional (3D) structure of the cochlea encased in the temporal bone requires structure-preserving imaging approaches. The cochlea has been studied with 2D imaging approaches such as conventional histology1 and transmission electron microscopy2 which offer high 2D resolution, but are susceptible to slicing and staining artifacts. Furthermore, coverage of a large volume is extremely tedious. Light sheet fluorescent microscopy (LSFM)3 offers 3D imaging of the cochlea, but requires tissue clearing which takes time and is also associated with tissue shrinkage and structural changes. Magnetic resonance imaging (MRI)4 on the other hand is non-destructive, but does not resolve cells.
X-ray phase-contrast computed tomography (XPCT) allows for non-destructive imaging of the human temporal bone or excised cochlea and is compatible with different tissue preparation techniques such as staining with osmium tetroxide (OTO) which increases contrast for lipid-rich tissue, dehydration in an increasing ethanol series to increase contrast between tissue and solvent and even tissue clearing allowing multi-modal imaging with LSFM3. There are a number of XPCT-studies on rodent and primate animal models used in auditory research both in unstained3,5,6,7,8 and OTO- or iodine-stained specimen9,10,11,12. In the translation from rodent to non-human primate to human cochleae, the sample size increases and thus requires a wider field-of-view (FOV), a higher photon energy, and eventually also adaptation of the phase-retrieval schemes. Human temporal bones have been studied in a number of XPCT-studies both at synchrotron (SR) sources, including both unstained and non-decalcified1,13,14,15,16,17,18, unstained and paraffin embedded19, as well as OTO stained samples5,20. Tackled questions include the evaluation of cochlear duct length and tonotopic mapping14,15,21 and analysis of the cochlear anatomy for therapeutic intervention16,17,22. Human cochleae have also been studied with μ-CT liquid embedded and unstained13, OTO- or iodine-stained11,12,17 and dried23.
Modern fourth generation synchrotron sources such as the Extremely Brilliant Source (EBS) at the European Synchrotron Radiation Facility (ESRF) offer highest brilliance and coherence even at comparatively high photon energies, allowing the imaging of large intact human organs with μm resolution. The HiP-CT protocol24 describes optimal sample preparation. In order to reduce sample movement and bubble formation and to increase contrast organs are transferred to 70% ethanol (EtOH) and mounted in agarose. At the test beamline BM05, brain, heart kidney, spleen and lung25,26,27 have been studied. The new beamline BM1828 has been explicitly designed to scan very large samples with a polychromatic, high-energy beam exploiting propagation-based phase-contrast. Recently hearts29 and kidneys30 have been scanned at BM18.
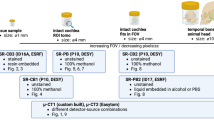
In this paper we report on post-mortem 3D imaging of excised human temporal bones and human cochleae using highly brilliant synchrotron radiation at the beamline BM18, GINIX instrument at beamline P10 and in-house μ-CT. We explore different sample mountings in PFA, PBS and 70% EtOH, as well the OTO stain for better visibility of the nervous tissue, see Fig. 1 for an overview of the samples considered for this manuscript. Importantly, along with the imaging approaches described below and the discussion of the results, we include all 3D datasets as a benchmark, for further tests of segmentation and morphometric measurements and for use in modeling sound transduction or fitting of implants.
A The 3D structure of the middle and inner ear, especially the organ of Corti (OoC) with the hair cells and their innervation by the spiral ganglion neurons are of interest for auditory research. B Sample preparation for XPCT experiments. Temporal bones were retrieved during routine autopsy and fixed in 4% paraformaldehyde (PFA) for several days. One temporal bone was kept in 4% PFA (H09). Another temporal bone was dehydrated in an increasing ethanol (EtOH) series up to 70% EtOH (H03). A third temporal bone was implanted with an optical cochlear implant (oCI) and kept in PBS. Two additional temporal bones got trimmed down to the cochlea of which one was decalcified and dehydrated to 70% EtOH (H01). The other cochlea was stained with osmium tetroxide (OTO) and kept in PBS (H02). All samples were mounted in cylindrical jars with agarose to avoid sample movement and degassed to avoid bubble formation. Fig. created with Bio Render.
Results
Experiment design and image chain
While absorption contrast in X-ray CT is well suited to image dense materials such as bones and metal implants, contrast for soft tissue can be enhanced by exploiting the sample-induced phase shift of (partially) coherent X-rays. Phase shifts arise from the decrement δ(r) of the X-ray refractive index n(r) = 1 − δ(r) + iβ(r) which is orders of magnitude larger for biological soft tissue than the imaginary part β(r) related to absorption. The sample-induced phase-shifts are converted into measurable intensity modulations by free-space propagation. Depending on the effective propagation distance zeff = z12/M, the effective pixelsize dxeff = dx/M and the photon wavelength λ, the imaging regime can be described by the unitless Fresnel number \(F={{\rm{dx}}}_{{\rm{eff}}}^{2}/({z}_{{\rm{eff}}}\lambda )\). Sample preparation is key for multiscale XPCT. Samples are partially dehydrated, kept in PBS or stained and mounted in cylindrical jars with agarose, see Fig. 1 for an overview of sample preparation and the Methods section for details. Sample containers as well as annotated photos of the BM18 beamline are shown in Fig. 2.
(1) BM18 beamline, ESRF, showing (A) exit window and sample stage and (B) sample stage and detector stage with different detector-lens combinations. The flatfield images are usually taken in a sample-free region of the sample jar. (2) μ-CT setup with source, sample stage and detector. The flatfield images are taken in a control jar that is filled with the same solvent as the sample jar. (3) Sample jars used for different sample sizes such as the excised cochlea and the temporal bone.
The BM18 beamline is located at the EBS storage ring. The detector stage houses several detector-lens combinations and can be translated to change the propagation distance z12. The BM18 source is a tripole wiggler with a fixed gap. The polychromatic energy E can be tuned between 50 and 280 keV by inserting polished attenuators. The polychromatic energy is carefully tailored in view of the object size, material composition, dose limit, and the dynamic range of the detector. Maintaining the dose limit is critical so that no bubbles are created which introduce streak artifacts and motion artifacts31. An effective energy Eeff can be calculated by taking into account the transmission of the entire optical pathway and detection parameters of the scintillator screen, exemplary calculated spectra are shown in the Supplementary Fig. 1.
For the large temporal bones, overview scans were recorded at a voxelsize dxeff = 6.2 μm in half-acquisition mode to almost double the detector’s horizontal FOV to 58 mm. To accommodate for the absorption gradient through the sample jar, the sample was shifted to the side of the beam profile. Flatfield images were recorded in the sample container in a sample-free region following the HiP-CT protocol24. Phase-reconstruction was performed with Paganin’s algorithm32. An illustrative projection image of the temporal bone and phase-retrieval are shown in Fig. 3. Region-of-interest (ROI) tomography of temporal bones and cochleae were performed with the rotation axis in the middle of the detector, placed in the middle of the symmetric beam profile at dxeff ∈ {1.8, 2.3} μm. In all configurations, accumulation of images can be exploited to increase signal-to-noise ratio. The μ-CT setup is shown in Fig. 2 (2). Scan times were significantly longer. Adapting the accumulation time to the required detail- and noise-level typically resulted in overnight scans between 12 and 16 h. The propagation distance z12 and the magnification M were set by moving both sample and detector stage. The flatfield was recorded in a separate container filled with the same solvent and mounted above sample jar.
In the following we present datasets of (i) human temporal bone in 4% PFA (H09), (ii) human temporal bone post-mortem implanted with oCI in PBS (H11), (iii) OTO stained cochlea in PBS (H02) and (iv) decalcified Cochlea in 70% EtOH (sample H01). See Tables 1, 2, 3 and 4 in the Methods section for detailed information on samples and scan parameters for both the synchrotron setup and μ-CT setup.
Human temporal bone in fixative solution
First, we showcase the multiscale imaging capability of BM18 for a human temporal bone immersed in 4% PFA and mounted in a jar of diameter 4 cm (sample H09). The overview scan was performed with dxeff = 6.2 μm in a half acquisition scheme. Subsequently a ROI tomography at the position of the cochlea was recorded at dxeff = 1.8 μm. Virtual slices through the reconstruction volume are shown in Fig. 4. The bony structures in the cochlea are represented well and at high detail level, see for example the fine structure in the surrounding bone. However, soft tissue such as the OoC is nearly invisible in this imaging configuration. As we show further below, the OoC becomes highly contrasted, however, when using heavy atom labeling (Fig. 7), and also when decalcified (Fig. 9). Note that (A-C) is oriented perpendicular to (D-F). The high contrast level and resolution facilitated fast segmentation based on region growing. Renderings of the segmented cochlea and stapes, as well as of the unsegmented temporal bone are shown in (G) and (H) respectively.
A Virtual slice through reconstruction volume at dxeff = 6.2 μm. B ROI tomogram recorded at the position of the cochlea at dxeff = 1.8 μm. C Zoom into (B). D Virtual slice through the reconstruction volume, perpendicular to (A). E ROI tomography at the position of cochlea. F Zoom into (E). G Volume rendering of the segmented cochlea and stapes. H Volume rendering of unsegmented temporal bone cut for unobstructed view. Scale bars 1 mm. (A–C) share the same orientation and are perpendicular to (D, E). The position of the respective perpendicular slices is indicated by arrows.
Human temporal bone with oCI
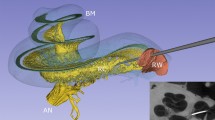
Next, we present datasets of a human temporal bone which had been implanted with an oCI post-mortem. The sample was immersed in PBS and mounted in a jar of diameter 7 cm (sample H11). The overview scan was recorded at dxeff = 6.2 μm in a half acquisition scheme. The same sample was scanned additionally at the μ-CT-setup, at dxeff = 30 μm and at dxeff = 15.6 μm, respectively. Since the sample had to be remounted during beamtime, the reconstruction volumes of these three datasets have been aligned manually. Virtual slices through the reconstruction volume are shown in Fig. 5. The fine bony structures of the cochlea are fairly represented and a high detail level in the surrounding bone is visible. Note that even in the ROI μ-CT dataset most of the fine lines are all likely to be bony trabeculae rather than unmineralized soft tissue such as membranes. At the same time, the surrounding bone shows a high detail level, although the SNR for the μ-CT is significantly lower than at BM18. Furthermore, the oCI implant results in a small but visible beam hardening artifact (see arrow). The high contrast in the SR-dataset allowed fast segmentation and volume rendering. The oCI and the three ossicles malleus, incus and stapes have been segmented with a region growing algorithm. The volume rendering in Fig. 6 shows the placement of the oCI in the basal turn of the cochlea. Supplementary Movie 1 presents an animation of the temporal bone.
A Volume rendering of the whole dataset. B Zoom into the middle and inner ear, clipping part of the dataset for an unobstructed view in the middle ear. The three ossicles are marked with arrows. C Volume rendering of segmented ossicles, oCI and cochlea. D Zoom into (B). Note the placement of the oCI's LEDs in the basal turn of the cochlea. E Virtual slice through the reconstruction volume showing the placement of the oCI in the basal turn of the cochlea. Scale bar 1 mm. A–D share the same orientation. See Supplementary Movie 1 for an animation.
OTO stained cochlea
Next, we illustrate the effect of the OTO staining on XPCT of the cochlea. An overview scan was performed with dxeff = 6.2 μm at an effective energy Eeff = 94 keV. Subsequently, a ROI tomography with dxeff = 2.3 μm was recorded at Eeff = 75 keV. The μ-CT scan was performed at dxeff = 7.2 μm and a tube voltage of 80 kV. Note that all datasets were registered manually because the sample had to be remounted between all measurements. Of each dataset a slice through the reconstructed volume and a zoom-in is shown in Fig. 7. The overall morphology of the cochlea is represented well in each dataset with the three scalae and separating membranes. The OTO stain increases contrast for lipid-rich tissue components such as the myelin sheaths in nervous tissue. Thus, the central nerve trunk in the modiolus and the innervation to the organ of Corti are highlighted. The μ-CT dataset has fairly good quality, while the SR-datasets have a better resolution and better SNR. The SR-dataset with the smallest voxelsize shows even more detail and has a higher SNR. In (B, D) Reissner’s membrane is observed to be ruptured, possibly either due to radiation damage or due to sample handling, i. e. due to bubble formation during degassing or simply remounting of the specimen in the becher. Out of these possibilities, mechanical damage by gas bubble formation seems most likely. Note the sample was first measured with the μ-CT, at which point Reissner’s membrane was still intact, and was then degassed again for prolonged time before the synchrotron recording. Volume renderings of the SR overview scan are shown in Fig. 8.
The OTO stain increases contrast for the lipid-rich nervous tissue. The reconstructed volumes obtained by different instruments and settings have been aligned virtually. A BM18 with dxeff = 2.3 μm. B Zoom into (A). C BM18 overview scan with dxeff = 6.2 μm and (D) Zoom into (C). E μ-CT with dxeff = 7.2 μm with (F) Zoom into (E). The red arrows point at Reissner’s membrane, which appears ruptured in the BM18 data, while it is intact in the prior recorded inhouse μ-CT scan. Note also that the gray values are inverted with respect to standard radiography images, i. e. dark corresponds to high density. Scale bars: (left) 1 mm, (right) 0.25 mm.
Decalcified human cochlea
To enhance contrast for soft tissue and to resolve the membranes and the organ of Corti in the cochlea, we have also imaged a decalcified human cochlea (H01) with XPCT. The human cochlea was removed during autopsy and fixed in 4% PFA for several days. After removal of the surrounding bone, the cochlea was decalcified in 10% EDTA for several weeks. The decalcified cochlea was dehydrated in an increasing Ethanol series up to 70% EtOH.
For a polychromatic high-energy beamline like BM18, imaging a weakly absorbing and rather small sample poses a challenge in terms of not oversaturating the detector and not exceeding the X-ray dose on the sample. Furthermore, we reduced the photon energy to have potentially more contrast for soft tissue. The planned installation of a chopper at BM18 which was not available yet during our beamtime would certainly help to solve this problem. We recorded the decalcified cochlea in the horizontalt tail of the BM18 beam with an effective energy Eeff = 64 keV and a voxelsize dxeff = 1.8 μm. In order to pave a link to higher resolution, we have also scanned this decalcified cochlea during a beamtime at the GINIX instrument33, beamline P10, PETRA III at DESY in Hamburg. The GINIX instrument was operated in a parallel beam configuration without collimating optics as described in detail in Frohn et al.34. The Energy was set to E = 20 keV with a channelcut monochromator. The tomogram was recorded in a continuous rotation at dxeff = 650 nm with a FOV of 1 mm × 1.5 mm. Phase retrieval was performed with a CTF-based approach35,36. With an exposure time of 35 ms, a single tomogram is recorded in 2 min and larger volumes can be covered by stitching, see Tab. 3 for a summary of the acquisition parameters.
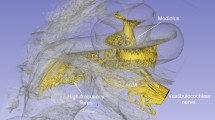
Virtual slices through the reconstruction volume of both scans are shown in Fig. 9. Filtering in Fourier space was applied to both datasets in order to reduce intensity fluctuations and stripes. This BM18 dataset shows more detail than the prior ones of non-decalcified samples. The soft tissue such as the membranes between the cochlear ducts are represented well. In the GINIX dataset with even higher resolution even cellular structures such as the organ of Corti are recognizable.
A Virtual slice through the reconstruction volume acquired at BM18 with dxeff = 2.26 μm. Scalebar 500 μm. B Virtual slice trough reconstruction volume acquired at GINIX with dxeff = 0.65 μm. The three scalae scala vestibuli (SV), scala media (SM) and scala tympani (ST) are separated by the Reissner’s membrane (RM) and the basilar membrane (BM) with the Organ of Corti (OoC). Scalebar 50 μm.
Discussion
We have imaged human temporal bones and cochleae with SR and laboratory μ-CT, at different size and preparation of samples, and correspondingly different instrumental settings. Accordingly, the image formation involved different contributions of absorption- and phase-contrast. The phase shift was accounted by a Fourier filter based phase retrieval with adapted parameters based on the Paganin approach derived for a homogeneous object and monochromatic radiation. Despite the fact that strictly speaking these conditions are not fulfilled, the resulting image quality was exceptionally good. The choice of spectra, the instrumental settings and parameters, and the detection technology were all instrumental for the unprecedented image quality obtained. To this end, the tabulated parameters, sample preparation and imaging protocol can serve as a reference for future extension and use, in particular of studies of human temporal bone and cochlea, e. g. for post-mortem analysis of cochlear pathology or implant development.
The high contrast for mineralized tissue can aid in recent studies such as attempts to quantify the bone density in patients with otosclerosis37, and 3D analysis of the neo-ossification of the temporal bone in implant patients38.
Figure 5 in particular shows that optimized μ-CT instrumentation which is more accessible for a preclinical setting will be able to address 3D imaging needs already with a surprisingly high image quality. In this sense, the SR results which still represent an unrivaled benchmark, can be partially translated to compact laboratory scale. At the same time, SR data can provide ground truth for machine learning approaches in image reconstruction and image processing of 3D tomographic data. BM18 as the state-of-the-art SR imaging beamline for whole organ imaging provides data with significantly enhanced visibility for fine details of membranes and the organ of Corti. At the same time, the soft tissue within the cochlea is still too poorly represented due to limited phase-contrast when surrounded by the mineralized and more absorbing components of the temporal bone. Higher image quality for soft nervous tissue therefore requires heavy metal staining, at least when imaged with the necessarily high photon energies to penetrate the temporal bone. To this end, the OTO stain and cochlea preparation which we have already introduced in11 comes out at surprising image quality. Even without segmentation, the different contrast values allow for renderings which already represent the structure of the auditory nerve, at least to some extent. At the same time, we have in this work not yet fully exploited the potential of preparing the entire human cochlea by decalcification and selective OTO staining in the organ of Corti, or even decalcified and unstained temporal bones in 70% ethanol.
Higher phase-contrast for unstained soft tissue can be achieved by lowering the photon energy, which becomes possible for decalcified temporal bone. Note as well that for a polychromatic high-energy beamline like BM18, imaging a weakly absorbing and rather small sample can pose a challenge in terms of not oversaturating the detector and not exceeding the X-ray dose on the sample. This is accentuated when reducing the photon energy to increase the contrast for soft tissue. The planned installation of a chopper at BM18 which was not available yet during our beamtime would solve this problem, since spectral tailoring and control of primary beam intensity would then be decoupled. In this way, it should become possible to collect data on temporal bone and cochlea with voxel size of 1 μm to 2 μm in phase contrast mode, with potentially huge biological impact, e.g., in view of localizing and counting hair cells, spiral ganglion neurons, and studies of their innervation. While there is in principle no physical obstacle aside from the necessary implementation of instrumentation, this has not yet been achieved in this work. At the same time, we stress that also at the current setting interesting biology of temporal bone becomes possible at the whole organ level, for example quantification of the bone density in patients with otosclerosis, or 3D analysis of the neo-ossification of the temporal bone in implant patients.
In conclusion, combined and synergistic use of laboratory μ-CT instrumentation at optimized settings and dedicated whole organ SR beamlines with large FOVs such as BM18 opens up 3D imaging of human temporal bone for a wide range of biomedical research projects. It also provides data for teaching otorhinolaryngology, for 3D simulation of micromechanics of middle and inner ear, for research addressing hearing disorders and loss, and for implant development. Along with the imaging protocols of this work, all 3D data as well as the raw data is made publicly available as benchmark to compare image quality, as test data to develop further 3D image processing tools, possibly including machine learning, and finally, above all, for future use for biomedical research on the human inner ear. Most interestingly is the fact, that the presented approach and its future extension can bridge histological and micro-anatomical length scales. The 3D XPCT volume data is available at GRO.data (dataset DOI 10.25625/V3YSYD)39.
Methods
Sample preparation
Temporal bones and cochleae studied here have been collected at the University Medical Center Göttingen (UMG), under proposals #22/1/19 and # 2/8/19 (“analysis of (neuro)degeneration in the cochlea”), approved by the ethics committee of UMG, as well as under #2020P000508 (“Otopathology of the Human Temporal Bone”), approved by the institutional review board (IRB) of the Massachusetts Eye and Ear Infirmary, including appropriate pre-mortem human consent forms and all subsequent procedures for technical processing and HIPAA-compliant data storage. Specimen have been immersed in 4% PFA or PBS without staining except for one cochlea which was OTO stained. For a better overview the specimen are numbered and summarized in Table 1, and the preparation protocols are illustrated in Fig. 1. The cochlea sample H02 was removed at autopsy 14 h post mortem, immersion fixed in 4% PFA for several days and then stained with osmium tetroxide (OTO, OsO4), by immersing in 10 ml of 2% OTO in deionized H20 at room temperature for 48 h. Round and oval windows were opened and the osmium solution was flushed through the scalae 3 times per day by applying gentle negative pressure near each window. The OTO binds to lipids and is a staining method widely used in electron microscopy40. The temporal bone samples H01, H03-H11 have been retrieved at routine autopsy and immersion fixed in 4% PFA for several days. In one of the temporal bone samples (H11) we inserted an optical cochlea implant via the round window, see also3. We used an oCI prototype designed for human application which contained 49 micro-LEDs on a strip. The insertion procedure was identical to that of a conventional cochlea implant: After opening of the mastoid cortical bone, a classic mastoidectomy was performed. After that, the round window was exposed by using a posterior tympanotomy approach. The bone covering the round window was removed and thus the round window membrane could be identified and opened. The oCI was then fully inserted into the scala tympani and fixed in position with surgical cement. All samples have been fixed with agarose in cylindrical jars of suitable diameters according to the so-called HiP-CT protocol24. Due to movement during transport and different requirements to the sample holder, the samples needed to be remounted in new plastic cups between measurements with the inhouse μ-CT and the BM18 beamtime.
Beamline BM18, EBS, ESRF
The source of the BM18 beamline28 is a tripole wiggler with a fixed gap. In the central pole (1.56 T), there is higher energy than in the side poles (0.85 T) and this can be exploited to tune the beam intensity and lateral profile on the sample either to measure at a lower intensity or to accommodate for absorption gradients in the sample during off-axis tomography. The polychromatic energy can be tuned between 50 and 280 keV by polished attenuators (i. a. C, SiO2, Al2O3, Al, Ti, Cu, Mo, Ag, W, Au) of different thicknesses. Taking into account the transmission of the entire optical pathway including the sample and the scintillator an effective energy Eeff can be calculated which corresponds roughly to a monochromatic energy. The 350 mm wide beam at the sample position and different detector configurations allow voxel sizes in the range of 0.7 μm to 80 μm and sample sizes up to 50 cm vertically by 30 cm in diameter for 30 kg maximum. The future large sample stage should make possible to accommodate samples of 2.5 m vertically by 1.4 m in diameter for 300 kg maximum. For image acquisition we used the PCO edge 4.2 with pixelsize dx = 6.5 μm equipped with a threefold Hasselblad tandem optics (100 mm/300 mm) and a 200 μm LuAG:Ce scintillator. Further we used the IRIS 15 detector with dx = 4.25 μm equipped with a zoom optic or demagnifying zoom (dzoom) optic and 0.1 mm or 2 mm LuAG:Ce scintillators respectively. The tomographic scans have been either performed with the rotation axis placed in the middle of the detector or with the rotation axis shifted to one side of the detector (so-called half acquisition). Half acquisition is used when the sample is larger than the effective detector width and allows almost twice the FOV in a 360° scan.
The empty beam correction has been carried out either in air for small samples or in the sample environment (jar and solvent) without the sample (so-called Hierarchical Phase-Contrast Tomography or HiP-CT25). Image reconstruction was performed using PyHST2 (ESRF), using the Paganin algorithm32 for phase retrieval and performing a subsequent tomographic reconstruction with the filtered backprojection. Parameters for each scan are summarized in Tables 2 and 3.
In-house μ-CT
Tomographic scans were acquired using a μ-CT instrument (EasyTOM, RX Solutions) equipped with a microfocus source (Hamamatsu L12161-07, W Target, 5–50 μm spotsize) and a flat panel detector (1440 px × 1704 px, dx = 127 μm). As above, empty beam correction has been carried out either in air for small samples or in the sample environment for large samples. The tomographic cone beam reconstruction was performed with the software of the instrument. The acquisition parameters and the parameters of the reconstruction volume for the data presented here are tabulated in Table 4.
Segmentation and rendering
The segmentation with the thresholding and region growing tools as well as the rendering of these segmentations has been performed with VGStudio Max (Hexagon AB, Stockholm, Sweden). The renderings and the animation have been created with Siemens Cinematic Anatomy (Siemens Healthineers AG, Erlangen, Germany). The renderings of the stained cochlea have been created using the 3D volumetric visualization framework NVIDIA Index (NVIDIA Corporation, Santa Clara, CA, USA).
Data availability
The 3D volume data will be available upon publication at GRO.data (https://doi.org/10.25625/V3YSYD).The raw beamtime data will become available 2026 at ESRF (dataset https://doi.org/10.15151/ESRF-ES-1223491086).
References
Mei, X. et al. Vascular supply of the human spiral ganglion: novel three-dimensional analysis using synchrotron phase-contrast imaging and histology. Sci. Rep. 10, 5877 (2020).
Vogl, C., Neef, J. & Wichmann, C. Methods for multiscale structural and functional analysis of the mammalian cochlea. Mol. Cell. Neurosci. 120, 103720 (2022).
Keppeler, D. et al. Multiscale photonic imaging of the native and implanted cochlea. Proc. Natl. Acad. Sci. 118, e2014472118 (2021).
Thylur, D. S., Jacobs, R. E., Go, J. L., Toga, A. W. & Niparko, J. K. Ultra-high-field magnetic resonance imaging of the human inner ear at 11.7 Tesla. Otol. Neurotol. 38, 133 (2017).
Müller, B. et al. Anatomy of the murine and human cochlea visualized at the cellular level by synchrotron-radiation-based micro-computed tomography. In Developments in X-ray Tomography V, vol. 6318, 26–34, https://doi.org/10.1117/12.680540 (SPIE, 2006).
Rau, C., Hwang, M., Lee, W.-K. & Richter, C.-P. Quantitative X-ray tomography of the mouse cochlea. PLoS ONE 7, e33568 (2012).
Bartels, M., Hernandez, V. H., Krenkel, M., Moser, T. & Salditt, T. Phase contrast tomography of the mouse cochlea at microfocus X-ray sources. Appl. Phys. Lett. 103, 083703 (2013).
Töpperwien, M. et al. Propagation-based phase-contrast X-ray tomography of cochlea using a compact synchrotron source. Sci. Rep. 8, 4922 (2018).
Richter, C.-P. et al. Fluvastatin protects cochleae from damage by high-level noise. Sci. Rep. 8, 3033 (2018).
Richter, C.-P. et al. Evaluation of neural cochlear structures after noise trauma using X-ray tomography. In Developments in X-ray Tomography IX, vol. 9212, 213–219, https://doi.org/10.1117/12.2062385 (SPIE, 2014).
Schaeper, J. J., Liberman, M. C. & Salditt, T. Imaging of excised cochleae by micro-CT: staining, liquid embedding, and image modalities. J. Med. Imaging 10, 053501 (2023).
Glueckert, R. et al. Visualization of the membranous labyrinth and nerve fiber pathways in human and animal inner ears using MicroCT imaging. Front. Neurosci. 12, 501 (2018).
Elfarnawany, M. et al. Micro-CT versus synchrotron radiation phase contrast imaging of human cochlea. J. Microsc. 265, 349–357 (2017).
Koch, R. W., Elfarnawany, M., Zhu, N., Ladak, H. M. & Agrawal, S. K. Evaluation of cochlear duct length computations using synchrotron radiation phase-contrast imaging. Otol. Neurotol. 38, e92–e99 (2017).
Li, H. et al. Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Sci. Rep. 11, 4437 (2021).
Li, H. et al. Unlocking the human inner ear for therapeutic intervention. Sci. Rep. 12, 18508 (2022).
Li, H. et al. Vestibular organ and cochlear implantation—a synchrotron and micro-CT Study. Front. Neurol. 12, 663722 (2021).
Iyer, J. S. et al. Visualizing the 3D cytoarchitecture of the human cochlea in an intact temporal bone using synchrotron radiation phase contrast imaging. Biomed. Opt. Express 9, 3757–3767 (2018).
Schmutzhard, J. et al. The cochlea in fetuses with neural tube defects. Int. J. Dev. Neurosci. 27, 669–676 (2009).
Schmutzhard, J. et al. Pelizaeus Merzbacher disease: morphological analysis of the vestibulo-cochlear system. Acta Oto-Laryngol. 129, 1395–1399 (2009).
Helpard, L., Li, H., Rask-Andersen, H., Ladak, H. M. & Agrawal, S. K. Characterization of the human helicotrema: implications for cochlear duct length and frequency mapping. J. Otolaryngol. - Head. Neck Surg. = Le. J. D.’oto-Rhino-Laryngologie Et. De. Chirurgie Cervico-Facial. 49, 2 (2020).
Li, H. et al. Synchrotron radiation-based reconstruction of the human spiral ganglion: implications for cochlear implantation. Ear Hearing 41, 173–181 (2020).
Sismono, F. et al. Synchrotron radiation X-ray microtomography for the visualization of intra-cochlear anatomy in human temporal bones implanted with a perimodiolar cochlear implant electrode array. J. Synchrotron Radiat. 28, 327–332 (2021).
Brunet, J. et al. Preparation of large biological samples for high-resolution, hierarchical, synchrotron phase-contrast tomography with multimodal imaging compatibility. Nat. Protoc. 18, 1441–1461 (2023).
Walsh, C. L. et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat. Methods 18, 1532–1541 (2021).
Ackermann, M. et al. The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling. eBioMedicine 85, 104296 (2022).
Xian, R. P. et al. A multiscale X-ray phase-contrast tomography dataset of a whole human left lung. Sci. Data 9, 264 (2022).
Cianciosi, F., Buisson, A.-L., Tafforeau, P. & Van Vaerenbergh, P. BM18, the new ESRF-EBS beamline for hierarchical phase-contrast tomography. In: Proc. 11th Mechanical Engineering Design of Synchrotron Radiation Equipment and Instrumentation, Jul 2021, Chicago, United States. pp.MOIO02 (2021).
Brunet, J. et al. Multidimensional analysis of the adult human heart in health and disease using hierarchical phase-contrast tomography (HiP-CT). Radiology 2024 312:1, https://doi.org/10.1148/radiol.232731 (2023).
Rahmani, S. et al. Micro to macro scale analysis of the intact human renal arterial tree with Synchrotron Tomography, https://doi.org/10.1101/2023.03.28.534566 (2023).
Xian, R. P. et al. A closer look at high-energy X-ray-induced bubble formation during soft tissue imaging. J. Synchrotron Radiat. 31, 566–577 (2024).
Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R. & Wilkins, S. W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40 (2002).
Salditt, T. et al. Compound focusing mirror and X-ray waveguide optics for coherent imaging and nano-diffraction. J. Synchrotron Radiat. 22, 867–878 (2015).
Frohn, J. et al. 3D virtual histology of human pancreatic tissue by multiscale phase-contrast X-ray tomography. J. Synchrotron Radiat. 27, 1707–1719 (2020).
Cloetens, P. et al. Holotomography: quantitative phase tomography with micrometer resolution using hard synchrotron radiation X rays. Appl. Phys. Lett. 75, 2912–2914 (1999).
Zabler, S., Cloetens, P., Guigay, J.-P., Baruchel, J. & Schlenker, M. Optimization of phase contrast imaging using hard X rays. Rev. Sci. Instrum. 76, 073705 (2005).
Quesnel, A. M. et al. Computed tomography density as a bio-marker for histologic grade of otosclerosis: a human temporal bone pathology study. Otol. Neurotol. 43, e605 (2022).
Geerardyn, A., Zhu, M., Verhaert, N. & Quesnel, A. M. Intracochlear trauma and local ossification patterns differ between straight and precurved cochlear implant electrodes. Otol. Neurotol. 45, 245 (2024).
Schaeper, J. J. et al. Replication data for: 3D imaging of the human temporal bone by X-ray phase-contrast tomography. GRO.data. https://doi.org/10.25625/V3YSYD (2024).
Liberman, M. C., Dodds, L. W. & Pierce, S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J. Comp. Neurol. 301, 443–460 (1990).
Campenhausen, E. et al. ESRF beamtime MD1374: 3d virtual histology of the human cochlea. European Synchrotron Radiation Facility. https://doi.org/10.15151/ESRF-ES-1223491086 (2026).
Acknowledgements
The experiment was carried out under approved proposal MD1374 at the beamline BM18 at ESRF, France (dataset https://doi.org/10.15151/ESRF-ES-1223491086)41. We thank Jakob Reichmann, Sarah Damerow and Eva Freiin von Campenhausen for help with sample embedding and measurements during the beamtime. Further, we thank René Müller for expert technical assistance in dissecting temporal bones. We also acknowledge fruitful interaction within the Human Organ Atlas Hub (HOAHub) Initiative. The research was funded by EXC 2067/1-390729940: Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells of the Deutsche Forschungsgemeinschaft (DFG). Important instrumental developments at BM18 have been funded by the Chan Zuckerberg Initiative (CZI).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.J.S. and T.S. designed and carried out the X-ray experiments. Human temporal bones and cochleae have been provided by T.M., C.A.K., C.S., C.T. and M.C.L. C.K. implanted one of the human temporal bones with an oCI. P.T. performed image reconstruction, J.J.S. data analysis. T.M., C.S. and M.C.L. supervised the work on samples, T.S. the imaging. J.J.S. and T.S. prepared the manuscript, with contributions from all authors. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schaeper, J.J., Tafforeau, P., Kampshoff, C.A. et al. 3D imaging of the human temporal bone by X-ray phase-contrast tomography. npj Imaging 3, 21 (2025). https://doi.org/10.1038/s44303-025-00086-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44303-025-00086-y