Abstract
Carrier transport and recombination at the buried interface of perovskite have seriously restricted the further development of inverted perovskite solar cells (PSCs). Herein, an interfacial dipolar chemical bridge strategy to address this issue is presented. 2-(Diphenylphosphino) acetic acid (2DPAA) is selected as the linker to reconstruct the interfacial dipole, which effectively enlarges the interfacial dipole moment to 5.10 D and optimizes to a positive dipole orientation, thereby accelerating vertical hole transport, suppressing nonradiative recombination and promoting the perovskite crystallization. The champion inverted device yields a high power conversion efficiency (PCE) of 26.53% (certified 26.02%). Moreover, this strategy is extended to the wide-bandgap perovskite and large-area devices, which delivers high PCEs of 22.02% and 24.11%, respectively. The optimized devices without encapsulation also demonstrate great long-term shelf and operational stability. Our work highlights the importance of interfacial dipole moment and orientation at the buried interface to realize efficient and stable inverted PSCs.
Similar content being viewed by others
Introduction
Among various photovoltaic (PV) technologies, organic-inorganic hybrid perovskite solar cells (PSCs) have the greatest potential because of their high absorption coefficient, long carrier diffusion length, low exciton binding energy and large-scale solution preparation1,2. Owing to the rapid development of self-assembled monolayers (SAMs), inverted (p-i-n structure) PSCs have surpassed normal (n-i-p structure) PSCs in terms of both power conversion efficiency (PCE) and device stability3. Currently, the highest PCE of inverted PSCs is certified over 26%, which is attributed to solving some limitations, such as disordered assembly of SAMs and small interfacial dipole moment4. Jen et al. employed a solvent washing strategy to remove excess molecules from the initial coating or solution incubation, which reduced the degree of molecular aggregation on the surface5. Recently, Yan et al. adopted a Co-SAM strategy (mixing phosphorylcholine chloride and (4-(3,6-dimethyl-9H-carbazol-9-yl)butyl)phosphonic acid (Me-4PACz)) to improve the monolayer coverage and reduce the leakage current6. In addition, the small interfacial dipole moment could be enlarged by manipulating the molecular structure of SAMs. Zhu et al. introduced a noncoplanar screw-shaped dibenzo(c,g)carbazole (DBC) unit as the core to balance the negative effect from the dipole moment of the methoxy group. Thus, a SAM ((4-(3,11-dimethoxy-7H-dibenzo(c,g)carbazol-7-yl)butyl)phosphonic acid, MeO-4PADBC) with a large dipole moment (2.4 D) was obtained to improve the band alignment and minimize the energy loss7. Following this, Jen et al. adjusted the dipole moment (3.93 and 4.29 D) and the Lewis basicity by incorporating cyclic disulfide or two flanking thiomethyl groups into the SAM, resulting in a suitable energetic alignment and high-quality crystallization of the perovskite8. It was obvious that these strategies could efficiently improve open-circuit voltage (VOC) and fill factor (FF) of PSCs. However, researchers were mostly concerned molecular arrangement and dipole moment, ignoring the deflecting orientation of the overall interfacial dipole, which always pointed to hole transport layer (HTL) due to the chemisorption model of SAMs9. As reported, the physical property of the substrate surface and the buried perovskite film were affected by the interfacial dipole orientation to some extent, such as the work function10. Especially, the buried perovskites were trend to be a n-type feature due to the amorphous perovskite and uncontrolled surface termination types, which impeded carrier transport and aggravated interfacial carrier recombination11,12. The misalignment of dipole orientation at the buried interface would exacerbate this problem, thus having a potential negative impact on the device performance and stability.
The total dipole pointed towards the perovskites called “positive dipole”, whereas “negative dipole” represented the total dipole pointed outside the perovskites10. A positive dipole orientation usually shifted the local vacuum level upwards, thereby causing an increase in the work function of the buried perovskite surface and adjusting it to be a more p-type feature13,14,15. This was beneficial for carrier separation and transport at the buried interface. In contrast, a negative dipole orientation had the opposite effects. The rational interfacial dipole orientation at the buried interface should be the positive dipole, thus efficiently ordering the interfacial band energy alignment and enlarging the VOC10,14. However, the influence of interfacial dipole orientation still has not been perceived to date on accelerating charge transport and improving photovoltaic performance, especially for the buried perovskite surface. Therefore, obtaining an optimal dipole orientation even with a larger interfacial dipole moment through a simple but efficient strategy, which is desirable for further improving the efficiency and reproducibility of inverted PSCs, is still a challenge.
In this study, we introduce a feasible interfacial dipolar chemical bridge of 2-(diphenylphosphino) acetic acid (2DPAA) into the buried interface of perovskites to reconstruct the interfacial dipole. The pre-buried 2DPAA strongly anchors on the SAM surface via phosphorylation and chemically link to the perovskite through a coordination effect, which effectively enlarge the interfacial dipole moment to 5.10 D. To our excited, this modulation changes the interfacial dipole orientation to a desirable direction with a positive dipole, which has never been reported to date. This reconstructed interfacial dipole also accelerates hole transport, releases the interfacial residual stress, induces high-quality perovskites and reduces trap-assisted nonradiative recombination at the buried interface. As a result, the champion device achieves an impressive PCE of 26.53% (certified 26.02%), with a remarkable VOC of 1.197 eV. Moreover, this strategy is extended to wide-bandgap and large-area (1 cm2) devices, resulting in PCEs of 22.02% and 24.11%, respectively. The unencapsulated devices treated with 2DPAA also present attractive long-term shelf and operational stability. More than 95.4%, 81.2% and 94.3% of their initial PCEs are retained after long-term storage in a N2-filled glove box, continuous thermal ageing and light soaking at the maximum power point for 1200 h, respectively.
Results
Effects of interfacial dipole reconstruction
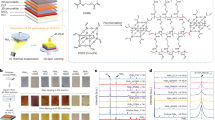
As had been proven, functional molecules containing –P, –N, –S and –O negative electron units could effectively passivate Lewis acid defects (such as undercoordinated Pb2+) by forming coordination covalent bonds, where the binding energy between –P units and the perovskite was the strongest16. Considering the high planarity and symmetry of the carbazole structure in SAMs, we thus selected non-coplanar diphosphate Lewis-base molecules, such as ethyldiphenylphosphine (EDPP) and 2DPAA, to serve as the interfacial dipolar chemical bridge7. We first employed density functional theory (DFT) to study the electrostatic potential (ESP) of both molecules. The electron-rich units of the carboxyl (–COOH) group and P atom in 2DPAA showed a negative ESP (red), whereas EDPP had only a single P atom to donate electrons (Fig. 1a and Supplementary Fig. 1). In addition, the model molecule of 2DPAA with –COOH could theoretically interact with the exposed hydroxyl (–OH) group via phosphorylation to strongly anchor on the surface of SAMs, which could enhance the adhesion and strengthen the mechanical toughness of PSCs compared with those of PSCs with EDPP17. We used a single-molecule structure to simulate the molecule characteristics18. The corresponding dipole moment of Me-4PACz deposited on the substrate was calculated to be 1.61 D (Fig. 1b), whereas that of Me-4PACz/2DPAA was 5.10 D, indicating that the interfacial dipole moment was enhanced when the substrate was covered by 2DPAA. This would induce a downward shift of the Fermi level (EF) of the buried perovskite surface19. Moreover, the dipole orientation could also be determined from the individual molecular model, the Me-4PACz/2DPAA compound exhibited a more vertical positive orientation pointing towards the perovskite film due to the fact that Lewis base groups in 2DPAA bonded with Pb2+ cations to afford a positive dipole9. This result was conducive to obtaining larger work function and accelerating transfer of holes from the perovskite layer to the HTL10,20.
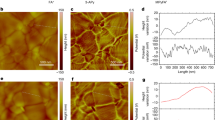
a Molecular structure, gaussian calculated ESP and molecular dipole of 2DPAA. b Gaussian structure optimized interfacial dipole moment and orientation of the Me-4PACz and Me-4PACz/2DPAA sample. c Schematic diagram of Me-4PACz adsorption on the substrate with and without 2DPAA treatment. d The optimal conformation of the bimolecular model adsorbed on the substrate (the arrow is the whole dipole orientation). e, f KPFM images of the control and 2DPAA-treated perovskite film at the buried interface. g CPD changes of both buried perovskite films.
To reflect the arrangement of SAMs on the substrate, we employed the density functional theory (DFT) to calculate the total system energy (Etotal) within various dipole arrangement conditions and further compare the energy difference (∆Ediff), where the Etotal represented the energy required for the molecules to adsorb on the substrate and ∆Ediff = EtotalTarget - EtotalBlank. Following the principle of minimum energy7,21, the smaller ∆Ediff means that SAMs with the target configuration is more easily adsorbed on the substrate compared with the blank configuration. The arrangement of SAMs on the substrate were affected by the steric hindrance, π-π interaction, dipole interaction and so on22,23,24,25. Considering the complexity and irregularity of molecular arrangement, we have simplified the calculation model and used a bimolecular model to represent the self-assembly behaviour of Me-4PACz on the NiOx substrate3. The spatial arrangement of molecules could be categorized into two extreme configurations, one configuration was the atomic structures of Me-4PACz adsorbed in a perpendicular (Perp) orientation, whereas the other configuration was adsorbed in a planar or parallel (Para) orientation5. The Perp orientation contained an upward and downward configuration, whereas the Para orientation had a right and left configuration (Supplementary Fig. 2). As shown in Supplementary Fig. 3, the representative spatial configuration of the bimolecular model could be summarized into six types. We additionally assumed that Me-4PACz was anchored on the substrate through only a single active site (Fig. 1c), so that the unbonded –OH group would interact with 2DPAA when exposed to the surface. In general, configuration 1-1 was chosen to be the blank configuration for Me-4PACz. The corresponding ∆Ediff of various bimolecular models was calculated to be 0.015, 1.08, 0.57, −0.046 and 0.43 eV for the configuration 2-1, 3-1, 4-1, 5-1 and 6-1 (Supplementary Fig. 4), respectively. It was obvious that the spatial configuration 5-1 had the lowest ∆Ediff, indicative of the arrangement of Me-4PACz on the substrate was more inclined to be formed this configuration, which was in line with the reported results5,7,21. We then calculated the Etotal and ∆Ediff when covered by 2DPAA (Supplementary Fig. 5). As expected, the value of ∆Ediff for the configuration 5-2 was significantly lower than that of other configurations (Supplementary Fig. 6). The whole dipole orientation of the optimal bimolecular model (Fig. 1d) was calculated to be a positive orientation, which was in agreement with the individual molecular orientation.
Additionally, we measured X-ray reflectivity (XRR) to further reflect the actual arrangement of Me-4PACz on the substrate3. Supplementary Fig. 7a shows the reflectivity curves, the corresponding fit-derived electron density (ρe) profiles and schematic of the Me-4PACz arranged on the substrate. We obtained the actual configuration and arrangement of Me-4PACz that extracted from the ρe of this system. Supplementary Fig. 7b shows a characteristic minimum in ρe of 0.22 e Å−3 attributed to the stretched alkyl chain with the anchor group, whereas the minimum ρe of 0.35 e Å−3 attributed to the carbazolyl headgroup26. The length of the stretched alkyl chain with the anchor group was identified as 9.3 Å, which was not reached the theoretical length27,28. It was suggested that Me-4PACz was anchored on the substrate with an oblique orientation, which was approximately configuration 5-1. The corresponding tilt angle (α) of Me-4PACz was ~54.3° (the α of SAMs was calculated from the equation: α=cos-1(t/L), where t is the film thickness, L is the molecular length, Supplementary Note 1) relative to the substrate surface29. This result was matched well with the bimolecular model that the interfacial dipole orientation was pointed outside the buried perovskites. Overall, both theoretical and experimental results demonstrated that the initial dipole featured a negative orientation derived from the oblique arrangement of Me-4PACz, but the interfacial dipole orientation was turned into the positive orientation after the reconstruction of the interface dipole by 2DPAA.
To reveal the contribution of the reconstructed interfacial dipole, Kelvin probe force microscopy (KPFM) was adopted to probe the vibration of the surface potential of the buried perovskite film with and without 2DPAA treatment, namely, the control and 2DPAA samples, respectively (Fig. 1e, f and Supplementary Fig. 8)30. According to contact potential difference (CPD) measurements of the buried perovskite surface, the control film exhibited a higher CPD ≈ −260 mV, whereas the 2DPAA-treated film surface had a lower CPD (-525 mV). This meant that the 2DPAA-treated sample had a larger work function and stronger p-type feature, which were favourable for interfacial band alignment and hole transport31. Otherwise, the distribution of the CPD in the 2DPAA-treated sample was smoother than that in the control sample (Fig. 1g), which could be attributed to the enhanced uniformity of the surface potential arising from reconstruction of the interfacial dipole.
Ultraviolet photoelectron spectroscopy (UPS) together with ultraviolet-visible (UV-vis) absorption spectroscopy was further employed to investigate the band alignment at the buried interface (Supplementary Figs. 9 and 10)32. Fig. 2a shows the corresponding EF, valence band maximum (EVB) and conduction band minimum (ECB) of different samples. The energy differences between EVB and EF were calculated to be 1.39 eV and 1.03 eV for the control and 2DPAA-treated sample at the buried perovskite surface, respectively. The results indicated that the 2DPAA-treated sample had a more p-type surface than the control sample derived from the enlarger interfacial dipole moment and the positive dipole orientation, which was in line with the KPFM results18. We also investigated the physical characteristic of the substrate with 2DPAA treatment. The value of the conductivity (Supplementary Fig. 11 and Supplementary Note 2) and transmittance (Supplementary Fig. 12) for NiOx/Me-4PACz/2DPAA film was approximate to the result of NiOx and NiOx/Me-4PACz film. In addition, the reconstructed interfacial dipole with a positive dipole orientation had a opposite effect on the substrate, which could reduce the work function of the Me-4PACz/2DPAA film. Supplementary Fig. 13 shows the corresponding band energy alignment for the buried substrate film with and without 2DPAA treatment, where the effects on the interfacial carrier transport barrier were negligible due to the energy differences of the work function was only 0.05 eV. The influences of the enlarged interfacial dipole moment and the positive dipole orientation on the buried perovskite surface were more important than that those on the substrate (Supplementary Fig. 14), which was more conducive to the separation and transfer of light-generated holes for the buried perovskite.
a Energy level diagram of different films at the buried interface. b Partial enlarged FT-IR spectra of Me-4PACz, 2DPAA and Me-4PACz/2DPAA sample. c H NMR Liquid-state 1H nuclear magnetic resonance (NMR) spectra for Me-4PACz, 2DPAA and Me-4PACz/2DPAA (solvent: DMSO-d6) film. d The high-resolution P 2p orbitals for Me-4PACz, 2DPAA and Me-4PACz/2DPAA film. e, f High resolution of FT-IR spectra for PbI2, 2DPAA and 2DPAA + PbI2 film. g High resolution of XPS spectra of Pb 4 f for PbI2 and 2DPAA + PbI2 film. h, i AFM-IR morphology and the corresponding 1725.7 cm−1 (yellow) absorption images of the NiOx/Me-4PACz/2DPAA film.
Fourier transform infrared (FT-IR) spectroscopy was conducted to demonstrate the interaction of 2DPAA with Me-4PACz and the perovskite33. Fig. 2b and Supplementary Fig. 15 show that the carbonyl (C = O) stretching vibration peak derived from ester groups (–COOR) appeared at 1729 cm-1, which was clearly different from the C = O stretching peak derived from –COOH groups (1695 cm-1), indicating that 2DPAA could efficiently interact with the exposed –OH at the buried interface when Me-4PACz was assembled in a disordered manner on the substrate34. The corresponding reaction equation for the phosphorylation of 2DPAA with Me-4PACz was drawn in Supplementary Fig. 16, in which the compound 2 might not appear in the actual film preparation but present in the solution reaction. The shift of the characteristic proton signal from the –COOH group (δ = 12.4 ppm) in the liquid-state hydrogen proton nuclear magnetic resonance (1H NMR) spectrum, further confirmed the interaction between 2DPAA and Me-4PACz (Fig. 2c and Supplementary Fig. 17)35. X-ray photoelectron spectroscopy (XPS) was employed to determine the interactions at the buried interface36. As illustrated in Fig. 2d and Supplementary Fig. 18, the characteristic peak of P 2p at 134.18 eV corresponded to the C–O–P chemical bond, verifying the occurrence of phosphorylation between Me-4PACz and 2DPAA after annealing37. In addition, the IR stretching vibration peak of P–C shifted from 1431 to 1435 cm-1 (Fig. 2e and Supplementary Fig. 19), and the stretching vibration peak of C = O also shifted from 1695 to 1705 cm−1 (Fig. 2f), indicating that both –P and –COOH in 2DPAA could interact with the undercoordinated Pb2+ at the buried perovskite surface38. Meanwhile, the characteristic peak of Pb 4 f significantly shifted to a lower binding energy (Fig. 2g and Supplementary Fig. 20), which was mainly attributed to the interaction between 2DPAA and the undercoordinated Pb2+39.
High resolution mass spectrometry (HRMS) was subsequently used to verify the phosphorylation reaction. Since it was measured in mixed solution, biactive –OH sites of SAMs were all theoretically anchored to 2DPAA, and the final product tended to be compound 2. The m/z of [M-H]- calculated for C46H43NO5P3 was 782.24, which could be found in 782.2422 m/z (Supplementary Fig. 21)40. This result could effectively confirm the formation of compound 2, which was also the direct evidence of phosphorylation. Additionally, FT-IR was used to investigate the stretching vibration peak derived from ester groups for the NiOx/Me-4PACz, NiOx/2DPAA and NiOx/Me-4PACz/2DPAA film. Supplementary Fig. 22 shows the ester groups only appeared in NiOx/Me-4PACz/2DPAA film at 1725.7 cm−1, which is consistent with the ester group characteristic peak (Supplementary Fig. 23) of the commercial carbonic ester (diphenyl carbonate). These results all demonstrated the occurrence of phosphorylation between Me-4PACz and 2DPAA. To verify the feasibility of interfacial dipole reconstruction. We used the atomic force microscopy-infrared spectroscopy (AFM-IR) to obverse the IR stretching peak recorded at 1725.7 cm−1 corresponding to the ester group41,42. Figure 2h, i shows the homogeneous ester signal for NiOx/Me-4PACz/2DPAA film, which occupied over 50% of the total area, whereas the NiOx/Me-4PACz film has no signal (Supplementary Fig. 24). This result not only demonstrated the occurrence of phosphorylation between Me-4PACz and 2DPAA but also confirmed that the interfacial dipole was efficiently reconstructed by 2DPAA treatment.
As reported, there were several types of SAMs anchoring on the substrate, including double-bonded type, single-bonded type and unbonded type (Supplementary Fig. 25). The unbonded type of Me-4PACz was belong to the downward configuration, which was easily washing away due to the weak physical adsorption, whereas the double-bonded and single-bonded types of Me-4PACz were all belong to the upward configuration, which could be retained on the substrate surface due to strong chemisorption22,43. The double-bonded type was mainly depended on the number of –OH groups on the substrate surface and the single-bond type was dominant if the –OH group was insufficient. To exclude the effect of the unbonded type, we used IPA solution to wash away the downward configuration of Me-4PACz, and measured the corresponding FT-IR spectrum of the substrate with and without IPA washing. The corresponding PO32− stretching vibration peak (Supplementary Fig. 26a) at 1069 cm−1 was still existed, indicative of Me-4PACz was still strongly anchored on the substrate even after IPA cleaning process and the amount of the downward configuration was small41. XPS spectrum was further conducted to distinguish the single-bonded or double-bonded type of Me-4PACz. The characteristic peak of P 2p at 132.7 eV corresponding to the P–OH or at 133.8 eV corresponding to the P = O (Supplementary Fig. 26b), which was represented as the single-bonded mode and the double-bonded mode, respectively44,45. It was obvious that the single-bonded type of Me-4PACz was one of the main adsorption modes, which matched well with the results of FT-IR and AFM-IR. Those results demonstrated that the reconstruction of interfacial dipole was mainly happened between the single-bonded type of Me-4PACz and 2DPAA, instead of the rare downward configuration. Overall, 2DPAA efficiently bridged SAMs and the buried perovskite, thus reconstructing the buried interfacial dipole, which was consistent with the theoretical results. This strategy efficiently enhanced the adhesion between the SAM and perovskite, and reduced interfacial defects, which was very conducive to the formation of high-quality perovskite films.
Morphologies and crystallinities of perovskite films
To verify the effect of the restructured interfacial dipole on the quality of perovskite films, in-situ UV-vis absorption spectroscopy was performed to study the crystallization kinetics of perovskite films with and without 2DPAA treatment. Owing to the strong chemical interaction between 2DPAA and PbI2, the nucleation rate was significantly reduced in the process of saturated precipitation crystallization for the 2DPAA-treated sample (Fig. 3a, b and Supplementary Fig. 27), which was favourable for achieving a larger grain size and lower redundant PbI26. We then peeled off the buried interface (Supplementary Fig. 28) and observed an obvious increase in the grain size (Fig. 3c, d) along with a smaller roughness (Supplementary Fig. 29) based on the scanning electron microscopy (SEM) and atomic force microscopy (AFM) images of the perovskite films12. For the top perovskite film, AFM and SEM images showed that the 2DPAA-treated perovskite films also exhibited a larger grain size along with a smaller roughness compared with the control film (Supplementary Figs. 30 and 31). Depth-resolved grazing-incidence wide-angle X-ray scattering (GIWAXS) was further conducted to evaluate the crystallinity of perovskite films with and without 2DPAA treatment46. The diffraction rings at q values of 1 and 2 Å−1 corresponded to the (100) and (200) crystal planes of the α-phase perovskite, where the 2DPAA-treated perovskite film exhibited brighter diffraction rings than the control film (Fig. 3e, f)47. The results indicated that the crystallinity of the 2DPAA-treated perovskite was improved, which was well consistent with the enhancement of the X-ray diffraction (XRD) peaks (Supplementary Fig. 32). As confirmed, the crystal orientation of the perovskite films with 2DPAA treatment was regulated that along (110) crystal plane, which was benefit to forming high-crystallinity and high-quality perovskite films7,33. Additionally, grazing-incidence X-ray diffraction (GIXRD) was employed to explore the depth-dependent crystal structure information for the (200) plane of the perovskite. Supplementary Fig. 33 shows the results obtained at different ψ values from 0.2° to 1.4° for the samples with and without 2DPAA treatment. The diffraction peak at 2θ = 28.2° for the control film significantly shifted towards a smaller angle, whereas that for the 2DPAA-treated film slightly shifted. This result indicated that the residual strain could be released after the introduction of 2DPAA into the buried interface48. The value of σM was subsequently calculated (Fig. 3g, Supplementary Fig. 34 and Supplementary Note 3) by fitting 2θ = sin2ψ. The 2DPAA-treated film had a negative slope of −0.018, corresponding to a σM of 7.43 MPa. In contrast, the slope of the control film was −0.071, corresponding to a larger σM of 29.18 MPa. The results indicated that the surface residual stress of the perovskite film was efficiently released by the 2DPAA treatment, which was also the reason for the generation of high-quality perovskite films.
a, b In-situ UV-vis of perovskite films deposited on the control and 2DPAA-treated substrate. c, d Buried-viewed SEM images of the control and 2DPAA-treated perovskite films. e, f GIWAXS images of perovskite film on the control and 2DPAA-treated substrate, respectively. g The linear fit 2θ-sin2ψ curves corresponding to the GIXRD spectra for both devices.
Photovoltaic performance
To verify the effects of the interfacial dipole reconstruction strategy on the PV performance, we used a perovskite with a bandgap of 1.55 eV to fabricate inverted PSCs (Fig. 4a and Supplementary Fig. 35). As shown in Supplementary Fig. 36 and Supplementary Table 1, the concentration of 2DPAA was adjusted from 0 to 0.8 mg mL−1, and the optimal concentration was determined to be 0.5 mg mL−1. Fig. 4b shows that the control device exhibited a PCE of 24.02%, with VOC = 1.147 V, short-circuit current (JSC) = 25.89 mA cm−2, and FF = 80.88%. In contrast, the champion PCE of the device with 0.5 mg mL−1 2DPAA treatment significantly increased to 26.53% (certified 26.02%, Supplementary Fig. 37), with a VOC of 1.197 V, a JSC of 25.94 mA cm−2 and an FF of 85.43%, which is one of the highest efficiencies reported for inverted PSCs to date. The hysteresis effect index (HEI) for the best 2DPAA-treated device (Supplementary Fig. 38 and Supplementary Note 4) was only 4.2%, which was much lower than that of the control device (12.4%)49. The results of the frequency-dependent capacitance in devices were confirmed that the accumulation of carriers at the interface might be the main reason for the hysteresis in our devices50. The observed capacitive response at low frequencies (1 Hz) for the 2DPAA-treated device was significantly weaker than that of the control device (Supplementary Fig. 39), which was in good agreement with the reduced HEI. We measured the external quantum efficiency (EQE) spectra for both devices (Fig. 4c). The corresponding integrated JSC values were calculated to be 25.46 and 25.69 mA cm−2 for the control and 2DPAA-treated devices, respectively, which well matched the JSC values extracted from current density-voltage (J-V) curves51. Additionally, the 2DPAA-treated device showed a stable power output efficiency (SPO) of 26.22% at 1.06 V (Fig. 4d), which was better than that of the control device (23.64% at 1.02 V, Supplementary Fig. 40). A certified SPO efficiency of 25.75% was achieved after maximum output power point (MPP) tracking for 500 s under continuous AM 1.5 G illumination (Supplementary Fig. 41). To confirm the reproducibility, we prepared 40 individual PSCs for the different conditions (Fig. 4e). The EDPP-treated devices only exhibited slight improvements in the PV parameters, which derived from the weak interfacial interaction, whereas the 2DPAA-treated devices showed significant improvements in the PV parameters (Supplementary Fig. 42), especially in VOC and FF. To further verify the advantages of 2DPAA in improving the VOC and FF of devices, we subsequently fabricated wide-bandgap (1.68 eV) inverted PSCs and large-area devices via a similar strategy. The wide-bandgap (1.68 eV) PSCs with 2DPAA treatment achieved the best PCE of 22.02%, with a VOC of 1.24 V (Fig. 4f). The corresponding work function of the buried perovskite surface based on the wide-bandgap perovskite was increased from 4.45 eV to 4.87 eV (Supplementary Fig. 43), as well as the built-in voltage (Vbi) of the wide-bandgap device was also greatly increased from 1.12 eV to 1.18 eV (Supplementary Fig. 44), which derived from the enlarged interfacial dipole moment and the positive dipole orientation. On the other side, the PCE of the large-area (1 cm2) device also reached a high PCE of 24.11% (Fig. 4g). There was also a noticeable performance improvement when different SAMs were adopted (Fig. 4h, i). The results suggested that 2DPAA was an efficient and universal functional molecule for realizing interfacial dipole reconstruction to obtain high-efficiency inverted PSCs with a minimal VOC loss. To better reflect the advantages of this interfacial dipole reconstruction strategy, we prepared a series of blank samples, such as Co-SAM (Supplementary Fig. 45), only isopropyl alcohol solution (Supplementary Fig. 46) and only 2DPAA (Supplementary Fig. 47). All of these results followed the same trend that the device with 2DPAA treatment was significantly outperformed to that of these blank samples, which was attributed to the reconstructed interfacial dipole that could enlarge dipole moment and regulate dipole orientation.
a Schematic diagram of device structure. b J-V curves of the champion narrow-bandgap PSCs with and without 2DPAA treatment. c EQE curves and the corresponding integrated photocurrent densities for both devices. d Stabilized output efficiency of the 2DPAA-treated device at the maximum output power point under simulated 1 sun illumination. e The statistical distribution curves of PCE for both devices. f, g J-V curves of the champion wide-bandgap and large area PSCs with and without 2DPAA treatment. h, i J-V curves of the best performing PSCs of various SAMs with and without 2DPAA treatment, respectively.
To analyse the reasons for the improved PV performance of the devices in depth, Mott-Schottky (M-S) curves were first employed to extract the value of Vbi. Compared with the control device (1.10 V), the 2DPAA-treated device achieved a larger Vbi of 1.14 V (Fig. 5a). This result indicated that the driving force for charge-carrier separation and transport was enhanced due to the enlarged interfacial dipole moment and regulated interfacial dipole orientation, which was beneficial for achieving high VOC and FF52. We then performed transient photocurrent (TPC) measurements to quantify the average charge transit time (τave) after charge generation (Fig. 5b and Supplementary Table 2). τave decreased from 4.93 μs for the control device to 2.89 μs for the 2DPAA-treated device. Based on the above results, the corresponding stepped-up vertical transit mobilities (Supplementary Table 3 and Supplementary Note 5) were calculated to be 0.9 × 10-3 and 1.49 × 10-3 cm2 V−1 s−1, respectively, revealing a more efficient hole transport process along the out-of-plane direction for the 2DPAA-treated devices, which was account for the positive interfacial dipole orientation and thus providing an additional accelerating field53. In addition, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) were further employed to study the carrier transport dynamics. Fig. 5c shows faster PL quenching for the 2DPAA-treated film, indicative of the enhanced hole transport along with reduced nonradiative recombination17. The PL lifetimes of the perovskite films for the control and 2DPAA samples were calculated by fitting the TRPL decays (Fig. 5d, Supplementary Table 4 and Supplementary Note 6). The average PL decay lifetime of the 2DPAA-treated film reflected a longer carrier lifetime (121.3 ns) than that of the control film (69.0 ns)54. Similar results were also obtained by measuring the photocurrent density (Jph) versus the effective voltage (Veff)50. The Jph/Jsat (here, Jsat is the saturation photocurrent density) ratio for the 2DPAA-treated device was greater than that for the control device, whether under short-circuit conditions or at the maximum power point (Fig. 5e, Supplementary Table 5 and Supplementary Note 7), which could be attributed to the improved crystallinity, optimized band alignment and reduced interfacial defects55.
a M-S plots for the control and 2DPAA device. b TPC curves of the control and 2DPAA-treated device. c, d PL and TRPL spectra of the control and 2DPAA-treated perovskite film. e Characteristics of photocurrent density versus effective voltage (Jph-Veff) on a double logarithmic scale. f Light intensity dependence on VOC plots for both devices. g EIS spectra for both devices (inset: equivalent circuit). h TPV decay for the control and 2DPAA-treated device. i EL spectra of the control and 2DPAA-treated device.
In addition to the enhancement of hole extraction and transport, trap-assisted nonradiative recombination is another key factor determining the device performance. The light intensity dependence of the J-V characteristics was subsequently conducted. Fig. 5f and Supplementary Fig. 48 show the VOC and JSC versus logarithm of the light density curves, respectively. The 2DPAA-treated device exhibited a slope of 1.30 kT/q (here, k is Boltzmann’s constant, T is the temperature, and q is the elementary charge), whereas the control device had a larger slope of 1.86 kT/q (Supplementary Note 8). This result indicated that the nonradiative recombination at the buried interface was efficiently suppressed by 2DPAA treatment56. As confirmed by the results of the space charge-limited current (SCLC) method, the corresponding trap-state density (Supplementary Fig. 49 and Supplementary Note 9) for the 2DPAA-treated device was calculated to be 2.22 × 1015 cm−3 by fitting the I-V curve in the trap-filling limited region (II), which was significantly lower than that of the control device (3.88 × 1015 cm−3)57. We also performed electrochemical impedance spectroscopy (EIS) and fitted the spectra with the corresponding equivalent circuit models (Fig. 5g and Supplementary Table 6)58. The series resistance (Rs) and recombination resistance (Rrec) for the control device were 13.10 Ω and 513 Ω, whereas the 2DPAA-treated device exhibited a lower Rs (5.45 Ω) but a higher Rrec (886 Ω). The results indicated that carrier transport was faster and carrier recombination was more difficult in the 2DPAA-treated device than those in the control device, which was partially due to the additional driving force derived from the regulated interfacial dipole moment and orientation55,59. Similar results were also obtained based on the transient photovoltage (TPV) decay (Fig. 5f and Supplementary Table 7). Compared with the control device (13.90 μs), the device treated with 2DPAA had a prolonged photovoltage decay time (34.54 μs). Fig. 5i and Supplementary Table 8 show the external quantum efficiencies of electroluminescence (EQE-EL) of both devices, with EQEEL values of 0.54% and 1.59% for the control device and the 2DPAA-treated counterpart, respectively. The resulting EQEEL could further be transformed into ∆V3 (VOC loss from the nonradiative recombination) values of 134 and 106 mV, respectively60. The corresponding values of the three components of the VOC loss (∆V1, ∆V2 and ∆V3) were calculated based on detailed balance theory (Supplementary Note 10)61. The 2DPAA-treated device showed a minimum VOC loss of 358 mV, which is one of the lowest values reported to date for inverted PSCs with a bandgap of 1.55 eV. In short, the excellent PV performance of the as-modified inverted PSCs was mainly attributed to the following aspects: 1) The interfacial dipole moment and orientation were regulated by 2DPAA treatment, thereby improving the band alignment and accelerating hole transport at the buried interface. 2) The functional groups of 2DPAA efficiently interacted with perovskite defects, thus inhibiting trap-assisted nonradiative recombination and reducing the VOC loss. 3) The collaborative optimization of the interfacial dipole and buried defects effectively released residual strain and induced the formation of high-quality perovskite films.
Stability of perovskite films and devices
The stability of the PSCs under various conditions was evaluated. Compared with the control film, the perovskite film treated with 2DPAA had an increased surface water contact angle (Supplementary Fig. 50), which would protect the perovskite from moisture erosion. As shown in Fig. 6a, the 2DPAA-treated perovskite film remained in the black phase after 7 days (relative humidity (RH) ranging from 60% to 80% and a temperature of 25 °C), whereas the control film had completely degraded. The stability is also related to ion migration (Fig. 6b, c and Supplementary Note 11). Therefore, the corresponding migration activation energy (Ea) of the 2DPAA-treated device was calculated, and a value of 1.51 eV was obtained, which was higher than that of the control device (1.03 eV), indicating the inhibited ion migration behaviour in the modified device62. We then studied the shelf stability for both devices, 95.4% of the initial efficiency was retained for the unencapsulated 2DPAA-treated device after 1200 h of storage in a N2-filled glove box (Fig. 6d), with the T90 (T90 is the time for PSCs to degrade to 90% of their initial efficiency) value linearly extrapolated to of 4450 h. The thermal stability of the devices was measured in a N2-filled glove box at 85 °C (Supplementary Fig. 51), where the unencapsulated 2DPAA-treated device maintained 81.2% of its initial efficiency, outperforming the control device (59.2%). Additionally, we evaluated the operational stability of both devices under continuous MPP tracking. The unencapsulated 2DPAA-treated device maintained 94.3% of its initial efficiency after 1200 h of MPP tracking (Fig. 6e), whereas the control device only maintained 63.2% of its initial efficiency. The improved device stability was attributed to the high-quality perovskite film with fewer interfacial defects and released residual stress.
a The pictures of the control and 2DPAA-treated perovskite films aged in ambient air with RH of 60% ~ 80% and temperature of 25 °C. b, c Temperature-dependent conductivity of the control and 2DPAA-treated device. d The long-term shelf stability for the control and 2DPPA-treated device. e Dynamic MPP tracking of the unsealed control and 2DPAA-treated device under 1 sun illumination (white light LED).
Discussion
In this work, we presented an interfacial dipole reconstruction strategy to enlarge the interfacial dipole moment up to 5.10 D and simultaneously regulate the interfacial dipole orientation, thus providing a desirable dipole, by inserting 2DPAA as an interfacial dipolar chemical bridge. Theoretical and experimental results confirmed that the reconstruction of the interfacial dipole could optimize the band alignment and induce high-quality perovskite films, thus accelerating vertical hole transport and inhibiting nonradiative recombination at the buried interface. As a result, the 2DPAA-treated device delivered a champion PCE of 26.53% (certified 26.02%) for a narrow-bandgap (1.55 eV) perovskite. Additionally, PCEs of 22.02% and 24.11% were realized for a wide-bandgap (1.68 eV) and a large-area (1 cm2) inverted PSCs, respectively. Moreover, the optimized PSCs exhibited excellent stability even after long-term storage, continuous thermal ageing and MPP tracking, demonstrating great application potential in the future. Our results highlight the effectiveness of the buried interfacial dipole reconstruction strategy in realizing the highly efficient and stable inverted PSCs.
Methods
Materials
All chemicals were used as received: Lead(II) chloride (PbCl2, 99.9%), formamidinium iodide (FAI, 99.5%), lead(II) iodide (PbI2, 99.999%), cesium iodide (CsI, 99.99%) and magnesium fluoride (MgF2, 99.99%) were obtained from Xi’an Yuri Solar Co., Ltd. Nickel oxide nanoparticles (NiOx, 99.999%), fullerene-C60 (99.9%) and bathocuproine (BCP) were obtained from Advanced Election Technology Co., Ltd. (4-(3,6-Dimethyl-9H-carbazole-9-yl)butyl) phosphonic acid (Me-4PACz, 99%) was purchased from TCI (Shanghai) Development Co., Ltd. Ethyldiphenylphosphine (EDPP, 98%), 2-(diphenylphosphino) acetic acid (2DPAA, 97%), N,N-dimethylformamide (DMF, 99.9%), dimethyl sulfoxide (DMSO, 99.9%), anisole (99.7%) and isopropyl alcohol (IPA, 99.5%) were purchased from Adamas-beta.
Perovskite precursors
(1) Narrow-bandgap perovskite (FA0.95Cs0.05PbI3): 0.8 mmol CsI, 1.6 mmol PbI2, 0.08 mmol PbCl2, 0.2 mmol MACl and 1.52 mmol FAI were added in 1 mL DMF and DMSO (4:1, v/v) mixture solution, stirring at room temperature until 6 h, and then filtered by a 0.22 polytetrafluoroethylene membrane before use. (2) Wide-bandgap perovskite (Cs0.05(FA0.77MA0.23)0.95Pb(I0.77Br0.23)3): FAI, MABr, PbI2, PbBr2 and CsI were added in DMF and DMSO (4:1, v/v) mixture solvent with 2% excess of PbX2 (X=Br, I), which was stirred at room temperature overnight and then filtered by a 0.22 polytetrafluoroethylene membrane before use.
Device fabrication
Glass/FTO substrates were successively cleaned by detergent, deionized water, and ethanol by ultrasonication for 20 min in each solvent. Then, Glass/FTO substrates were blown with nitrogen gas. The cleaned FTO substrates were then treated with UV ozone for 15 min before use. After that, NiOx (10 mg mL−1 in H2O) solution was dynamically spin-coated on the FTO substrate at 2000 rpm for 30 s and annealed in air at 150 °C for 10 min. Me-4PACz (0.5 mg mL−1 in ethanol) was subsequently deposited on the NiOx substrate at 3000 rpm for 30 s and annealed in glove box at 100 °C for 10 min. Afterwards, 150 μL of IPA solution was dynamically spin-coated on Me-4PACz substrate to wash away the disordered molecular arrangement. Then, 60 μL of EDPP or 2DPAA solutions with different concentration in IPA were dynamically spin-coated on the substrate at 5000 rpm for 25 s and annealed at 100 °C for 5 min. For the deposition of narrow-bandgap perovskite layers, perovskite precursor solutions were deposited on the substrates by a consecutive two-step spin-coating process at 1000 and 5000 rpm for 8 and 25 s, respectively. At last, 150 µL of anisole was dropped onto the substrates at 10 s. A similar consecutive two-step spin-coating process at 1000 and 4000 rpm for 5 and 25 s (anisole was dropped at 5 s), respectively, was presented for the deposition of wide-bandgap perovskite layers. The as-prepared perovskite films were annealed at 120 °C for 15 min. Then, propane-1,3-diammonium iodide (0.5 mg mL−1 in IPA) solution was dynamically spin-coated on perovskite at 4500 rpm for 25 s and annealed at 100 °C for 10 min. C60 (20 nm), BCP (6 nm) and Ag (100 nm) were sequentially deposited onto the perovskite films by vacuum thermal evaporation (ca. 10−4 Pa) to end the device fabrication. In order to enhance the absorption of perovskite layer, 100 nm MgF2 layer was evaporated onto the front surface of FTO glass substrates.
Characterization
The surface morphology of perovskite film and cross-section were recorded on scanning electron microscope (SEM, thermo scientific Apreo 2 C). Topography images of perovskite films were recorded by using atomic force microscope (AFM, Bruker NanoIR3). The water contact angles (CA) of perovskite films were tested by SDC-350. The crystallinity was measured via X-ray diffraction (XRD) on Bruker AXS D8 Advance with a Co-K X-ray irradiation source operated at 40 kV and 40 mA. Fourier Transform Infrared (FT-IR) spectra were obtained from Vertex 70 infrared spectrometer (Bruker company, transmission mode, RES = 2 cm−1, scanning time = 16, scanning range = 400–2000 cm−1.). UV-vis absorption spectra were tested on the UV-3600i Plus. Photoluminescence (PL) and Time-resolve PL (TRPL) spectra were measured by PicoQuant FluoTime 510 nm laser excitation. X-ray photoelectron spectra were recorded on a Thermo Fisher Scientific ESCALAB 250Xi with a monochromatic Al Kα X-ray source (1486.6 eV) and an X-ray beam of around 900 μm (Czech Republic). NMR spectra were recorded with Bruker Avance NMR spectrometer (400 MHz). Mass spectra were obtained from Agilent G6546AA. Electrochemical impedance spectroscopy (EIS), Mott-Schottky (MS), transient photovoltage spectrum (TPV) and transient current spectrum (TPC) were measured on Paios (Fluxim). The J-V curves were collected on the reference silicon cell calibrated solar simulator, which was integrated with Keithley model 2400 under 100 mW cm−2 illumination and a simulated AM 1.5 G spectrum. The J-V curves were measured by forward (−0.1 V to 1.5 V forward bias) or reverse (1.5 V to −0.1 V) scans with a voltage step of 20 mV and a delay time of 100 ms for each point. The EQE spectra were obtained on the Enliterch QE-R system. The Ea was measured by temperature-dependent conductivity in dark, and the value was obtained by fitting the slope of the straight line in the high temperature region. The dynamic MPP tracking of the encapsulated devices was measured at 25 °C under a white light-emitting diode lamp (100 mW cm−2) with a sixteen-channel photovoltaic maximum power point tracking test system (YH-VMPP-16).
Simulation methods
The calculations were executed utilizing density functional theory (DFT) with the projector augmented wave (PAW) method, as implemented by the Vienna Ab Initio Simulation Package (VASP). The exchange-correlation potential was parameterized by the generalized gradient approximation (GGA) in the form of the Perdew-Burke-Ernzerhof (PBE) functionals. We adopted slab models to probe the influence of impurities on diverse perovskite surfaces. For geometry optimizations and self-consistent field calculations, a plane-wave expansion cutoff of 300 eV and a 3×3×1 k-point mesh for sampling the Brillouin zone were employed. The atomic positions within the structures were optimized until the maximum residual forces were below 0.03 eV/Å. Furthermore, the van der Waals corrections to the electronic energies were integrated using the D2 method.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. All other data are available from the corresponding authors on request. Source data are provided with this paper.
References
Dong, Q. F. et al. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 347, 967–970 (2015).
Howard, I. A. et al. Coated and printed perovskites for photovoltaic applications. Adv. Mater. 31, e1806702 (2019).
Li, M. et al. Self-assembled monolayers for interfacial engineering in solution-processed thin-film electronic devices: design, fabrication, and applications. Chem. Rev. 124, 2138–2204 (2024).
Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 384, 189–193 (2024).
Liu, S. et al. Buried interface molecular hybrid for inverted perovskite solar cells. Nature 632, 536–542 (2024).
Cao, Q. et al. Co-self-assembled monolayers modified NiOx for stable inverted perovskite solar cells. Adv. Mater. 36, 2311970 (2024).
Li, Z. et al. Stabilized hole-selective layer for high-performance inverted p-i-n perovskite solar cells. Science 382, 284–289 (2023).
Jiang, W. et al. Hole-selective contact with molecularly tailorable reactivity for passivating high-performing inverted perovskite solar cells. CCS Chem. 6, 1654–1661 (2024).
Ma, Y. et al. Recent progress in interfacial dipole engineering for perovskite solar cells. Nano-Micro Lett. 15, 173 (2023).
Canil, L. et al. Tuning halide perovskite energy levels. Energy Environ. Sci. 14, 1429–1438 (2021).
Park, K. et al. Surface defect engineering of metal halide perovskites for photovoltaic applications. ACS Energy Lett. 7, 1230–1239 (2022).
Gao, Z.-W. et al. Buried interface modification in perovskite solar cells: a materials perspective. Adv. Energy Mater. 12, 2104030 (2022).
Duan, J. et al. Effect of side-group-regulated dipolar passivating molecules on CsPbBr3 perovskite solar cells. ACS Energy Lett. 6, 2336–2342 (2021).
Hu, S. et al. Optimized carrier extraction at interfaces for 23.6% efficient tin-lead perovskite solar cells. Energy Environ. Sci. 15, 2096–2107 (2022).
Ansari, F. et al. Passivation mechanism exploiting surface dipoles affords high-performance perovskite solar cells. J. Am. Chem. Soc. 142, 11428–11433 (2020).
Li, C. et al. Rational design of lewis base molecules for stable and efficient inverted perovskite solar cells. Science 379, 690–694 (2023).
Chen, Y. et al. Synergistical transition metal ion Co-doping and multiple functional additive passivation for realizing 25.30% efficiency perovskite solar cells. Energy Environ. Sci. 16, 5243–5254 (2023).
Chen, Y. et al. Ion compensation of buried interface enables highly efficient and stable inverted MA-free perovskite solar cells. Adv. Funct. Mater. 32, 2206703 (2022).
Dong, K. et al. Impact of dipole effect on perovskite solar cells. ChemSusChem 17, e202301497 (2024).
Zhang, M. et al. Reconfiguration of interfacial energy band structure for high-performance inverted structure perovskite solar cells. Nat. Commun. 10, 4593 (2019).
Zhao, K. et al. peri-Fused polyaromatic molecular contacts for perovskite solar cells. Nature 632, 301–306 (2024).
Zhang, S. et al. Minimizing buried interfacial defects for efficient inverted perovskite solar cells. Science 380, 404–409 (2023).
Berg, I. et al. Self-assembled monolayers of N-heterocyclic olefins on Au (111). Angew. Chem. Int. Ed. 62, e202311832 (2023).
Jiang, W. et al. π-Expanded carbazoles as hole-selective self-assembled monolayers for high-performance perovskite solar cells. Angew. Chem. Int. Ed. 61, e202213560 (2022).
Berman, G. P. et al. Entangled spin states in self-assembled monolayer systems. Phys. Rev. B 66, 193406 (2002).
Schmaltz, T. et al. Low-voltage self-assembled monolayer field-effect transistors on flexible substrates. Adv. Mater. 25, 4511–4514 (2013).
Schuettfort, T. et al. Observation of a distinct surface molecular orientation in films of a high mobility conjugated polymer. J. Am. Chem. Soc. 135, 1092–1101 (2013).
Khassanov, A. et al. Structural investigations of self-assembled monolayers for organic electronics: Results from X-ray reflectivity. Acc. Chem. Res. 48, 1901–1908 (2015).
Jin, H. et al. X-ray studies of self-assembled organic monolayers grown on hydrogen-terminated Si (111). Langmuir 20, 6252–6258 (2004).
Nie, J. et al. Recent progress in regulating surface potential for high-efficiency perovskite solar cells. ACS Energy Lett. 9, 1674–1681 (2024).
Chen, Q. et al. Interfacial dipole in organic and perovskite solar cells. J. Am. Chem. Soc. 142, 18281–18292 (2020).
Chen, Y. et al. Design of an inorganic mesoporous hole-transporting layer for highly efficient and stable inverted perovskite solar cells. Adv. Mater. 30, 1805660 (2018).
Yang, J. et al. Crystallization tailoring of cesium/formamidinium double-cation perovskite for efficient and highly stable solar cells. J. Energy Chem. 48, 217–225 (2020).
Wang, Q. et al. Tailored succinic acid-derived molecular structures toward 25.41%-efficiency and stable perovskite solar cells. Adv. Mater. 36, 2307709 (2024).
Ji, X. et al. Target therapy for buried interface enables stable perovskite solar cells with 25.05% efficiency. Adv. Mater. 35, 2303665 (2023).
Chen, Y. et al. Reducing carrier transport barrier in anode interface enables efficient and stable inverted mesoscopic methylammonium-free perovskite solar cells. Chem. Eng. J. 425, 131499 (2021).
Bi, Z. et al. Structural evolution of phosphorus species on graphene with a stabilized electrochemical interface. ACS Appl. Mater. Inter. 11, 11421–11430 (2019).
Tan, Q. et al. Inverted perovskite solar cells using dimethylacridine-based dopants. Nature 620, 545–551 (2023).
Cheng, C. et al. A novel organic phosphonate additive induced stable and efficient perovskite solar cells with efficiency over 24% enabled by synergetic crystallization promotion and defect passivation. Nano Lett. 23, 8850–8859 (2023).
Zhou, H. et al. Glycol monomethyl ether-substituted carbazolyl hole-transporting material for stable inverted perovskite solar cells with efficiency of 25.52%. Angew. Chem. Int. Ed. 63, e202403068 (2024).
Park, S. M. et al. Low-loss contacts on textured substrates for inverted perovskite solar cells. Nature 624, 289–294 (2023).
Deng, J. et al. Side-group-mediated small molecular interlayer to achieve superior passivation strength and enhanced carrier dynamics for efficient and stable perovskite solar cells. Adv. Funct. Mater. 34, 2309484 (2024).
Wu, J. et al. Bisphosphonate-anchored self-assembled molecules with larger dipole moments for efficient inverted perovskite solar cells with excellent stability. Adv. Mater. 36, 2401537 (2024).
Wang, K. et al. Adsorption of phosphorylated chitosan on mineral surfaces. Colloid Surf. A 436, 656–663 (2013).
Granja, P. L. et al. Cellulose phosphates as biomaterials. II. Surface chemical modification of regenerated cellulose hydrogels. J. Appl. Polym. Sci. 82, 3354–3365 (2001).
Wu, X. et al. Backbone engineering enables highly efficient polymer hole-transporting materials for inverted perovskite solar cells. Adv. Mater. 35, 2208431 (2023).
Huang, Z. et al. Anion-π interactions suppress phase impurities in FAPbI3 solar cells. Nature 623, 531–537 (2023).
Li, Y. et al. In-situ-synthesized low-dimensional perovskite for >25% efficiency stable MA-free perovskite solar cells. Adv. Mater. 36, 2310711 (2024).
Meng, H. et al. Inhibition of halide oxidation and deprotonation of organic cations with dimethylammonium formate for air-processed p-i-n perovskite solar cells. Nat. Energy 9, 536–547 (2024).
Chen, Y. et al. Multilayer cascade charge transport layer for high-performance inverted mesoscopic all-inorganic and hybrid wide-bandgap perovskite solar cells. Sol. RRL 4, 2000344 (2020).
Yu, S. et al. Homogenized NiOx nanoparticles for improved hole transport in inverted perovskite solar cells. Science 382, 1399–1404 (2023).
Yu, X. et al. Green-solvent processable dopant-free hole transporting materials for inverted perovskite solar cells. Angew. Chem. Int. Ed. 62, e202218752 (2023).
Xu, H. et al. Constructing robust heterointerfaces for carrier viaduct via interfacial molecular bridges enables efficient and stable inverted perovskite solar cells. Energy Environ. Sci. 16, 5792–5804 (2023).
Chen, Y. et al. Interfacial contact passivation for efficient and stable cesium-formamidinium double-cation lead halide perovskite solar cells. iScience 23, 100762 (2020).
Yuan, R. et al. Boosted charge extraction of NbOx-enveloped SnO2 nanocrystals enables 24% efficient planar perovskite solar cells. Energy Environ. Sci. 14, 5074–5083 (2021).
Lv, Y. et al. High-efficiency perovskite solar cells enabled by anatase TiO2 nanopyramid arrays with an oriented electric field. Angew. Chem. Int. Ed. 59, 11969–11976 (2020).
Azmi, R. et al. Double-side 2-dimensional/3-dimensional heterojunctions for inverted perovskite solar cells. Nature 628, 93–98 (2024).
Tang, W. et al. Acetone-assisted precursor engineering enables low-temperature fabrication of CsPbI2Br perovskite for efficient solar cells. J. Power Sources 482, 228965 (2021).
Shi, Y. et al. Interfacial dipoles boost open-circuit voltage of tin halide perovskite solar cells. ACS Energy Lett. 9, 1895–1897 (2024).
Li, Z. et al. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 376, 416–420 (2022).
He, J. et al. Surface chelation of cesium halide perovskite by dithiocarbamate for efficient and stable solar cells. Nat. Commun. 11, 4237 (2020).
Wang, M. et al. Uracil induced simultaneously strengthening grain boundaries and interfaces enables high-performance perovskite solar cells with superior operational stability. Adv. Mater. 36, 2306415 (2024).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2022YFB4200500), the National Natural Science Foundation of China (Grant Nos. 22409020, 22379101 and 22075190), the Natural Science Foundation of Sichuan Province (Grant Nos. 2024NSFSC1163 and 2024NSFSC0001) and the Discipline Leading Innovation Team Project of Chengdu University of Technology (11400-000525-08).
Author information
Authors and Affiliations
Contributions
Y.C. conceived the idea and proposed the experimental design. Y.P. and Y.C. performed the device fabrication and the technology innovation. Y.P. characterized the material samples (AFM, SEM, KPFM, in-situ UV-vis and MPP test, etc.). C.L. and J.Z. assisted with the fabrication of wide-bandgap devices and J-V measurements. Y.W.D. and W.J.T. helped perform the GIWAXS, GIXRD, PL and TRPL measurements. Y.C. drafted the original manuscript. Y.C., Y.H.W. and Q.P. helped with the manuscript preparation. Y.C. and Q.P. supervised the project. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Chen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, Y., Chen, Y., Zhou, J. et al. Enlarging moment and regulating orientation of buried interfacial dipole for efficient inverted perovskite solar cells. Nat Commun 16, 1252 (2025). https://doi.org/10.1038/s41467-024-55653-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55653-5