Abstract
Transmembrane AMPA receptor regulatory proteins (TARPs) are claudin-like proteins that tightly regulate AMPA receptors (AMPARs) and are fundamental for excitatory neurotransmission. With cryo-electron microscopy (cryo-EM) we reconstruct the 36 kDa TARP subunit γ2 to 2.3 Å, which points to structural diversity among TARPs. Our data reveals critical motifs that distinguish TARPs from claudins and define how sequence variations within TARPs differentiate subfamilies and their regulation of AMPARs.
Similar content being viewed by others
Introduction
Information transfer in the brain occurs at specialized cellular junctions known as synapses, which act as neuronal communication hubs1. Most synapses are glutamatergic, where a pre-synaptic neuron releases glutamate (Glu), and a post-synaptic neuron receives Glu. AMPARs in the post-synaptic membrane bind Glu and initiate depolarization of the post-synaptic neuron through their Glu-gated cation channels1,2. TARPs are auxiliary subunits that regulate the trafficking, gating kinetics, and pharmacology of AMPARs2,3.
TARP regulatory subunits tightly regulate AMPAR function in the post-synaptic membrane, a critical aspect of the brain’s ability to fine-tune information processing1,2,3. There are six TARP subtypes (TARPγ2, γ3, γ4, γ5, γ7, γ8), split into type-I (TARPγ2, γ3, γ4, γ8) and type-II (TARPγ5, γ7) families. Generally, TARPs increase the conductance of AMPARs and enhance susceptibility to pharmacological channel block4,5, but type-I TARPs slow desensitization and deactivation kinetics, while type-II TARPs appear to have a negative effect on gating when compared to type-I TARPs2. Furthermore, structural differences between TARPs in the same class underlie sensitivity to certain classes of drugs targeted to AMPAR-TARP complexes6. Since the first TARP was identified a quarter century ago (TARPγ2, also known as stargazin)7, TARPs have been recognized as a crucial component of synaptic function1,8. While many foundational functional and cryo-EM studies have driven the field forward, the precise structural details of how TARPs regulate AMPARs remain ambiguous.
Cryo-EM studies of TARP subunits have advanced our understanding of TARP structure in the context of AMPAR complexes, but the intermediate resolution has historically precluded de novo building of TARP structures6,9,10,11,12,13,14,15,16,17. X-ray crystallography structures of TARP homologs, such as claudins, have been indispensable for modeling TARPs18. Claudins are cellular junction proteins that form paracellular barriers between epithelial and endothelial cells and are functionally distinct from TARPs19. The reliance on claudin structures for TARP modeling has hampered the identification of distinct structural features that (1) differentiate TARPs from claudins and (2) explain the regulatory potential of TARPs for AMPARs.
Here, we use cryo-EM to determine the structure of the prototypical TARP, TARPγ2. We identify motifs in TARPγ2 that distinguish TARP classes from one another and further differentiate TARPs from claudins. These structural features likely underlie modulatory effects exhibited by TARPs on AMPAR gating.
Results
Structure of TARPγ2
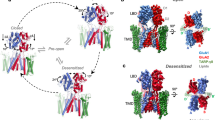
With cryo-EM, we reconstructed TARPγ2 to an overall resolution of 2.3 Å (2.0 Å–2.5 Å locally; Supplemental Fig. 1). Our data enables us to build most of the transmembrane ___domain (TMD) and extracellular ___domain (ECD) de novo (Fig. 1a and Table 1). The high resolution of our reconstruction enables identification of multiple distinct structural features in the TARPγ2 extracellular ___domain (ECD), which sits atop its tetraspanin transmembrane (TM) helical bundle comprised of transmembrane (TM) helices TM1-4 (Fig. 1a). The ECD is comprised of a five-stranded β-sheet and a single extracellular helix (ECH) that immediately precedes TM2. A previously identified disulfide bridge (DSB) between β3 (C67) and β4 (C77) strands in the ECD stabilizes the TARPγ2 ECD (Fig. 1b) and is conserved across all TARPs and the TARP-like claudins.
a Cryo-EM map of TARPγ2, colored rainbow from N-terminus, NT (blue) to C-terminus, CT (red). b Extracellular portion of the TARPγ2 model showing the β3-β4 DSB, loop anchor DSB, and TARP cleat. c Cartoon schematic of TARPγ2 structure highlighting key structural features that rigidify the entire ECD atop the tetraspanin TMD, colored as in (a).
We identify two moieties in our reconstruction of TARPγ2 that distinguish TARPs from claudins. First, a π-π-π stack, which we term the TARP cleat motif, secures the TARPγ2 ECD atop the TARPγ2 TMD (Fig. 1b). The cleat motif is formed by H60 (from β2), Y32 (TM1-β1 loop), and W178 (TM4). We also observe a second DSB in the ECD. This DSB, the loop anchor DSB, anchors the β1-β2 loop onto the β-sheet (Fig. 1b). The loop anchor DSB is made between C40 in the β1-β2 loop and C68 on β3. Altogether, these motifs rigidify the structure of TARPγ2 by providing additional structural interactions within the ECD and between the ECD and TMD (Fig. 1c).
Conservation of TARP features
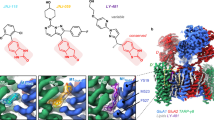
The TARP cleat motif is conserved in all TARPs and the TARP-like subunit germline-specific gene 1-like (GSG1L) (Fig. 2a) but is absent from all claudins (Supplemental Fig. 2). We also evaluated the conservation of the cleat motif through AlphaFold220 structure prediction. This suggests that the TARP cleat motif is present in all mammalian TARPs (Supplemental Fig. 3a). Interestingly, while the TARP cleat motif is conserved in all TARPs, the loop anchor DSB is not (Fig. 2a). Structure prediction in AlphaFold2 (Supplemental Fig. 3b) also points to the loop anchor DSB being conserved in type-I TARPs but not in type-II TARPs. Thus, while our structure pointed us to look at the conservation of the cleat motif and loop anchor DSB, this was already predicted by AlphaFold2 (Supplemental Fig. 3c).
a Multiple sequence alignment demonstrating the relative conservation of the TARP Cleat Motif, β3-β4 DSB, and Loop Anchor DSB between TARP family members. Intensity of the shade of purple represents the percent identity. Type-II TARPs are labeled in green. Loop Anchor DSB is unique to type-I and excluded from type-II TARPs. b Alignment of TARPγ2 structure in cyan with other TARP family members (TARPγ3, pink, PDB: 8C2H; TARPγ5, green, PDB: 7RZ5; TARPγ8, purple, PDB: 8AYN; GSG1L, red, PDB: 7RZ9). c Zoomed in view of TARP extracellular domains illustrating differing orientations in the β1-β2 loops. d View of the TARP cleat motif illustrating conservation among all TARP family members. e Model of predicted β1-β2 loop orientations between type-I and type-II TARPs illustrating distinct potential contacts between TARP subtypes and AMPARs.
Surprisingly, the TARP cleat motif and loop anchor DSB are within previous TARP structures but not identified. Previously determined structures of TARPs are overall like our structure of TARPγ2 (Fig. 2b), and the loop anchor DSB is within structures of TARPγ321 and TARPγ86,15,22, and even previously published structures of TARPγ210. However, it is absent, as expected, in the structure of the type-II TARP, TARPγ523,24 (Fig. 2c) and the TARP-like subunit GSG1L11,23 (Fig. 2c). In contrast, the TARP cleat motif is conserved in all TARPγ3, γ5, and γ8 subunit structures as well as GSG1L6,21,23 (Fig. 2d). We hypothesize that these structural details and their conservation were previously missed because of a lack of structural resolution.
The dichotomy in β1-β2 loop organization between type-I and type-II TARPs has significant functional implications. For example, type-II TARPs lack the loop anchor DSB and have been observed to directly interact with AMPAR subunits that are in the A and C positions when they occupy the “X” auxiliary subunit site11,23 (Fig. 2e). However, we expect that this is not possible for type-I TARPs in the “X” site given the presence of the loop anchor DSB, which locks in the β1-β2 loop in an orientation away from the A and C AMPAR subunit positions. However, if a type-I TARP occupies the “Y” TARP position (Fig. 2e), modulation of the AMPAR at subunit positions B or D by the β1-β2 loop is likely possible despite the loop anchor DSB, and is supported by observations in cryo-EM studies of type-I TARPs in complex with AMPARs21. Given the conformational changes associated with AMPAR gating, the presence or absence of the loop anchor DSB within type-I TARPs versus type-II TARPs could explain differences observed in electrophysiology experiments between chimeric constructs of the β1-β2 loop in type-I and type-II TARPs.
Role of loop anchor DSB in AMPAR regulation
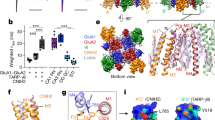
Based on this idea, we hypothesized that ablation of the loop anchor DSB from TARPγ2 would impair the modulatory nature of TARPγ2 on AMPARs. To test this hypothesis, we employed a concatenated cDNA construct encoding the AMPAR subunit GluA2 fused to TARPγ2 and a fluorescent GFP marker25 (Fig. 3a, Methods). Transfection of this cDNA into Expi293 cells produced functional receptors that showed whole-cell AMPAR responses consistent with the characteristic modulation of AMPAR gating by TARPγ2 (Fig. 3b). Interestingly, disrupting the TARPγ2 loop DSB by mutating C40 and C68 to serine (ΔDSB) resulted in reduced peak currents, likely attributed to decreased trafficking of the mutant channels to the cell surface. (Fig. 3b, c).
a Schematic representation of the GluA2 (blue)-TARPγ2 (cyan) fusion construct used for electrophysiology demonstrating the ___location of the ΔDSB mutation (pink). b Representative traces, c current densities (two-tailed Welch’s t-test, p = 0.0026), d percentages of desensitization (two-tailed Welch’s t-test, p = 0.0014) and e time constants of desensitization kinetics of AMPARs complexed with either WT or ΔDSB TARPγ2 during a 500 ms exposure to 1 mM Glutamate (two-tailed Welch’s t-test, p = 0.33). WT, black, n = 13; ΔDSB, pink, n = 9; Bars represent mean ± SEM; **p < 0.01. Source data are provided as a Source Data File.
To accurately measure desensitization, we focused on our analysis on channels with a peak current more than 200 pA. Perturbing the loop anchor DSB with ΔDSB substitutions increases the fraction of desensitized receptors compared to WT (Fig. 3d), indicating that the loop anchor DSB is a major contributor to the ability of TARPγ2 to enhance AMPAR activation. However, desensitization kinetics were unchanged in ΔDSB receptor complexes compared to WT TARPγ2-containing receptor complexes (Fig. 3e), akin to what has been previously reported for deletion of the entire β1-β2 loop11,26. We, therefore, conclude that the loop DSB is critical for the function of type-I TARPs, given that the loss of this single disulfide linkage phenocopies the loss of the entire β1-β2 loop.
Given this result, we considered why the loop anchor DSB might be critical to the type-I TARP function. Previous studies examining the role of the TARP extracellular domains on AMPAR modulation show that several extracellular motifs contribute to the overall modulatory function of type-I TARPs8,26,27,28 (Fig. 4). These features include (1) the β1-β2 loop, which interacts with AMPAR LBDs when TARPγ2 is at the “Y” position26,29; (2) the β4-TM2 linker, which interacts with the LBD when TARPγ2 is at the “X” position9,17,27,29; and (3) the TM3-β5 linker, which interacts with the S1-M1 and S2-M4 linkers when TARPγ2 is arranged at the “Y” position26 (Fig. 4). While the loss of any of these three features from TARPγ2 alters the population of desensitized receptors relative to WT TARPγ2, the β4-TM2 loop more profoundly influences AMPAR desensitization and modulates desensitization kinetics26,30. However, the exact roles of the TARP motifs are challenging to completely delineate because of the distinct “X” and “Y” positioning of the TARPs. Mutagenesis could affect TARPs at one site and not the other, making the functional roles not completely clear.
Cartoon representation of TARPγ2 showing extracellular features that are critical for AMPAR modulation. The β1-β2 loop, the Loop Anchor DSB, and the TM3-Β5 loop (pink) are predicted to modulate AMPAR gating from TARPs in the “Y” position and, when mutated, increase the fraction of desensitized AMPARs compared to WT TARPγ2. The β4-TM2 loop (purple) is predicted to modulate AMPAR gating from the “X” position and, in addition to regulating the fraction of desensitized AMPARs, also regulates τdes.
Discussion
To explain how these disparate structural features are all required for TARPγ2 to enhance activation via reducing the population of desensitized receptors, we propose a model in which these motifs orchestrate a network of extracellular interactions (Fig. 4). The loop anchor DSB enables the β1-β2 loop to interact with AMPARs while at the “Y” site. Loss of this DSB ablates the TARPγ2 effect on the activated state. On the other hand, the β4-TM2 loop, by which TARPγ2 interacts with AMPARs while at the “X” site, is also critical for enhancing AMPAR activation. Thus, the presence of both motifs at disparate sites around the AMPAR is critical for the effect of TARPs on AMPAR activation by limiting the population of desensitized receptors. Meanwhile, the β4-TM2 loop affects the desensitization kinetics via mechanisms that are not completely clear. This may explain how TARP stoichiometry can tune AMPAR function through differential occupancy of the “X” and “Y” sites. Supporting this idea is that type-II TARPs and GSG1L modulate AMPARs via the β1-β2 loop while occupying the “X” site. The TARP cleat fixes the orientation of the TARP ECD atop the transmembrane helical bundle. A high-resolution structural analysis of how the TARP motifs change during AMPAR gating is required to fully delineate these details.
The TARP cleat motif also plays a significant role in distinguishing TARPs from claudins. Both TARPs and claudins share the same overall structural fold (i.e., tetraspanin with a five-stranded extracellular β-sheet). However, claudins have strong oligomerization properties, where they self-oligomerize to form paracellular barriers. A similar phenomenon has not been reported for TARP proteins. We propose that the TARP cleat motif plays a role in preventing oligomerization in TARPs, enabling their complexation with AMPARs and other synaptic proteins.
In sum, we report the structure of TARPγ2, and how precise moieties in the ECD account for tune AMPAR function. In addition, we precisely define how TARPs are differentiated from claudins, which may explain the critical point of divergence between the structurally related proteins that are functionally distinct. Our findings provide a framework for future studies to understand the function of TARPs and foundations to target TARPs in structure-based drug design against AMPAR-related neurological disorders.
Methods
Image processing
The initial stages of cryo-EM sample preparation and data collection were carried out on the GluA2-TARPγ2 complex25. From this data, a 2.80 Å AMPAR-TARPγ2 local map (Supplemental Fig. 1a), symmetry expansion was used to refine the structure of TARPγ2. To achieve this, we applied C4 symmetry to the AMPAR-TARP particles (Supplemental Fig. 1a). We masked one TARPγ2 in the AMPAR-TARPγ2, then inverted this mask, and subtracted the inverted mask from all particle images. We then used the subtracted particle images, coupled with the original TARPγ2 mask (non-inverted) applied to the complete AMPAR-TARPγ2 complex cryo-EM map reference to refine the final cryo-EM reconstruction of TARPγ2 (Supplemental Fig. 1b).
Model building, refinement, and structural analysis
Coot31 was used to build a polyalanine chain into TARPγ2 map. Bulky resides from sequence information were used to anchor the building. A previously determined structure of TARPγ2 (pdb 5WEO) and a structure predicted from AlphaFold2 (AlphaFold Protein Structure Database, #AF-O88602) were used as reference. Isolde32 and Phenix33 were used to refine the model. Quality of the model was assessed with MolProbity34. Visualizations and ___domain measurements were performed in ChimeraX35. The software was compiled and accessed via the SBGrid Consortium36.
Sequence analysis
All sequence alignments were done with ClustalW37 and analyzed in Jalview38.
Structure prediction
TARP structure predictions of TARPγ2, γ3, γ4, γ5, γ7, γ8 of human, rat, mouse species were used from AlphaFold2. For each TARP subunit structure prediction, the respective amino acids corresponding to the cleat motif and disulfide bridge were determined. Cleat motif measurements were taken by calculating the distance between the Cα’s of histidine to tyrosine and Cα’s of tyrosine to tryptophan. Calculations were performed using the Biopython.PDB package.
AlphaFold2 accession numbers of models: AF-Q9Y698, AF-A0JNG9, AF-O88602, AF-Q71RJ2, AF-Q9JJV5, AF-Q0VD05, AF-O60359, AF-Q8VHX0, AF-A0A3Q1LKG2, AF-Q9JJV4, AF-Q8VHW9, AF-Q9UBN1, AF-E1BEI3, AF-Q8VHW4, AF-Q8VHW8, AF-Q9UF02, AF-E1BIG3, AF-P62956, AF-P62957, AF-P62955, AF-Q8WXS5, AF-F1MV40, AF-Q8VHW2, AF-Q8VHW5.
Electrophysiological recordings
40 ml of Expi293 Gnti- (Gibco, A39240) cells at a concentration of 1.75*106 cells/ml were transfected by mixing 8 μg GluA2-Tarpγ2 (WT) or GluA2-ΔDSB plasmid with 40 μl of polyethylenimine (PEI; Polysciences, 24765) diluted in cell culture media. Cells were incubated with DNA:PEI complexes at 37 °C for 24 h, followed by a further 48 h incubation at 30 °C to facilitate the mutant channel trafficking to the cell surface. Cells were then added to 12-mm coverslips coated with 0.1 mg/ml poly-l-lysine for recordings. Whole-cell patch-clamp recordings were performed using pulled borosilicate glass (Sutter Instrument). Pipettes with 2–5 MΩ resistance were filled with internal solution (mM): 110 CsF, 30 CsCl, 4 NaCl, 0.5 CaCl2, 10 HEPES and 5 EGTA (adjusted to pH 7.4 with CsOH). The extracellular solution (ECS) consisted of (mM): 150 NaCl, 3 KCl, 2 CaCl2 and 10 HEPES adjusted to pH 7.4 with NaOH. Cells were lifted and exposed with ECS that contained 1 mM glutamate for 500 ms intervals using the SF-77C perfusion fast-step system (Warner Instruments), which was set up as previously described39. ECS without glutamate was applied for 2 s between each 500 ms interval. Recordings were performed using Axopatch 700B amplifier and Digidata 1550B (Molecular Devices) at −60 mV hold potential and acquired at 1 kHz using pCLAMP10.7 software (Molecular Devices). Data analyses were focused on the peak currents above 200 pA to ensure an accurate measurement of desensitization and its kinetics.
The percentage of GluA2-Tarpγ2 desensitization was calculated as depicted below:
Desensitization kinetics (τdesensitization) were obtained from fitting traces to a standard first-order Chebyshev exponential with a 4-pt smoothing filter (Clampfit 10.7).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM reconstruction is deposited into the Electron Microscopy Data Bank (EMDB) at accession number EMD-43242. The structural model generated from cryo-EM is deposited in the Protein Data Bank (pdb) at accession number 8VHV. Electrophysiology data are provided as a Source Data File. Source data are provided with this paper.
References
Diering, G. H. & Huganir, R. L. The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018).
Hansen, K. B. et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharm. Rev. 73, 298–487 (2021).
Twomey, E. C., Yelshanskaya, M. V. & Sobolevsky, A. I. Structural and functional insights into transmembrane AMPA receptor regulatory protein complexes. J. Gen. Physiol. 151, 1347–1356 (2019).
Brown, P. M. G. E., McGuire, H. & Bowie, D. Stargazin and cornichon-3 relieve polyamine block of AMPA receptors by enhancing blocker permeation. J. Gen. Physiol. 150, 67–82 (2017).
Carrillo, E. et al. Memantine inhibits calcium-permeable AMPA receptors. Preprint at https://doi.org/10.1101/2024.07.02.601784 (2024).
Zhang, D. et al. Modulatory mechanisms of TARP γ8-selective AMPA receptor therapeutics. Nat. Commun. 14, 1659 (2023).
Letts, V. A. et al. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. 19, 340–347 (1998).
Tomita, S., Shenoy, A., Fukata, Y., Nicoll, R. A. & Bredt, D. S. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology 52, 87–91 (2007).
Twomey, E. C., Yelshanskaya, M. V., Grassucci, R. A., Frank, J. & Sobolevsky, A. I. Elucidation of AMPA receptor-stargazin complexes by cryo-electron microscopy. Science 353, 83–86 (2016).
Twomey, E. C., Yelshanskaya, M. V., Grassucci, R. A., Frank, J. & Sobolevsky, A. I. Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 549, 60–65 (2017).
Twomey, E. C., Yelshanskaya, M. V., Grassucci, R. A., Frank, J. & Sobolevsky, A. I. Structural bases of desensitization in AMPA receptor-auxiliary subunit complexes. Neuron 94, 569–580.e5 (2017).
Twomey, E. C., Yelshanskaya, M. V., Vassilevski, A. A. & Sobolevsky, A. I. Mechanisms of channel block in calcium-permeable AMPA receptors. Neuron 99, 956–968.e4 (2018).
Zhao, Y., Chen, S., Swensen, A. C., Qian, W.-J. & Gouaux, E. Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364, 355–362 (2019).
Yu, J. et al. Hippocampal AMPA receptor assemblies and mechanism of allosteric inhibition. Nature 594, 448–453 (2021).
Herguedas, B. et al. Mechanisms underlying TARP modulation of the GluA1/2-γ8 AMPA receptor. Nat. Commun. 13, 734 (2022).
Chen, S. et al. Activation and desensitization mechanism of AMPA receptor-TARP complex by Cryo-EM. Cell 170, 1234–1246.e14 (2017).
Zhao, Y., Chen, S., Yoshioka, C., Baconguis, I. & Gouaux, E. Architecture of fully occupied GluA2 AMPA receptor-TARP complex elucidated by cryo-EM. Nature 536, 108–111 (2016).
Suzuki, H. et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 344, 304–307 (2014).
Zihni, C., Mills, C., Matter, K. & Balda, M. S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 17, 564–580 (2016).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Zhang, D. et al. Structural mobility tunes signalling of the GluA1 AMPA glutamate receptor. Nature 1–6 https://doi.org/10.1038/s41586-023-06528-0 (2023).
Zhang, D., Watson, J. F., Matthews, P. M., Cais, O. & Greger, I. H. Gating and modulation of a hetero-octameric AMPA glutamate receptor. Nature 594, 454–458 (2021).
Klykov, O., Gangwar, S. P., Yelshanskaya, M. V., Yen, L. & Sobolevsky, A. I. Structure and desensitization of AMPA receptor complexes with type II TARP γ5 and GSG1L. Mol. Cell 81, 4771–4783.e7 (2021).
Gangwar, S. P. et al. Modulation of GluA2-γ5 synaptic complex desensitization, polyamine block and antiepileptic perampanel inhibition by auxiliary subunit cornichon-2. Nat. Struct. Mol. Biol. 30, 1481–1494 (2023).
Hale, W. D. et al. Allosteric competition and inhibition in AMPA receptors. Nat. Struct. Mol. Biol. 1–11 https://doi.org/10.1038/s41594-024-01328-0 (2024).
Riva, I., Eibl, C., Volkmer, R., Carbone, A. L. & Plested, A. J. Control of AMPA receptor activity by the extracellular loops of auxiliary proteins. eLife 6, e28680 (2017).
Dawe, G. B. et al. Distinct structural pathways coordinate the activation of AMPA receptor-auxiliary subunit complexes. Neuron 89, 1264–1276 (2016).
Cais, O. et al. Mapping the interaction sites between AMPA receptors and TARPs reveals a role for the receptor N-terminal ___domain in channel gating. Cell Rep. 9, 728–740 (2014).
Tomita, S. et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058 (2005).
Hawken, N. M., Zaika, E. I. & Nakagawa, T. Engineering defined membrane-embedded elements of AMPA receptor induces opposing gating modulation by cornichon 3 and stargazin. J. Physiol. 595, 6517–6539 (2017).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Cryst. D. 60, 2126–2132 (2004).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D. Struct. Biol. 74, 519–530 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Cryst. D. 75, 861–877 (2019).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M., Barton, G. J. Jalview Version 2 - A multiple sequence alignment editor and analysis workbench. Bioinformatics. 25, 1189–1191 (2009).
Li, R.-C., Ben-Chaim, Y., Yau, K.-W. & Lin, C.-C. Cyclic-nucleotide–gated cation current and Ca2+-activated Cl current elicited by odorant in vertebrate olfactory receptor neurons. Proc. Natl Acad. Sci. USA 113, 11078–11087 (2016).
Acknowledgements
We thank members of the Twomey and Huganir labs for insightful discussions, and Junhua Yang and Niki Gooya for technical assistance with electrophysiological recordings. All cryo-EM data was collected at the Beckman Center for Cryo-EM at Johns Hopkins with assistance from D. Sousa and D. Ding. E.C.T. is supported by National Institutes of Health (NIH) grant R35GM154904, the Searle Scholars Program (Kinship Foundation #22098168) and the Diana Helis Henry Medical Research Foundation (#142548). R.L.H. is supported by NIH grants R01 NS036715 and R01 MH112152. Z.Q. is supported by NIH grants R35 GM124824, R01 NS118014, and RF1 NS134549. W.D.H. is supported by NIH grant K99 MH132811.
Author information
Authors and Affiliations
Contributions
E.C.T. and R.L.H. supervised all aspects and planning of this research. E.C.T., A.M.R., and W.D.H. designed the project. E.C.T. and W.D.H. wrote the manuscript with input from all authors. W.D.H. prepared samples for cryo-EM, collected cryo-EM data, processed cryo-EM data, analyzed data, and built models with E.C.T. A.M.R. assisted with analysis, structure prediction, model building, and in uncovering the conserved TARP motifs. C.R.W. performed biochemistry and imaging experiments with W.D.H. critical for the manuscript review. N.K. performed the electrophysiological experiments and analyzed the data. Z.Q. supervised the electrophysiological study and wrote the results with N.K.
Corresponding authors
Ethics declarations
Competing interests
R.L.H. is a scientific cofounder and Scientific Advisory Board member of Neumora Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrew Plested, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hale, W.D., Romero, A.M., Koylass, N. et al. Structure of transmembrane AMPA receptor regulatory protein subunit γ2. Nat Commun 16, 671 (2025). https://doi.org/10.1038/s41467-025-56027-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56027-1