Abstract
Bacterial outer membrane vesicles (OMVs) are nano-sized structures derived from the outer membrane of Gram-negative bacteria, which have emerged as key players in host-pathogen interactions, yet their potential as biomarkers remains largely unexplored due to the difficulty of identification in complex biological samples. Here we show an approach for detecting and quantifying bacterial OMVs in blood using a Polymyxin B-fluorescein probe (PmBF), which targets bacterial lipopolysaccharides (LPS). The probe selectively labels OMVs, enabling their differentiation from host extracellular vesicles and quantitative analysis using nano-flow cytometry. In male mouse models of pneumonia, we observe elevated serum PmBF+ EVs as early as 6 h post-infection, preceding positive blood cultures. In clinical samples, PmBF+ EVs show superior performance for diagnosing bacterial infections and differentiate them from virus or mycoplasma infections. Our findings highlight circulating PmBF+ EVs as promising biomarkers of bacterial infections.
Similar content being viewed by others
Introduction
Bacterial infections remain a leading cause of morbidity and mortality worldwide1,2,3, especially Gram-negative strains such as Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), Pseudomonas aeruginosa (P. aeruginosa), and make up the majority of the 2024 WHO Bacterial Priority Pathogens List4. Timely and accurate diagnosis is crucial for optimal patient outcomes and appropriate antibiotic use5. However, current diagnostic biomarkers exhibit significant limitations. Host-response markers, such as C-reactive protein (CRP) and procalcitonin (PCT), often lack specificity for bacterial infections and may not show significant elevation in early or localized infections6,7. Moreover, these markers can be elevated in non-bacterial conditions, including virus infections8,9,10, mycoplasma infections11,12,13 and non-infectious inflammatory conditions14,15,16,17,18,19. Pathogen-derived markers, such as bacterial antigens and nucleic acids, offer higher specificity but can be insensitive in early stages or low bacterial load conditions20. Additionally, they may not reliably distinguish between active infection and colonization21. The exploration of more precise biomarkers is thus imperative.
Outer membrane vesicles (OMVs) are nanoscale, spherical, bilayered structures derived from the outer membrane of Gram-negative bacteria22. These vesicles are actively released by bacteria during normal growth and in response to environmental stressors, and contain a diverse array of bioactive components, including proteins, lipids, nucleic acids, and virulence factors23,24,25. Accumulating evidence suggests that OMVs play crucial roles in bacterial physiology, pathogenesis, and intercellular communication. For instance, OMVs have been shown to mediate horizontal gene transfer26,27,28, facilitate biofilm formation29,30, and promote bacterial survival under adverse conditions31,32,33. Moreover, OMVs have been implicated in the modulation of host-pathogen interactions by delivering bacterial effectors to host cells, stimulating innate immune responses, and promoting inflammation, highlighting their potential involvement in the pathogenesis of bacterial infections34,35,36,37. Even studies found that OMVs can appear at the site of bacterial infections38,39,40,41,42,43. However, their direct association with bacterial infections and their clinical utility as biomarkers remain largely unclear.
Analyzing OMVs in host biological samples is a necessary prerequisite for studying their functions in the host and verifying their potential as biomarkers. However, ideal methods are currently lacking. One major challenge is the overwhelming presence of host-derived extracellular vesicles (EVs) in biological fluids, which outnumber bacterial OMVs and share similar physicochemical properties, making differentiation difficult44. Another critical issue is the low abundance of OMVs in biological specimens, requiring highly sensitive analytical methods for reliable quantification45,46. Moreover, a huge number of impurity components with similar particle size to OMVs in the blood, especially in disease states, such as lipoproteins, will leads to erroneous readings and affects clinical validity47,48,49,50. Thus, further solutions are needed to address these complexities in biological samples. Addressing these challenges requires the development of innovative tools and approaches to comprehensively explore the roles and functions of OMVs in bacterial infections.
In this work, we develop a highly specific and sensitive method for detecting and quantifying bacterial OMVs in complex biological samples based antibiotics fluorescein probe (Polymyxin B-FITC, PmBF)). Using this approach, we demonstrate the presence of bacterial OMVs in circulation during early stages of infection, positive rate higher than blood cultures, highlighting their potential as early biomarkers for bacterial infections. These findings provide fresh insights into host-pathogen interactions and open avenues for improving early diagnosis of bacterial infections Fig. 1.
PmBF selectively tags OMVs, enabling their distinction from host extracellular vesicles and facilitating quantitative analysis via nano-flow cytometry. In male mouse models of lung infection, bacteria release OMVs into the circulation and can be detected. In clinical evaluations, PmBF+ EVs consistently demonstrate superior diagnostic performance, effectively distinguishing bacterial infections from viral or mycoplasma infections. This underscores the potential of PmBF+ EVs as reliable and biomarkers for bacterial infections (Created with BioRender.com).
Results
PmBF Specifically Recognizes and Quantitatively Detects OMVs
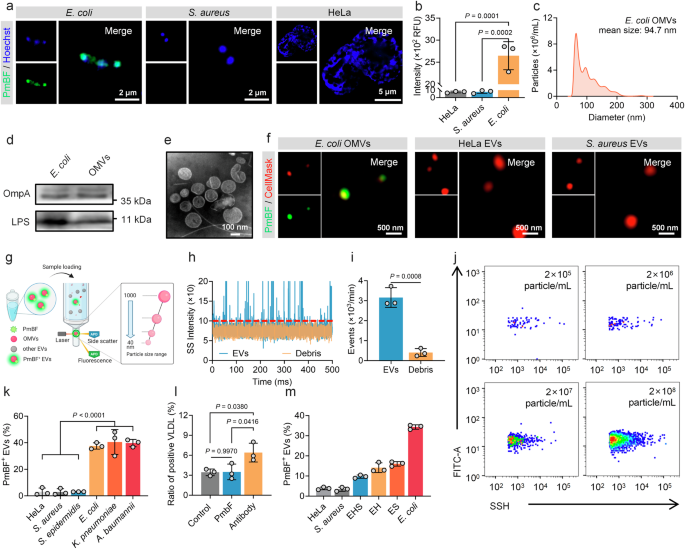
Accurate differentiation between OMVs and human EVs is a necessary prerequisite for their precise analysis. To address this challenge, we sought a molecule with high specificity for bacterial structures. Antibiotics, known for their selective targeting of microbial cells, were considered ideal options. Through preliminary literature research, Polymyxin B (PmB) emerged as a promising option due to its unique mechanism of action. This cyclic cationic polypeptide antibiotic, produced by Paenibacillus polymyxa, exerts its antibacterial effect by binding to lipopolysaccharides (LPS) on the outer membrane of Gram-negative bacteria51,52,53. We thus synthesized a fluorescent probe, PmB-FITC (PmBF), by conjugating fluorescein isothiocyanate (FITC) to the N-terminal of PmB. MALDI-TOF mass spectrometry analysis Supplementary Figs. 1a–c displayed successful coupling of PmB and FITC. Fluorescence detection and absorption spectrum of PmB before and after FITC marking similarly confirmed that the bound fluorescent probes retained their fluorescent properties Supplementary Fig. 1d. The bactericidal activity of PmBF was also evaluated, revealing a reduction in its efficacy compared to PmB Supplementary Fig. 1e. This reduction may be attributed to the structural modification introduced by FITC, which could induce conformational changes in PmB, thereby impairing its ability to insert into the bacterial membrane and exert its bactericidal function. To assess the selectivity of PmBF, we incubated it with Gram-negative bacteria (Escherichia coli, E. coli), Gram-positive bacteria (Staphylococcus aureus, S. aureus), and mammalian cells (HeLa). Super-resolution Fig. 2a and confocal images Supplementary Fig. 2 showed that PmBF signals co-localized only with E. coli, while no significant PmBF signal observed in S. aureus and HeLa. Quantitative fluorescence analysis corroborated these findings Fig. 2b, suggesting that PmBF selectively targets Gram-negative E. coli.
a Super-resolution images of Gram-negative bacteria, Gram-positive bacteria and mammal cell after incubation with PmBF for 1 h. b Fluorescence intensity of each group were detected by cytation 5, RFU: Relative Fluorescence Units (Bars represent the mean ± SD, n = 3 biological replicates). c Nanoparticles tracking analysis (NTA) of E. coli OMVs. d Western blot images of E. coli and OMVs, the protein loading amount was 50 μg for the bacterial samples and 20 μg for the EVs sample. e Transmission electron microscopy (TEM) images of E. coli OMVs. f Super-resolution images of E. coli OMVs, HeLa EVs and S. aureus EVs labeled with CellMask™ Deep Red plasma membrane stain (red) and PmBF (green). g Schematic representation of nano-flow cytometry (Created with BioRender.com). h, i Natural extracellular vesicles (control group) and debris sample detection signal and concentrations detected based on nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). j Quantitative analysis of E. coli OMVs with different concentration gradients by nano-flow cytometry. k Quantitative analysis of different vesicles after PmBF labelling by nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). l Quantitative analysis of positive VLDL after labeling by PmBF or antibody probes, control group was treated with PBS (Bars represent the mean ± SD, n = 3 biological replicates). m PmBF probe was used to detect E. coli OMVs within a mixture of various vesicle types (S. aureus and HeLa EVs). Group HeLa: 100% HeLa EVs; Group S. aureus: 100% S. aureus EVs; Group EHS: 40% E.coli OMVs + 30% HeLa EVs + 30% S. aureus EVs; Group EH: 50% E.coli OMVs + 50% HeLa EVs; Group ES: 50% E.coli OMVs + 50% S. aureus EVs; Group E. coli: 100% E.coli OMVs (Bars represent the mean ± SD, n = 3 biological replicates). b, k, l were determined by one-way ANOVA with multiplicity adjusted P value; i was determined by a two-tailed unpaired t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
To confirm that PmBF achieves specific targeting through LPS, we treated E. coli with a specific antibody against LPS to competitive combined with LPS before PmBF labeling. Fluorescence imaging revealed a significant reduction in fluorescence intensity after LPS blocking Supplementary Fig. 3, indicating that PmBF’s binding to E. coli is mediated by its specific interaction with LPS. This finding further supports the specificity of PmBF for Gram-negative bacteria and suggests its potential utility in targeting OMVs, which also contain LPS.
We next investigated the ability of PmBF to target OMVs. EVs from E. coli, S. aureus and HeLa cells were isolated from the culture media by ultracentrifugation. These EVs were characterized by nanoparticle tracking analysis (NTA), western blotting (WB), and transmission electron microscopy (TEM). NTA revealed mean particle sizes of 94.7 nm, 112.4 nm, and 103.5 nm for EVs from E. coli, S. aureus, and HeLa cells, respectively Fig. 2c and Supplementary Figs. 4a, b. WB confirmed the presence of specific markers for each type of EV: OmpA and LPS for E. coli OMVs, LTA for S. aureus EVs, and TSG101 and CD81 for HeLa EVs Fig. 2d and Supplementary Figs. 4c, d, Supplementary Fig. 12. TEM imaging revealed the typical spherical structure of EVs Fig. 2e and Supplementary Figs. 4c, d. These results indicated the successful isolation of EVs by ultracentrifugation.
To evaluate PmBF’s selective binding to OMVs and visualize this interaction, EVs pre-stained with CellMask™ Deep Red plasma membrane stain were incubated with PmBF and imaged using a super-resolution microscope. PmBF signals were well colocalized with CellMask-stained OMVs from E. coli, while no significant PmBF signals were detected in the S. aureus or HeLa EV groups Fig. 2f and Supplementary Fig. 4e. These findings affirmed PmBF’s capability to specifically target E. coli-derived OMVs. Further, to accurately quantify OMVs labeled with PmBF, we introduced the nano-flow cytometry Fig. 2g, an instrument capable of analyzing nanoparticles ranging from 40 to 1000 nm. In addition, a key metric for evaluating the performance of an EV detection instrument is its ability to distinguish EVs from contaminants, such as membrane debris, which is a major source of interference and may bind to free probes, leading to false positives. To assess whether the nano-flow cytometry detection platform can exclude interference from such contaminants, we treated E. coli OMVs with Triton X-100 to generate a preparation of vesicle fragments. As shown in Fig. 2h, the signal from the debris samples was significantly lower than that of natural OMVs, with the particle number decreasing approximately 7.78 times Fig. 2i, indicating that the nano-flow cytometry can effectively exclude membrane debris for the quantitative analysis of OMVs.
Currently, OMVs are thought to be widespread in human biofluids and play key roles in various biological processes, but specific data on OMV concentrations remain limited. Most studies rely on methods like ELISA, Western blot, or Limulus amoebocyte lysate (LAL) assay, which often provide overall LPS concentrations of OMVs, not OMVs particle concentrations51,52,53,54,55,56. In our team’s previous study, we found that serum OMVs in healthy adults accounted for roughly 3% to 4% of overall EVs, approximately 3 × 107 to 4 × 1010 particles mL-157. Additionally, Gram-negative bacterial culture supernatants report OMV concentrations averaging 3.26 × 1012 particles mL-158. Here, we evaluated the sensitivity of the nano-flow cytometry platform, which was capable of detecting OMVs at concentrations as low as 2 × 105 particles mL-1 Fig. 2j, while WB detected bands only at OMV concentrations above 2 × 109 particle mL-1 Supplementary Fig. 5a. This highlighting the sensitivity and potential of nano-flow cytometry for OMV detection in these samples.
Based on this platform, We used different concentrations of PmBF probes to label the E. coli OMVs of to explore the optimal labeling concentration of PmBF, and the results are shown in Supplementary Fig. 5b. The positive rate of PmBF labeling tended to stabilize when the concentration of PmBF exceeded 5 μM. To balance labeling efficiency with minimizing non-specific binding and to maximize the signal-to-noise ratio, we selected 5 μM as the working concentration for all subsequent experiments. Following, we further expanded the types of EVs, including those derived from mammal cells, Gram-negative, and Gram-positive bacteria, to investigated the labeling efficiency of PmBF. All EVs were tested by the nano-flow cytometry after PmBF labeling. Figure 2k showed that OMVs displayed high labeling efficiency (E. coli 37.53%, K. pneumoniae 40.50%, A. baumannii 39.73%) then mammalian cells and Gram-positive bacteria-derived EVs (HeLa 3.07%, S. aureus 2.93%, S. epidermidis 3.07%). The lower labeling efficiency observed in HeLa, S. aureus, and S. epidermidis EVs might be due to nonspecific adsorption. These results further confirmed that OMVs could be specifically targeted by PmBF and quantitative analysis when combined with nano-flow cytometry.
To further evaluate PmBF’s specificity for OMVs, we investigated its ability to resist interference from lipoproteins, which are potential sources of nonspecific labeling in human samples47,48,49,50. We compared the labeling ratios of PmBF to an LPS antibody-based assay and a control group using VLDL liquid without any probes. High labeling ratios were observed in the LPS antibody group, while PmBF and the control group exhibited similar low ratios Fig. 2l. Moreover, to evaluate whether PmBF can detect OMVs in mixtures containing different types of EVs, we mixed E. coli OMVs with different types of EVs derived from HeLa or S. aureus at varying ratios (100% HeLa EVs; Group S. aureus: 100% S. aureus EVs; Group EHS: 40% E. coli OMVs + 30% HeLa EVs + 30% S. aureus EVs; Group EH: 50% E. coli OMVs + 50% HeLa EVs; Group ES: 50% E. coli OMVs + 50% S. aureus EVs; Group E. coli: 100% E. coli OMVs). The mixed samples were then analyzed to assess the ability of our method to detect and quantify OMVs. The results demonstrated that, regardless of the proportion of OMVs in the mixtures, our labeling method accurately reflected the relative proportions of OMVs Fig. 2m. Recovery rates were consistent across different mixing ratios, highlighting the sensitivity and specificity of the method in detecting OMVs in mixed EV samples. This finding demonstrates that PmBF has a higher ability to resist interference from lipoproteins and other types of EVs, highlighting its improved specificity for OMVs.
PmBF Labeling Preserves OMVs Integrity and Biological Activity
Maintaining the physical, biological, and functional integrity of OMVs during the labeling process is essential for reliable investigations of their roles in various physiological and pathological processes. To validate the suitability of PmBF for OMVs studies, we comprehensively assessed its biocompatibility, focusing on the preservation of the native properties and functions of OMVs.
First, we examined whether the labeling process alters the fundamental properties of OMVs, including their particle size, concentration, and morphology. NTA and TEM revealed that PmBF labeling preserved the size distribution and morphology of OMVs Supplementary Fig. 6a–c, presumably due to PmBF’s small molecular weight, which allows efficient labeling without causing significant physical changes to the vesicles. Additionally, we assessed the stability of PmBF-OMVs over time. The size and concentration of the PmBF-OMVs remained stable for 7 days at 4 °C, comparable to the stability of unlabeled OMVs Supplementary Fig. 6d, e. This finding demonstrated that PmBF labeling does not compromise the physical integrity of OMVs during both short-term and prolonged storage. To investigate the stability of PmBF labeling, we measured the fluorescence intensity of PmBF-OMVs over a period of 7 days Supplementary Fig. 6f. The fluorescence intensity exhibited minimal fluctuations, confirming the stable labeling capacity of PmBF probes. Furthermore, we evaluated whether PmBF labeling affects the protein composition and biological function of OMVs. Western blot analysis showed that PmBF labeling did not alter the protein composition of OMVs Supplementary Figs. 6g and 12.
Given that PmB primarily binds to the negatively charged LPS of Gram-negative bacteria59,60, we performed zeta potential measurements before and after PmBF labeling, to investigate whether the labeling concentration of the PmBF probe affects the zeta potential of OMVs. The results revealed that PmBF labeling did not significantly alter the zeta potential of OMVs Supplementary Fig. 7a. To further validate this finding, we labeled bacteria with PmBF, followed by zeta potential analysis. The results consistently showed that PmBF also didn’t cause noticeable changes in the zeta potential of the bacteria under markering concentration Supplementary Fig. 7b. To further confirm whether the observed results were affected by FITC conjugation, we conducted additional experiments using a gradient of PmB and PmBF concentrations to treat E. coli. The results showed that varying concentrations of PmB and PmBF did not significantly alter bacterial concentrations Supplementary Fig. 7c. These findings further confirmed that the probe had negligible impact on the integrity of OMVs and bacteria, indicating that PmBF labeling did not significantly interfere with the physical properties of OMVs under the experimental conditions.
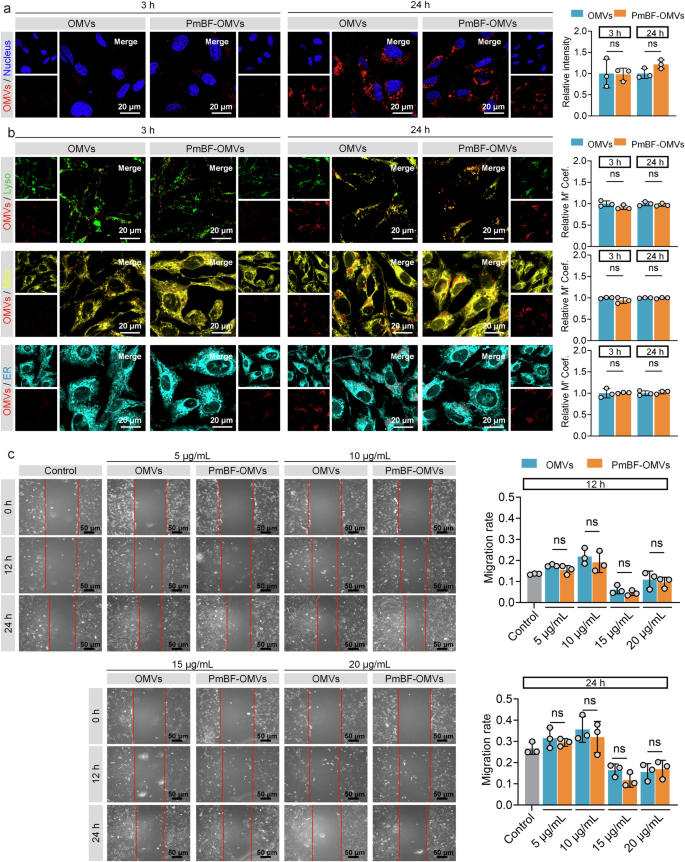
For the biological activity, we assessed the OMVs by examining their effects on BEAS-2B cell proliferation. Both OMVs and PmBF-OMVs exhibited comparable effects on BEAS-2B cell proliferation at 12 h and 24 h post-coincubation Supplementary Fig. 7d. To directly observe whether PmBF labeling affects the interaction between OMVs and cells, we stained natural OMVs or PmBF-OMVs with CellMask™ Deep Red plasma membrane stain and tracked their cellular uptake. Both natural OMVs and PmBF-OMVs were successfully internalized by cells after co-incubation for 3 or 24 h, as visualized through fluorescence imaging Fig. 3a and Supplementary Fig. 7e. Quantitative fluorescence analysis further confirmed that the cellular uptake levels of natural OMVs and PmBF-OMVs were comparable, with no significant differences observed. Further, to investigate whether the intracellular distribution of OMVs is affected by PmBF labeling, different cellular organelles were stained and co-localization analysis with internalized OMVs was performed Fig. 3b and Supplementary Fig. 7f. The results showed that internalized OMVs primarily localized within lysosomes, and the distribution pattern of PmBF-OMVs was consistent with that of natural OMVs. While our current experimental setup does not allow us to definitively isolate the effect of PmbF labeling on each specific pathway, the observation that the overall internalization of OMVs is not significantly affected by PmbF labeling suggests that the major uptake mechanisms, including receptor-mediated endocytosis, are likely not substantially inhibited.
a Confocal imaging of cellular internalization of natural OMVs and PmBF-OMVs, along with quantitative analysis of fluorescence intensity, blue: nucleus; red: OMVs (Bars represent the mean ± SD, n = 3 biological replicates). b Confocal imaging of the colocalization of natural OMVs and PmBF-OMVs with different cellular organelles, accompanied by colocalization coefficients (Lyso: lysosomes, green; Mito: mitochondria, yellow; ER: endoplasmic reticulum, cyan; OMVs: red) (Bars represent the mean ± SD, n = 3 biological replicates). c Scratch experiment of BEAS-2B cells incubated with gradient concentration OMVs or labeled OMVs for 12 or 24 h, control group treated by PBS (Bars represent the mean ± SD, n = 3 biological replicates). a–c were determined by a two-tailed unpaired t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
Additionally, to assess the potential impact of PmbF labeling on the biological activity of OMVs, we performed a wound healing scratch assay. We chose this assay based on previous studies demonstrating that OMVs can modulate cell migration and because it provides a relatively simple and quantitative readout of a complex cellular process. The results, presented in Fig. 3c, showed no significant difference in migration rates between BEAS-2B cells treated with natural OMVs and those treated with PmbF-labeled OMVs across all concentrations tested. These indicate that PmBF labeling preserves the biological activity of OMVs. Collectively, the above results highlight the stability and non-disruptive nature of PmBF labeling.
Circulating OMVs Serve as an Early Markers of Bacterial Infections
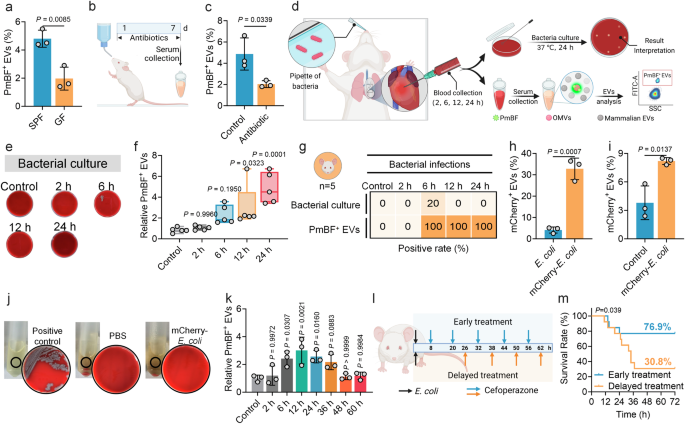
Previous studies have confirmed the presence of a small proportion of bacteria-derived OMVs in mouse and human serum57. Here, to validate whether the developed antibiotic-based fluorescent probe can detect OMVs in serum, we selected germ-free (GF, without any bacterial colonization) mice and specific pathogen-free (SPF, with normal commensal microbiota) male mice as research models. Since GF mice are theoretically devoid of bacteria, they were used as a reference to compare the detection results with those from SPF mice. The results showed that 4.80% of PmBF+ EVs on average were detected in serum from SPF mice, which was significantly higher than the 1.97% detected in GF mice Fig. 4a. To further confirm that the observed differences were caused by bacteria present in the mice, we administered a combination of antibiotics to eliminate bacteria from the mice Fig. 4b, confirmed by the remarkable reduction in fecal bacteria counting Supplementary Fig. 8a. Subsequent analysis revealed that after bacterial clearance, the proportion of positive EVs in the treated mice was significantly reduced to 2.03% Fig. 4c, near the levels of GF mice. These results demonstrate that our developed method can effectively identify OMVs in serum and suggest a correlation between the presence of OMVs in mouse serum and the bacterial load within the body.
a Analysis of circulating PmBF+ EVs levels in germ-free (GF) and conventional mice (SPF) mice by nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). b Schematic diagram of the construction of a mouse model of colonized bacteria clearance, d: days (Created with BioRender.com). c Analysis of circulating PmBF+ EVs levels in mice models before (Control group) or after intestinal flora cleared (Bars represent the mean ± SD, n = 3 biological replicates). d Schematic diagram of the construction of mouse models of bacterial infections and blood were collected from mice after intranasally injection with 8 × 107 CFU bacteria at 2, 6, 12, 24 h (Created with BioRender.com). e Blood bacterial culture plates for mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS. f Quantitative analysis of circulating PmBF+ EVs changes in mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS (The center line of each box indicates the median. The bottom and top bonds of the box show the 25th and 75th percentiles, respectively. Whiskers extend to the minimum and maximum values, n = 5 biological replicates). g Comparison of bacterial cultures and circulating OMVs positivity rates in mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS (Elements Created with BioRender.com). h Expression analysis of mCherry in wide-type or mCherry-E. coli derived OMVs (Bars represent the mean ± SD, n = 3 biological replicates). i Analysis of circulating mCherry+ EVs in mice models infected with mCherry-E. coli, control group mice was treated with PBS. The Y-axis indicates the percentage of serum mCherry+ EVs to total EVs. (Bars represent the mean ± SD, n = 3 biological replicates). j Blood bacterial culture plates as well as LB medium. Positive control: live mCherry-E. coli; PBS: blood of mice treated by PBS; mCherry-E. coli: blood of mice treated by mCherry-E. coli. No viable bacterial colonies were observed in group PBS and mCherry-E. coli. k Dynamic monitoring of circulating PmBF+ EVs levels at different time points post-infection in mice. Antibiotic treatment was initiated at 12 h post-infection, with administration every 12 h thereafter (Bars represent the mean ± SD, n = 3 biological replicates). l Illustration of the effect of different points in time of treatment on mouse infection models survival rate (Created with BioRender.com). m Mouse survival curves. a, c, h, i was determined by a two-tailed unpaired t-test; f, k were determined by one-way ANOVA with multiplicity adjusted P value; Survival analysis was performed using the Log-rank (Mantel-Cox) test to compare the survival curves between groups (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
We further tested this correlation in bacterial infections. We developed a male mouse model of lung infection by intranasally introducing two most common pathogens, E. coli or K. pnenmoniae Fig. 4d. Blood samples were collected at different time points post-infection for bacterial culture and serum EVs detection. In the E. coli infection group, serum PmBF+ EVs levels significantly increased at 6, 12 and 24 h post-infection Figs. 4e, f, while only one mouse had a positive bacterial culture at 6 h. The positive rates of serum PmBF+ EVs were consistently higher than those of bacterial cultures Fig. 4g. Commonly used inflammatory markers, CRP and PCT, showed variable elevations Supplementary Figs. 8b, c, with PCT not significantly increasing at 6, 12, and 24 h and CRP not significantly increasing at 12 h. Similarly, in the K. pneumoniae infection group, PmBF+ EVs were showed elevated trends at 6, 12 and 24 h post-infection, with all negative blood bacterial cultures Supplementary Figs. 8d–f. To further assess the specificity of PmBF for detecting Gram-negative bacterial infections, we developed a mouse infection model using the Gram-positive bacterium Streptococcus pneumoniae (S. pneumoniae). Circulating EVs were analyzed 12 h post-infection. As shown in Supplementary Fig. 8g, the levels of PmBF+ EVs in the serum of S. pneumoniae-infected mice remained unchanged. These results provide additional evidence supporting the specificity of PmBF for the diagnosis of Gram-negative bacterial infections.
Additionally, to investigate the bacterial load required to affect circulating OMVs levels, we infected mice with varying bacterial concentrations and measured circulating OMVs. The results revealed that at a bacterial load of 8 × 106 CFU, circulating OMVs levels in some mice began to plateau. At bacterial loads down to 8 × 105 CFU, circulating OMVs levels in all mice were comparable to those of uninfected controls Supplementary Fig. 8h.
To determine whether these elevated circulating PmBF+ EVs originated directly from the infecting bacteria at the site of infection, rather than from increased intestinal OMV release due to potential gut barrier dysfunction, we engineered an E. coli strain expressing an mCherry-OmpA fusion protein. Red fluorescence intensity was significantly elevated in mCherry-E. coli compared to wild type E. coli Supplementary Fig. 9, indicating the successful plasmid introduction. Further validation revealed that OMVs from mCherry-E. coli carried mCherry, as evidenced by a higher fluorescent rate Fig. 4h. In the lung infection model, mice intranasally administered with mCherry-E. coli exhibited an average of 8.2% mCherry+ vesicles in serum at 6 h post-infection, significantly higher than in PBS-treated mice Fig. 4i. Similarly, no viable mCherry-E. coli were cultured from the blood of infected mice Fig. 4j. Taken together, these findings demonstrate that the elevated circulating PmBF+ OMVs mainly originated from the infecting bacteria and highlight the potential of PmBF+ EVs as sensitive markers for bacterial infections.
Further, to investigate the dynamic changes in circulating OMVs levels during bacterial infection and their association with infection resolution, we infected mice with bacteria and monitored circulating OMVs levels over time. Antibiotic treatment was initiated at 12 h post-infection to assess the impact of infection remission on OMVs dynamics. As shown in Fig. 4k, circulating OMVs levels began to rise at 6 h post-infection and peaked at 12 h, indicating an active bacterial infection. Upon initiation of antibiotic treatment, circulating OMVs levels gradually decreased over time. As the treatment progresses, OMVs levels declined gradually compared to the peak levels, and by 36-48 h, they returned to baseline levels comparable to those in uninfected controls. These results suggest that circulating OMVs levels dynamically reflect the progression of bacterial infection and respond to effective antibiotic treatment, highlighting their potential as biomarkers for monitoring infection and treatment efficacy.
To investigate the impact of early diagnosis on therapeutic outcomes, we constructed mouse lung infection models and initiated antibiotic treatment at different time points, with dosing every 12 h, and observed the survival rates Fig. 4l. In the delayed treatment group, the survival rate dropped to 30.8% at 32 h, while it was effectively increased to 76.9% in the early treatment group Fig. 4m. These findings demonstrate the importance of early intervention in improving prognosis. Notably, in these mouse lung infection models, PmBF+ EVs were detectable in the circulation as early as 6 h post-infection, preceding positive bacterial cultures and significant elevations in conventional inflammatory markers. This early detection of PmBF+ EVs highlights their potential to facilitate timely diagnosis and guide early treatment decisions, which can significantly improve patient outcomes.
Clinical Utility of PmBF+ EVs as Biomarkers for Bacterial Infections
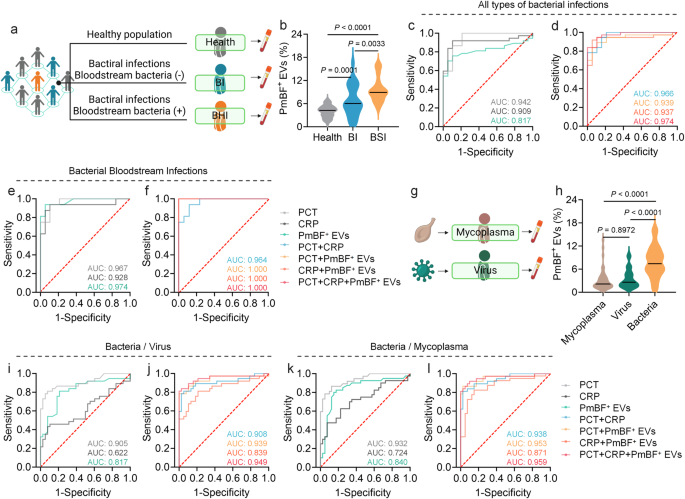
To explore the clinical potential of PmBF+ EVs as a biomarker, we analyzed serum samples from healthy individuals and patients with bacterial infections, both with negative (BI) and positive (BSI) bloodstream cultures Fig. 5a and Supplementary Table 1. Serum EVs were isolated and characterized by NTA, WB, and TEM Supplementary Figs. 10 and 12. As showed in Fig. 5b, the PmBF+ EVs levels in patient serum were significantly elevated compared to healthy controls (mean 7.90% vs. 4.00%), with BSI patients exhibiting higher levels (mean 9.61%, range 5.00-15.90%) than BI patients (mean 6.75%, range 0.70-16.50%), suggesting a correlation between PmBF+ EV levels and infection severity.
a Clinical samples collection of healthy people and patients with different types of bacterial infections (Created with BioRender.com). b Detection of circulating PmBF+ EVs levels in the health screening population and patients with different types of bacterial infections (The horizontal line indicates the median, Health: n = 60 biological replicates; BI: n = 24 biological replicates; BSI: n = 16 biological replicates). c, d ROC curves for the diagnosis of all types of infections by PmBF+ EVs, CRP, PCT alone and in combination. e, f ROC curves for the diagnosis of bacterial bloodstream infections by PmBF+ EVs, CRP, PCT alone and in combination. g Clinical samples collection of patients with mycoplasma or virus infections (Created with BioRender.com). h Detection of circulating PmBF+ EVs levels in patients with mycoplasma, virus or bacterial infections (include bacterial infections with positive or negative blood cultures) (The horizontal line indicates the median, Mycoplasma: n = 40 biological replicates; Virus: n = 38 biological replicates; Bacteria: n = 40 biological replicates). i, j ROC curve for differential diagnosis between bacterial or virus infections. k, l ROC curve for differential diagnosis between bacterial or mycoplasma infections. b, h were determined by one-way ANOVA with multiplicity adjusted P value (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
To further investigate the diagnostic performance of PmBF+ EVs, receiver operating characteristic (ROC) curve analyses were conducted. For all bacterial infections and bloodstream infections specifically, the areas under the curve (AUC) were 0.817 and 0.974, respectively Fig. 5c, e. Notably, combining PmBF+ EVs with PCT and CRP further improved the AUC for bacterial infection diagnosis Fig. 5d, particularly for bacterial bloodstream infections Fig. 5f.
Finally, we investigated the potential of PmBF+ EVs in differentiating bacterial infections from viral and mycoplasma infections Fig. 5g and Supplementary Table 1. Circulating PmBF+ EV levels were significantly higher in patients with bacterial infections compared to those with virus or mycoplasma infections Fig. 5h. ROC analyses for differential diagnosis yielded AUCs of 0.817 (bacteria vs. virus) and 0.840 (bacteria vs. mycoplasma) for PmBF+ EVs alone Fig. 5i, k. Combining PmBF+ EVs with PCT and CRP further improved the AUCs to 0.949 and 0.959, respectively, highlighting the potential of PmBF+ EVs as a biomarker for the differential diagnosis of bacterial infections.
Discussion
OMVs have emerged as key players in host-pathogen interactions, yet their potential as biomarkers remains largely unexplored due to challenges in specific detection. Recent advancements in OMVs detection encompass a wide range of innovative approaches, including DNA aptamer-based assays, LPS antibody-based methods, nanoplasmonic sensors, and aggregation-induced emission bioprobes. However, each of these approaches has limitations. DNA aptamer-based assays and LPS antibody methods may suffer from cross-reactivity and reduced specificity in complex biological environments. Similarly, aggregation-induced emission bioprobes, while demonstrating high sensitivity, often exhibit suboptimal specificity, particularly in complex biological matrices. Nanoplasmonic sensors are characterized by high cost and complex operation, susceptibility to environmental factors, and stringent sample processing requirements. These challenges highlight the unmet need for robust, specific, and broadly applicable methods for OMVs detection in biological samples57,61,62.
By employing PmB, an antibiotic known for its high affinity to bacterial LPS63,64, we have developed a highly specific molecular probe for OMVs detection in complex biological samples. When used in conjunction with nano-flow cytometry technology, our probe enables not only reliable distinction between bacterial OMVs and host-derived extracellular vesicles, but also precise quantification of bacterial OMVs in these complex mixtures. This specificity is particularly important given the significant overlap in size and structure between OMVs and host extracellular vesicles, a well-documented challenge in biomarker discovery. Compared to conventional antibody-based methods, the PmBF probe offers enhanced performance, particularly in reducing cross-reactivity. Prior studies have shown that antibody-based approaches can exhibit non-specific binding to other components or even host proteins, leading to false positives57. In contrast, the smaller molecular size of PmB compared to antibodies may improve its binding kinetics and accessibility to LPS on OMVs, enhancing both specificity and sensitivity. Moreover, the chemical stability of PmB and its compatibility to fluorescent labeling contribute to the robustness and versatility of our method.
Bacterial infection diagnostics rely on markers such as CRP, PCT, and white blood cell (WBC) counts. While these markers are widely used, they suffer from limitations, including low specificity for bacterial infections, delayed elevation following infection onset, and inability to distinguish between bacterial and non-bacterial infections. Traditional blood culture, the gold standard, is time-consuming and often yields false negatives in patients with low bacterial loads or prior antibiotic use. Here, our results demonstrate the potential of this method based on PmBF for early diagnosis of bacterial infections. In animal models, we observed increased levels of PmBF+ EVs as early as 6 h post-infection, preceding both blood cultures and common inflammatory markers. This early detection capability could improve the rapid diagnosis of bacterial infections, potentially leading to earlier treatment initiation. This advantage is crucial for improving patient outcomes, as extensive research has shown that delays in antibiotic administration during bacterial infections significantly worsen prognosis65,66,67. The clinical data from our study also demonstrate its value in real-world settings. PmBF+ EVs levels were significantly elevated in patients with bacterial infections, including those with negative blood cultures. This suggests our method could identify infections that might be missed by traditional diagnostic techniques. Furthermore, compared to conventional markers, OMVs offer unique advantages, including their direct origin from bacteria, which provides higher specificity. This allows for earlier and more precise differentiation between bacterial and non-bacterial infections, which is crucial for determining the necessity of antibiotic treatment and, when appropriate, facilitating its prompt initiation. Moreover, the quantitative nature of our method may provide insights into infection severity, potentially allowing for more personalized infectious disease management.
There are indeed some limitations of our study. The clinical validation was conducted on a relatively small cohort, focusing primarily on E. coli and K. pneumoniae infections. Larger, more diverse studies will be necessary to fully establish the utility of this approach across a wider range of pathogens, including Gram-positive bacteria and fungi. Additionally, PmB-resistant bacteria, due to surface LPS modifications, may exhibit reduced binding capacity to PmB, potentially limiting the applicability of this method. Future studies are needed to further evaluate the suitability of PmBF for detecting resistant strains and to optimize probe design to expand its diagnostic range. Further characterization and more comprehensive experiments are still needed in the future to thoroughly evaluate the effects of PmBF labeling on OMVs. Moreover, the nano-flow cytometry’s cost and the need for specialized operation currently limit its feasibility for point-of-care or at-home diagnostics. Future work should prioritize developing accessible, user-friendly technologies to broaden clinical applications. Generally, future research should focus on expanding the range of pathogens studied, including antibiotic-resistant strains, investigating the potential of this method in monitoring treatment efficacy and predicting clinical outcomes, and exploring the use of this technology in other biological fluids.
In summary, we have developed a approach for specifically detecting and quantifying OMVs in complex biological samples. By combining a PmB-based fluorescent probe with advanced nanoparticle analysis technology, we demonstrated the presence of OMVs in blood samples from both animal models and human patients, revealing their potential as early indicators of bacterial infections. Our findings uncovered a significant increase in circulating PmBF+ EVs during the early stages of infection, preceding traditional diagnostic markers. Of clinical significance, we showed that circulating PmBF+ EVs could serve as a promising biomarker for the early diagnosis and differentiation of bacterial infections. Our methodology thus offers a valuable platform for advancing our understanding of bacterial infections and potentially improving their management in clinical settings.
Methods
Specific pathogen free (SPF) eight-week-old male BALB/c mice were purchased from Guangdong Medical Laboratory Animal Center; Germ free (GF) eight-week-old male BALB/c mice were purchased from GemPharmatech. The animal procedures were performed with ethical compliance and approved by the Laboratory Animal Management Center, Southern Medical University with an approval number of SMUL2022002. All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011) and the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020). All the animal experiments complied with institutional guidelines. SPF mice were group housed 4 mice per cage in a specific pathogen-free environment temperature (22–26 °C) and humidity (40–70%) house rooms on a 12 h light, 12 h dark cycle (Feed: BEIJING HFK BIOSCIENCE, 1025). GF mice were housed in sterile isolators (Feed: XIETONG.ORGANISM, XTI01ZJ-010). According to the Animal Ethics Committee guidelines, mice that experience weight loss exceeding 20% or display symptoms such as hunched posture, impaired locomotion, or respiratory distress should be promptly euthanized using CO2 gas. Otherwise, the mice were euthanized at the conclusion of the experiment. All blood samples were obtained from Nanfang Hospital, Southern Medical University, and approved by the Medical Ethic Committee of Nanfang Hospital, Southern Medical University, with an approval number of NFEC-2022-001. All participants/their Legally Authorised Representatives have given written consent for their data to be used in the scientific research. Participants received no compensation for their participation. Detailed materials are provided in Supplementary Table 2.
Synthesis of PmBF probe
Firstly, 5 mg of PmB sulphate was weighed and dissolved in phosphate buffer with pH=9.5. The free FITC dissolved in DMSO was added into the PmB sulfate solution at the mole ratio of FITC: PmB sulfate = 3:1, and the reaction was carried out at room temperature (RT) under light-shielded conditions for 2 h. Finally, acetone was added to the reaction solution to remove the free FITC, and the remaining solution was evaporated under reduced pressure for later use. Probes and raw materials are subsequently subjected to mass spectrometry analysis (Bruker, MALDI-TOF).
Mass spectrometry analysis
MALDI-TOF mass spectrometry was employed to analyze PmB, free FITC and PmBF, and using a Bruker MALDI-TOF instrument ( = 1 biological replicates). PmB and PmBF were dissolved in ultrapure water at 0.5 mg/mL, while free FITC was dissolved in dichloromethane at the same concentration. The matrix used was 2,5-dihydroxybenzoic acid (DHB). A 1 µL aliquot of each sample was mixed with 1 µL of matrix solution on a MALDI plate and allowed to co-crystallize at room temperature. The instrument was operated in positive ion mode, and spectra were collected in the range of 300 to 2000 m/z. Data processing for peak identification and quantification was performed using Bruker’s FlexAnalysis software, ensuring precise molecular characterization of PmB, PmBF, and free FITC.
Fluorescence intensity detection by Cytation 5
Different bacteria or mammalian cell labeled by PmBF probes were centrifuged at 3000 g for 30 min 3 times to remove free probes. Control group were treated by same volume of solvent DMSO. The washed precipitate was resuspended using 200 μL PBS and added to a 96-well plate. The 96-well plate was placed into the Multi-Mode Microplate Reader with Cellular Imaging (BioTek, Cytation 5) for 10 s shaking time, followed by detected with the excitation wavelength of 488 ± 10 nm and the emission wavelength of 530 ± 10 nm.
Bacteria culture and extracellular vesicles isolation
Escherichia coli (ATCC25922, Serotype: O6, Biotype 1), Klebsiella pneumoniae (ATCC13883, Serotype: 3), Acinetobacter baumannii (ATCC19606), Staphylococcus aureus (ATCC25923), Staphylococcus epidermidis (ATCC12228) and streptococcus pneumoniae (ATCC49619) were obtained from the American Type Culture Collection (ATCC) and stored at -80°C. Gram-negative bacteria were cultured in Luria-Bertani (LB) broth and Gram-positive bacteria in Brian Heart Infusion (BHI) broth. Briefly, bacteria were grown overnight in broth in a shaking incubator (37 °C, 220 rpm). Afterward, the bacteria-containing medium was diluted by 50-folds into fresh medium and further grown to the early stationary phase for latter isolation. Cultures of bacteria were centrifuged at 3000 g for 30 min and 12000 g for 30 min. Next, the supernatants were filter through a polyethersulfone (PES) membranes filter (0.22 μm pore-size) to remove cell debris, and then ultracentrifuged for 70 min at 135000 g to pellet EVs and resuspended by PBS to ultracentrifuged again. All centrifugation steps were performed at 4 °C. Pelleted EVs were resuspended in 1 mL PBS per 500 mL culture medium for nano-flow cytometry (nanoFCM) and 200 μL per 500 mL for WB. The nano-flow cytometry results were analyzed using NanoFCM Profession V1.0.
Mammalian cell derived extracellular vesicle isolation
HeLa cells (Jennio Biotech, JNO-H0276, verified by STR profiling) were grown in 1640 medium supplemented with 10% Fetal bovine serum (FBS), 100 IU ml-1 Penicillin and 100 µg ml-1 Streptomycin in 37 °C, 5% CO2. After the cells were cultured to 80%, the serum-free medium was replaced for 12 h to starve the cells, and then the supernatant was collected after culturing for 12 h using the medium containing FBS-Without EXOSOME. The collected supernatants were centrifuged at 300 g, 2000 g and 10,000 g for 10 min, 20 min and 30 min respectively. Afterward, the collected supernatants were ultracentrifuged for 70 min at 135000 g to pellet EVs and resuspended by PBS to ultracentrifuged again. All centrifugation steps were performed at 4 °C. Pelleted EVs were resuspended in 1 mL PBS per 500 mL culture medium for nano-flow cytometry (nanoFCM) and 200 μL per 500 mL for WB. The nano-flow cytometry results were analyzed using NanoFCM Profession V1.0.
Serum total extracellular vesicle isolation
The whole blood samples were standard 1 h at room temperature and then centrifuged at 300 g for 10 min to obtain serum. The serum were centrifuged at 2000 g for 20 min and 10,000 g for 30 min to remove impurities such as cellular debris. Afterward, the collected supernatants were ultracentrifuged for 35 min at 110000 g to pellet EVs and resuspended by PBS to ultracentrifuged again. All centrifugation steps were performed at 4 °C. Pelleted EVs were resuspended in 100 μL PBS.
Western blotting (WB)
All bacteria and EVs were dissolved in loading buffer and boiled for 15 min. The total protein concentration was measured by BCA assay. For the WB characterization experiment, the protein loading amount was 50 μg for the bacterial samples and 20 μg for the EVs sample. Samples were separated by 12% SDS-polyacrylamide gel electrophoresis (30 min at 80 V for compression and 60 min at 120 V for separation) and transferred to polyvinylidene fluoride (PVDF) membranes for 90 min at 200 mA. Afterward, blocked the membranes by 5% bovine serum albumin (BSA) at room temperature for 2 h or 4 °C overnight. Blots were incubated overnight with primary antibodies. Mouse monoclonal to E. coli LPS, Rabbit monoclonal to LTA, Rabbit monoclonal to CD81, Rabbit monoclonal to CD9, Rabbit monoclonal to TSG 101, Rabbit anti-Rickettsia conorii ompA Polyclonal antibody, were used with 1:500 dilution. Incubation with secondary antibodies was performed with 1:5000 dilution after extensive washing of the membranes in 5% TBS-Tween20. After washing, Enhanced chemiluminescence (ECL) Kit HRP was added, and imaging was performed using Snapandgo. Blot images are collected using Multifunctional Fluorescence Imaging System (BIO-OI, OI600SE-MF). Antibodies information were listed in the supplementary Table 2.
Nanoparticle tracking analysis (NTA)
NTA was performed to determine the size and concentration of the EVs using NanoSight NS300 instrument (Malvern, UK). Standard measurement was adjusted. The precipitated EVs were evenly dissolved in PBS and then diluted to the concentration between 1 × 107 mL-1 to 1 × 109 mL-1, which were loaded into a 1 mL syring. The samples were injected into the sample cell at the syringe speed of 40 µL s-1. The NanoSight NS300 captured three 30 s sample videos, which were analyzed by using NanoSight Software NTA3.2. The detection threshold was adjusted to include as many particles as possible with the restrictions that 10–100 red crosses were counted while only <10% were not associated with distinct particles. Blue cross count was limited to 5.
Preparation of OMV debris samples using Triton X-100
OMVs were disrupted into fragments using Triton X-100. Briefly, purified OMVs were diluted in PBS to a final concentration of 109 particles mL-1. Triton X-100 was prepared at a 1:100 dilution in the OMV suspension. The mixture was gently mixed and incubated at 37 °C for 30 minutes to facilitate vesicle disruption. Following incubation, the sample was centrifuged at 110000 g for 35 min at 4 °C to remove any insoluble debris. The supernatant, containing OMV membrane Debris, was carefully collected and used immediately for downstream analyses.
Coomassie brilliant blue staining
All fractions and pellets were dissolved in a loading buffer and boiled for 10 min. The total protein concentration was measured by BCA assay. Samples were separated by 12% SDS-polyacrylamide gel electrophoresis (30 min at 80 V for compression and 60 min at 120 V for separation) and staining by Coomassie brilliant blue for 10 min. Gels images are collected using a Multifunctional Fluorescence Imaging System (BIO-OI, OI600SE-MF).
EV labeling
EVs suspensions were incubated with PmBF probes (5 μM) at 37 °C for 30 min and then ultracentrifuged at 110000 g for 35 min to remove free probes before detection by nano-flow cytometry (nanoFCM). The nano-flow cytometry results were analyzed using NanoFCM Profession V1.0. The labeling efficiency results were presented as the percentage of PmBF-positive particles relative to the total side scatter events. The gating strategy was shown in Supplementary Fig. 11.
Serum PmBF+ EVs detection
For serum PmBF+ EVs detection of human or mice, 100 μL serum was used to for subsequent testing. The serum were first centrifuged at 2000 g for 20 min and 10000 g for 30 min to remove impurities such as cellular debris. Pre-processed serum were treated by PmBF directly for 30 min at 37 °C, and then ultracentrifuged twice to remove free probes and resuspend EVs samples using PBS. Samples were detected by nano-flow cytometry (nanoFCM). The nano-flow cytometry results were analyzed using NanoFCM Profession V1.0. We calculated the reference range (μ ± 1.96σ) for serum PmBF+ EVs levels in the control group of mice using the formula bellow, and any results exceeding this range were considered positive (Box 1).
Cell and bacteria imaging
Cells or bacteria were dissolved in PBS and prepared to OD600 = 1 for further used. All samples were co-incubated with PmBF for 1 h and then washed three times using PBS. To obsearve the fluorescence intensity, cells or bacteria were seeded onto 20 mm confocal dishes and stained with Hoechst 33342 for 15 min before imaging. All fluorescent images were obtained by Laser Scanning Confocal Microscope (Olympus FV3000, objective lenses: NA 1.42, WD 0.15 mm) or Super-Resolution Microscope (Nikon N-SIM + , objective lenses: 100x SR Apochromat TIRF Objective). The excitation wavelength for PmBF is 488 nm and for Hoechst 33342 is 405 nm. Images processing is performed using Olympus FV31S-SW for confocal images or NIS-Elements Viewer for super-resolution images. The confocal images fluorescence intensity were analyzed by ImageJ (ImageJ 1.54 d; Java 1.8.0_345).
EVs super-resolution imaging
EVs were pre-stained with CellMask™ Deep Red Plasma Membrane Stains on 1:1000 dilution for 10 min before labeling by PmBF for 15 min. Labeling-EVs were ultracentrifuged at 110000 g for 35 min to remove free probes. The examples were adsorbed by confocal plate substrates coated with polylysine and then exposed to 488 nm (PmBF) and 647 nm (CellMask™ Plasma Membrane Stains) lasers under Super-Resolution Microscope (Nikon N-SIM + , objective lenses: 100x SR Apochromat TIRF Objective). to obtain the EV images. Images processing is performed using NIS-Elements Viewer.
LPS blocking assay
To assess the blocking effect of LPS on PmBF labeling, a monoclonal antibody targeting LPS (20 μg mL-1) was incubated with bacteria at 37 °C for 1 h. After incubation, unbound antibodies were thoroughly removed by washing the samples three times with PBS to nsure minimal background interference. Subsequently, PmBF (5 μM) was added to the bacterial samples and incubated at 37 °C for 30 minutes to allow labeling, unbound probes were thoroughly removed by washing the samples three times with PBS. Following labeling, fluorescence signals were visualized using a confocal microscope (Olympus FV3000) and quantitatively analyzed using intensity were analyzed by ImageJ (ImageJ 1.54 d; Java 1.8.0_345).
Cell proliferation assays
BEAS-2B cells (Jennio Biotech, JNO-H0002, verified by STR profiling) were seeded onto 96-well plates (5000 per well) and cultured for 24 h in 37 °C, 5% CO2 following by coincubating with natural OMVs or labeled-OMVs (10 μg mL-1) for 24 h. Cell proliferation was assessed after different treatments using a Cell Counting Kit-8 (CCK8).
Scratch assay
BEAS-2B cells (Jennio Biotech, JNO-H0002, verified by STR profiling) were seeded into 6-well plates and cultured until they reached 90% confluence. A linear scratch was then created on the cell monolayer using a sterile 200 μL pipette tip. The medium was replaced with fresh serum-free medium containing different concentrations of OMVs or PmbF-labeled OMVs (5, 10, 15, and 20 μg/mL). Images of the scratch were captured at 0, 12 and 24 h using an inverted microscope. The scratch area was measured using ImageJ software (ImageJ 1.54 d; Java 1.8.0_345), and the cell migration rate was calculated.
Mouse model of bacterial infections
E. coli was dissolved in saline to make a suspension. Taking 20 μL of bacteria suspension and slowly pipette a drop onto the nostril. After the liquid was inhaled into the nostril, pipette the next drop until all the 20 μL was delivered. Repeat the process with another 20 μL of suspension on the other nostril. Mice were treated with 8 × 107 CFU per 20 g. Blood was collected at 0 (control), 2, 6, 12 and 24 h after treatment for bacterial culture and serum OMV levels detection. We calculated the reference range (μ ± 1.96σ) for serum OMV levels in the control group of mice using the formula below, and any results exceeding this range were considered positive (Box 1).
Detection of mCherry-positive EVs in mouse serum
Mice were infected with mCherry-E. coli (8 × 107 CFU per 20 g), and blood was collected via cardiac puncture 6 h post-infection. A total of 100 μL of serum was obtained from each sample. The serum was pre-processed by sequential centrifugation at 2000 g for 20 minutes and 10,000 g for 30 minutes at 4 °C to remove impurities. Total EVs were then isolated from the serum by ultracentrifugation (110000 g for 35 min at 4 °C) and resuspended in 100 μL of PBS. To label mCherry-positive EVs, the resuspended EVs were incubated with anti-mCherry green fluorescent antibody for 30 minutes at 37 °C. Unbound antibodies were removed by a second ultracentrifugation step. The EV pellet was resuspended in 100 μL of PBS and analyzed using nano-flow cytometry (nanoFCM) to determine the percentage of mCherry-positive EVs among the total EV population. The nano-flow cytometry results were analyzed using NanoFCM Profession V1.0.
The survival rate of mouse models
Mouse models of bacteria infections were divided into early treatment group and delayed treatment group, treated with 1.5 × 108 CFU per 20 g nasally. early treatment group were treated with Cefoperazone (0.16 mg per 200 μL each time at 12 h intervals, intraperitoneal injection) at 8 h after infection, while delayed treatment group was started at 26 h.
Intestinal flora clearance mouse models
Mice were gavaged with a mixture of ampicillin (200 mg mL-1), vancomycin (100 mg mL-1), neomycin (200 mg mL-1), gentamycin (200 mg mL-1), and erythromycin (200 mg mL-1) for 7 days.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 10.1.2 software (GraphPad Software, Inc., San Diego, CA, USA) or SPSS 27. Appropriate tests were applied in analyzing these data, meeting assumptions of the statistical methods. P value less than 0.05 was considered to be significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the paper and its supplementary information files. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. Source data are provided with this paper. MALDI-TOF mass spectrometry analysis data of FITC, PmB, and the synthesized PmBF can be downloaded from https://www.scidb.cn/en/anonymous/NlpCbnV1. https://doi.org/10.57760/sciencedb.22390. Source data are provided with this paper.
References
GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet 400, 2221–2248 (2022).
Vincent, J.-L. et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 323, 1478–1487 (2020).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet 395, 200–211 (2020).
World Health Organization, WHO bacterial priority pathogens list, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance (https://www.who.int/publications/i/item/9789240093461).
Timsit, J.-F., Ruppé, E., Barbier, F., Tabah, A. & Bassetti, M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med 46, 266–284 (2020).
Ng, I. K. & Goh, W. G. Interpretation of acute phase reactants (C-reactive protein and procalcitonin) in children with pneumonia. Trop. Doct 54, 394–395 (2024).
Kubo, K. et al. Benefits and harms of procalcitonin- or C-reactive protein-guided antimicrobial discontinuation in critically ill adults with sepsis: A systematic review and network meta-analysis. Crit. Care Med 52, e522–e534 (2024).
Karasu, M. et al. The relationship between nuclear factor-kappa B and Inhibitor-Kappa B parameters with clinical course in COVID-19 patients. Mol. Biol. Rep. 51, 813 (2024).
Kosmider, E. et al. Observational study of effects of HIV acquisition and antiretroviral treatment on biomarkers of systemic immune activation. PLoS One 19, e0288895 (2024).
Sun, X., Tang, J., Lu, J., Zhang, H. & Li, C. Development and validation of a prediction model for mortality in critically ill COVID-19 patients. Front Cell Infect. Microbiol 14, 1309529 (2024).
Pan, T., Guo, X., Yang, D., Ding, J. & Chen, C. Expression and significance of procalcitonin, leukotriene B4, serum amyloid A, and C-reactive protein in children with different types of pneumonia: An observational study. Med. (Baltim.) 103, e37817 (2024).
Li, J., Zhang, H., Guo, J. & Ma, X. Clinical features of mycoplasma pneumoniae pneumonia in children without fever. BMC Pediatr. 24, 52 (2024).
Papan, C. et al. Combinatorial host-response biomarker signature (BV score) and its subanalytes TRAIL, IP-10, and CRP in children with Mycoplasma pneumoniae community-acquired pneumonia. J. Infect. Dis. 230, e247–e253 (2023).
Lu, S. et al. Correlation between PCT, 25(OH)D, PTX-3, AMS levels and the severity of diabetic ketoacidosis complicated by pancreatitis. BMC Endocr. Disord. 21, 136 (2021).
Chen, J. et al. Early diagnostic value of plasma PCT and BG assay for CRBSI after OLT. Transpl. Proc. 43, 1777–1779 (2011).
Zhang, Q., Zuo, Y. & Xu, M. The correlation of serum vaspin, S100A12 and PCT levels with the severity of ulcerative colitis and its clinical significance. Am. J. Transl. Res 13, 7914–7920 (2021).
Saadat, S. H., Javanbakht, M. & Shahyad, S. Brain-derived neurotrophic factor and C-reactive protein (CRP) biomarkers in suicide attempter and non-attempter major depression disorder (MDD) patients. Ann. Gen. Psychiatry 23, 27 (2024).
Utsumi, M. et al. Albumin-lymphocyte-globulin-c-reactive protein index as a novel prognostic biomarker for hepatocellular carcinoma after hepatectomy. Dig. Surg. 41, 161–170 (2024).
Liu, Y. et al. Association of c-reactive protein/albumin ratio with mortality in patients with traumatic brain injury: A systematic review and meta-analysis. Heliyon 10, e33460 (2024).
Wilson, M. R. et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N. Engl. J. Med. 380, 2327–2340 (2019).
Filkins, L. M., Bryson, A. L., Miller, S. A. & Mitchell, S. L. Navigating clinical utilization of direct-from-specimen metagenomic pathogen detection: clinical applications, limitations, and testing recommendations. Clin. Chem. 66, 1381–1395 (2020).
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol 17, 13–24 (2019).
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol 21, 415–430 (2023).
Wen, M. et al. Bacterial extracellular vesicles: A position paper by the microbial vesicles task force of the chinese society for extracellular vesicles. Interdiscip. Med. 1, e20230017 (2023).
Sartorio, M. G., Pardue, E. J., Feldman, M. F. & Haurat, M. F. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev. Microbiol 75, 609–630 (2021).
Dorward, D. W. & Garon, C. F. DNA is packaged within membrane-derived vesicles of gram-negative but not gram-positive bacteria. Appl Environ. Microbiol 56, 1960–1962 (1990).
Crispim, J. S. et al. Desulfovibrio alaskensis prophages and their possible involvement in the horizontal transfer of genes by outer membrane vesicles. Gene 703, 50–57 (2019).
Bitto, N. J. et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 7, 7072 (2017).
Turnbull, L. et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7, 11220 (2016).
Cooke, A. C., Nello, A. V., Ernst, R. K. & Schertzer, J. W. Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS One 14, e0212275 (2019).
Prados-Rosales, R. et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol. 196, 1250–1256 (2014).
Rakoff-Nahoum, S., Coyne, M. J. & Comstock, L. E. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol. 24, 40–49 (2014).
Dürwald, A. et al. Reaching out in anticipation: bacterial membrane extensions represent a permanent investment in polysaccharide sensing and utilization. Environ. Microbiol 23, 3149–3163 (2021).
Pathirana, R. D. & Kaparakis-Liaskos, M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell Microbiol 18, 1518–1524 (2016).
Díaz-Garrido, N., Badia, J. & Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 10, e12161 (2021).
Muraca, M., Putignani, L., Fierabracci, A., Teti, A. & Perilongo, G. Gut microbiota-derived outer membrane vesicles: under-recognized major players in health and disease? Discov. Med. 19, 343–348 (2015).
Shen, Y. et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 (2012).
DeVoe, I. W. & Gilchrist, J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J. Exp. Med. 141, 297–305 (1975).
Craven, D. E. et al. Adherence of isolates of neisseria meningitidis from patients and carriers to human buccal epithelial cells. J. Infect. Dis. 142, 556–568 (1980).
Fiocca, R. et al. Release of helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188, 220–226 (1999).
Keenan, J. et al. A role for the bacterial outer membrane in the pathogenesis of helicobacter pylori infection. FEMS Microbiol Lett. 182, 259–264 (2000).
Vidakovics, M. L. et al. B cell activation by outer membrane vesicles-a novel virulence mechanism. PLoS Pathog. 6, e1000724 (2010).
Ren, D. et al. Characterization of extended co-culture of non-typeable haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med (Maywood) 237, 540–547 (2012).
Fernández-Rhodes, M. et al. New origins of yeast, plant and bacterial-derived extracellular vesicles to expand and advance compound delivery. Int J. Mol. Sci. 25, 7151 (2024).
Xie, J., Haesebrouck, F., Van Hoecke, L. & Vandenbroucke, R. E. Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends Microbiol 31, 1206–1224 (2023).
Xie, J., Li, Q., Haesebrouck, F., Van Hoecke, L. & Vandenbroucke, R. E. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 40, 1173–1194 (2022).
van der Pol, E. et al. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomedicine 14, 801–810 (2018).
Dragovic, R. A. et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7, 780–788 (2011).
Yuana, Y., Levels, J., Grootemaat, A., Sturk, A. & Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell Vesicles 3, 10.3402 (2014).
Böing, A. N. et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 3, 10.3402 (2014).
Habier, J. et al. Extraction and analysis of RNA isolated from pure bacteria-derived outer membrane vesicles. Methods Mol. Biol. 1737, 213–230 (2018).
Choi, J.-P., Jeon, S. G., Kim, Y.-K. & Cho, Y. S. Role of house dust mite-derived extracellular vesicles in a murine model of airway inflammation. Clin. Exp. Allergy 49, 227–238 (2019).
Tulkens, J. et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 69, 191–193 (2020).
Mosby, C. A., Bhar, S., Phillips, M. B., Edelmann, M. J. & Jones, M. K. Interaction with mammalian enteric viruses alters outer membrane vesicle production and content by commensal bacteria. J. Extracell. Vesicles 11, e12172 (2022).
Liu, Q. et al. Outer membrane vesicles derived from salmonella typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of salmonella enterica serovars in the mouse model. Int J. Med Microbiol 306, 697–706 (2016).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Ou, Z. et al. Single-particle analysis of circulating bacterial extracellular vesicles reveals their biogenesis, changes in blood and links to intestinal barrier. J. Extracell. Vesicles 12, e12395 (2023).
De Langhe, N. et al. Mapping bacterial extracellular vesicle research: insights, best practices and knowledge gaps. Nat. Commun. 15, 9410 (2024).
Tran, T. B. et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int J. Antimicrob. Agents 48, 592–597 (2016).
Velkov, T., Thompson, P. E., Nation, R. L. & Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med Chem. 53, 1898–1916 (2010).
Shin, H.-S., Gedi, V., Kim, J.-K. & Lee, D.-K. Detection of Gram-negative bacterial outer membrane vesicles using DNA aptamers. Sci. Rep. 9, 13167 (2019).
Ou, Z. et al. High-performance tracking of bacterial extracellular vesicles in living systems using an aggregation-induced emission luminogen. Chem. Eng. J. 446, 136847 (2022).
Goode, A., Yeh, V. & Bonev, B. B. Interactions of polymyxin B with lipopolysaccharide-containing membranes. Faraday Discuss 232, 317–329 (2021).
Slingerland, C. J., Kotsogianni, I., Wesseling, C. M. J. & Martin, N. I. Polymyxin stereochemistry and its role in antibacterial activity and outer membrane disruption. ACS Infect. Dis. 8, 2396–2404 (2022).
Bonine, N. G. et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am. J. Med Sci. 357, 103–110 (2019).
Zasowski, E. J. et al. A systematic review of the effect of delayed appropriate antibiotic treatment on the outcomes of patients with severe bacterial infections. Chest 158, 929–938 (2020).
Kemmler, C. B. et al. Delays in antibiotic redosing: Association with inpatient mortality and risk factors for delay. Am. J. Emerg. Med. 46, 63–69 (2021).
Acknowledgements
This work was supported by the National Science Fund for Distinguished Young Scholars (82025024, L.Z.); the Key Project of the National Natural Science Foundation of China (82230080, L.Z.); the National Natural Science Foundation of China (82302593, Z.O. and 82272438, B.S.); Guangdong Natural Science Fund for Distinguished Young Scholars (2023B1515020058, B.S.); the Natural Science Foundation of Guangdong Province (2023A1515012512, Z.O.); the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2022J001, B.S.); the Open Topic Funding Program of the Affiliated Qingyuan Hospital (Qingyuan People’s Hospital), Guangzhou Medical University (202301-202, J.L.); The Key Research and Development Program Project of Jiangxi Province (20232BBG70019, B.S.). Figures 1, 2g, 4b, d, g, l, 5a, g and Supplementary Fig. 8f were created with BioRender (Created in BioRender. Ou, Z. (2025) https://BioRender.com/d03p476).
Author information
Authors and Affiliations
Contributions
Z.O., B.S., and L.Z. contributed with conception and design of the work. Q.L. executed the experimental work, data acquisition and analysis. J.L, D.T., Q.W. and W.Y. collected clinical samples. B.H. analyzed the results of the PmBF characterization. Y.W., XY.H. and XX.H. contributed with animal work and data acquisition. B.R. contributed with EVs isolation work. Z.O., B.S., and L.Z. substantially revised the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Terri N. Ellis, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Ou, Z., Lin, J. et al. Specific labeling of outer membrane vesicles with antibiotic-conjugated probe reveals early bacterial infections in blood. Nat Commun 16, 3535 (2025). https://doi.org/10.1038/s41467-025-58676-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58676-8