Abstract
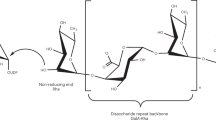
Rhamnogalacturonan I (RGI) is a structurally complex pectic polysaccharide with a backbone of alternating rhamnose and galacturonic acid residues substituted with arabinan and galactan side chains. Galactan synthase 1 (GalS1) transfers galactose and arabinose to either extend or cap the β-1,4-galactan side chains of RGI, respectively. Here we report the structure of GalS1 from Populus trichocarpa, showing a modular protein consisting of an N-terminal ___domain that represents the founding member of a new family of carbohydrate-binding module, CBM95, and a C-terminal glycosyltransferase family 92 (GT92) catalytic ___domain that adopts a GT-A fold. GalS1 exists as a dimer in vitro, with stem domains interacting across the chains in a ‘handshake’ orientation that is essential for maintaining stability and activity. In addition to understanding the enzymatic mechanism of GalS1, we gained insight into the donor and acceptor substrate binding sites using deep evolutionary analysis, molecular simulations and biochemical studies. Combining all the results, a mechanism for GalS1 catalysis and a new model for pectic galactan side-chain addition are proposed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The diffraction data and crystallographic models that support the findings of this study are available at the Protein Data Bank (https://www.rcsb.org/) under PDB accession codes 8D3T and 8D3Z for GalS1 in the apo form and the GalS1 bound to Mn2+, respectively. The SAXS data and model have been deposited and are available from the SIMPLE SAXS (https://simplescattering.com) database under the accession code XSMHXTBH. Other data that support the findings of this study and any computer code used herein are available from the corresponding author upon request. Source data are provided as part of this paper.
Code availability
The software used for analysis of the crystallographic data is freely available online or from the authors of each software package. Any computer code used herein is available from the corresponding author upon request.
References
Carpita, N. C. Update on mechanisms of plant cell wall biosynthesis: how plants make cellulose and other (1->4)-β-D-glycans. Plant Physiol. 155, 171–184 (2011).
Zabotina, O. A., Zhang, N. & Weerts, R. Polysaccharide biosynthesis: glycosyltransferases and their complexes. Front. Plant Sci. 12, 625307 (2021).
Rini, J. M. & Esko J. D. in Essentials of Glycobiology 3rd edn (eds Varki, A. et al.) Ch. 6 (Cold Spring Harbor Laboratory Press, 2017).
Na, L., Li, R. & Chen, X. Recent progress in synthesis of carbohydrates with sugar nucleotide-dependent glycosyltransferases. Curr. Opin. Chem. Biol. 61, 81–95 (2021).
Liwanag, A. J. M. et al. Pectin biosynthesis: GALS1 in Arabidopsis thaliana is a β-1,4-galactan β-1,4-galactosyltransferase. Plant Cell 24, 5024–5036 (2012).
Ebert, B. et al. The three members of the Arabidopsis glycosyltransferase family 92 are functional β-1,4-galactan synthases. Plant Cell Physiol. 59, 2624–2636 (2018).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. & Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 (2014).
Atmodjo, M. A., Hao, Z. & Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 64, 747–779 (2013).
Luis, A. S. & Martens, E. C. Interrogating gut bacterial genomes for discovery of novel carbohydrate degrading enzymes. Curr. Opin. Chem. Biol. 47, 126–133 (2018).
Laursen, T. et al. Bifunctional glycosyltransferases catalyze both extension and termination of pectic galactan oligosaccharides. Plant J. 94, 340–351 (2018).
Harholt, J., Suttangkakul, A. & Vibe Scheller, H. Biosynthesis of pectin. Plant Physiol. 153, 384–395 (2010).
Gorshkova, T. et al. Aspen tension wood fibers contain β-(1—> 4)-galactans and acidic arabinogalactans retained by cellulose microfibrils in gelatinous walls. Plant Physiol. 169, 2048–2063 (2015).
Ulvskov, P. et al. Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta 220, 609–620 (2005).
Obro, J. et al. Simultaneous in vivo truncation of pectic side chains. Transgenic Res. 18, 961–969 (2009).
Mellerowicz, E. J. & Gorshkova, T. A. Tensional stress generation in gelatinous fibres: a review and possible mechanism based on cell-wall structure and composition. J. Exp. Bot. 63, 551–565 (2012).
Zykwinska, A., Thibault, J.-F. & Ralet, M.-C. Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J. Exp. Bot. 58, 1795–1802 (2007).
Lin, D., Lopez-Sanchez, P. & Gidley, M. J. Binding of arabinan or galactan during cellulose synthesis is extensive and reversible. Carbohydr. Polym. 126, 108–121 (2015).
McCartney, L., Steele-King, C. G., Jordan, E. & Knox, J. P. Cell wall pectic (1->4)-beta-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 33, 447–454 (2003).
McCartney, L., Ormerod, A. P., Gidley, M. J. & Knox, J. P. Temporal and spatial regulation of pectic (1–>4)-beta-D-galactan in cell walls of developing pea cotyledons: implications for mechanical properties. Plant J. 22, 105–113 (2000).
Klaassen, M. T. & Trindade, L. M. RG-I galactan side-chains are involved in the regulation of the water-binding capacity of potato cell walls. Carbohydr. Polym. 227, 115353 (2020).
Culbertson, A. T., Ehrlich, J. J., Choe, J. Y., Honzatko, R. B. & Zabotina, O. A. Structure of xyloglucan xylosyltransferase 1 reveals simple steric rules that define biological patterns of xyloglucan polymers. Proc. Natl Acad. Sci. USA 115, 6064–6069 (2018).
Urbanowicz, B. R. et al. Structural, mutagenic and in silico studies of xyloglucan fucosylation in Arabidopsis thaliana suggest a water-mediated mechanism. Plant J. 91, 931–949 (2017).
Rocha, J. et al. Structure of Arabidopsis thaliana FUT1 reveals a variant of the GT-B class fold and provides insight into xyloglucan fucosylation. Plant Cell 28, 2352–2364 (2016).
Smith, P. J. et al. Enzymatic synthesis of artificial polysaccharides. ACS Sustain. Chem. Eng. 8, 11853–11871 (2020).
Loqué, D., Scheller, H. V. & Pauly, M. Engineering of plant cell walls for enhanced biofuel production. Curr. Opin. Plant Biol. 25, 151–161 (2015).
Prabhakar, P. K. et al. in Methods in Cell Biology Vol. 160 (eds Anderson, C. T. et al.) 145–165 (Academic Press, 2020).
Montanier, C. et al. Circular permutation provides an evolutionary link between two families of calcium-dependent carbohydrate binding modules. J. Biol. Chem. 285, 31742–31754 (2010).
Cid, M. et al. Recognition of the helical structure of beta-1,4-galactan by a new family of carbohydrate-binding modules. J. Biol. Chem. 285, 35999–36009 (2010).
Macquet, A., Ralet, M. C., Kronenberger, J., Marion-Poll, A. & North, H. M. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 48, 984–999 (2007).
Haughn, G. & Western, T. Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front. Plant Sci. 3, 00064 (2012).
Venditto, I. et al. Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc. Natl Acad. Sci. USA 113, 7136–7141 (2016).
Taujale, R. et al. Deep evolutionary analysis reveals the design principles of fold A glycosyltransferases. eLife 9, e54532 (2020).
Neuwald, A. F. A Bayesian sampler for optimization of protein ___domain hierarchies. J. Comput. Biol. 21, 269–286 (2014).
Sheikh, M. O. et al. Rapid screening of sugar-nucleotide donor specificities of putative glycosyltransferases. Glycobiology 27, 206–212 (2017).
Tsutsui, Y., Ramakrishnan, B. & Qasba, P. K. Crystal structures of β-1,4-galactosyltransferase 7 enzyme reveal conformational changes and substrate binding. J. Biol. Chem. 288, 31963–31970 (2013).
Venkat, A. et al. Modularity of the hydrophobic core and evolution of functional diversity in fold A glycosyltransferases. J. Biol. Chem. 298, 102212 (2022).
Prabhakar, P. K., Rao, K. K. & Balaji, P. V. The Cys78–Asn88 loop region of the Campylobacter jejuni CstII is essential for α2,3-sialyltransferase activity: analysis of the His85 mutants. J. Biochem. 156, 229–238 (2014).
Prabhakar, P. K., Srivastava, A., Rao, K. K. & Balaji, P. V. Monomerization alters the dynamics of the lid region in Campylobacter jejuni CstII: an MD simulation study. J. Biomol. Struct. Dyn. 34, 778–791 (2016).
Lunin, V. V. et al. Molecular mechanism of polysaccharide acetylation by the Arabidopsis xylan O-acetyltransferase XOAT1. Plant Cell 32, 2367–2382 (2020).
Wang, H.-T. et al. Rational enzyme design for controlled functionalization of acetylated xylan for cell-free polymer biosynthesis. Carbohydr. Polym. 273, 118564 (2021).
Zhang, Y. et al. Roles of active site tryptophans in substrate binding and catalysis by α-1,3 galactosyltransferase. Glycobiology 14, 1295–1302 (2004).
van der Veen, B. A. et al. Hydrophobic amino acid residues in the acceptor binding site are main determinants for reaction mechanism and specificity of cyclodextrin-glycosyltransferase. J. Biol. Chem. 276, 44557–44562 (2001).
Yang, T. et al. Hydrophobic recognition allows the glycosyltransferase UGT76G1 to catalyze its substrate in two orientations. Nat. Commun. 10, 3214 (2019).
Abbott, D. W. & van Bueren, A. L. Using structure to inform carbohydrate binding module function. Curr. Opin. Struct. Biol. 28, 32–40 (2014).
Oka, N., Mori, S., Ikegaya, M., Park, E. Y. & Miyazaki, T. Crystal structure and sugar-binding ability of the C-terminal ___domain of N-acetylglucosaminyltransferase IV establish a new carbohydrate-binding module family. Glycobiology 32, 1153–1163 (2022).
Valdez, H. A. et al. Role of the N-terminal starch-binding domains in the kinetic properties of starch synthase III from Arabidopsis thaliana. Biochemistry 47, 3026–3032 (2008).
Noguchi, J. et al. Crystal structure of the branching enzyme I (BEI) from Oryza sativa L. with implications for catalysis and substrate binding. Glycobiology 21, 1108–1116 (2011).
Breton, C., Mucha, J. & Jeanneau, C. Structural and functional features of glycosyltransferases. Biochimie 83, 713–718 (2001).
Lowe, J. B. & Varki, A. in Essentials of Glycobiology (eds Varki, A. et al.) Ch. 17 (Cold Spring Harbor Laboratory Press, 1999).
Kellokumpu, S., Hassinen, A. & Glumoff, T. Glycosyltransferase complexes in eukaryotes: long-known, prevalent but still unrecognized. Cell. Mol. Life Sci. 73, 305–325 (2016).
Boeggeman, E. E., Ramakrishnan, B. & Qasba, P. K. The N-terminal stem region of bovine and human β1,4-galactosyltransferase I increases the in vitro folding efficiency of their catalytic ___domain from inclusion bodies. Protein Expr. Purif. 30, 219–229 (2003).
Grabenhorst, E. & Conradt, H. S. The cytoplasmic, transmembrane, and stem regions of glycosyltransferases specify their in vivo functional sublocalization and stability in the Golgi. J. Biol. Chem. 274, 36107–36116 (1999).
Ramakrishnan, B., Balaji, P. V. & Qasba, P. K. Crystal structure of beta1,4-galactosyltransferase complex with UDP-Gal reveals an oligosaccharide acceptor binding site. J. Mol. Biol. 318, 491–502 (2002).
Ramakrishnan, B., Boeggeman, E. & Qasba, P. K. Binding of N-acetylglucosamine (GlcNAc) β1–6-branched oligosaccharide acceptors to β4-galactosyltransferase I reveals a new ligand binding mode. J. Biol. Chem. 287, 28666–28674 (2012).
Gámez-Arjona, F. M. & Mérida, Á. Interplay between the N-terminal domains of Arabidopsis starch synthase 3 determines the interaction of the enzyme with the starch granule. Front. Plant Sci. 12, 704161 (2021).
Christiansen, C. et al. The carbohydrate-binding module family 20–diversity, structure, and function. FEBS J. 276, 5006–5029 (2009).
Hedin, N., Velazquez, M. B., Barchiesi, J., Gomez-Casati, D. F. & Busi, M. V. CBM20CP, a novel functional protein of starch metabolism in green algae. Plant Mol. Biol. 108, 363–378 (2022).
Zhu, Y., Zhang, M., Kelly, A. R. & Cheng, A. The carbohydrate-binding ___domain of overexpressed STBD1 is important for its stability and protein-protein interactions. Biosci. Rep. 34, e00117 (2014).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Cornil, I., Kerbel, R. S. & Dennis, J. W. Tumor cell surface beta 1-4-linked galactose binds to lectin(s) on microvascular endothelial cells and contributes to organ colonization. J. Cell Biol. 111, 773–781 (1990).
Raz, A. & Lotan, R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 6, 433–452 (1987).
Moremen, K. W. et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 14, 156–162 (2018).
Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl Acad. Sci. USA 99, 13419–13424 (2002).
Pereira, J. H., McAndrew, R. P., Tomaleri, G. P. & Adams, P. D. Berkeley Screen: a set of 96 solutions for general macromolecular crystallization. J. Appl. Crystallogr. 50, 1352–1358 (2017).
Winter, G., Lobley, C. M. & Prince, S. M. Decision making in xia2. Acta Crystallogr. D 69, 1260–1273 (2013).
Hendrickson, W. A. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science 254, 51–58 (1991).
Terwilliger, T. C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D 65, 582–601 (2009).
Terwilliger, T. C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D 64, 61–69 (2008).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr .D 68, 352–367 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Classen, S. et al. Implementation and performance of SIBYLS: a dual endstation small-angle X-ray scattering and macromolecular crystallography beamline at the Advanced Light Source. J. Appl. Crystallogr. 46, 1–13 (2013).
Hura, G. L. et al. Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS). Nat. Methods 6, 606–612 (2009).
Guinier, A. & Fournet, G. Small Angle Scattering of X-rays (John Wiley & Sons, 1955).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Schneidman-Duhovny, D., Hammel, M., Tainer, John, A. & Sali, A. Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys. J. 105, 962–974 (2013).
Schneidman-Duhovny, D., Hammel, M. & Sali, A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 38, W540–W544 (2010).
Svergun, D. I., Petoukhov, M. V. & Koch, M. H. J. Determination of ___domain structure of proteins from X-ray solution scattering. Biophys. J. 80, 2946–2953 (2001).
Volkov, V. V. & Svergun, D. I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 36, 860–864 (2003).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Neuwald, A. F. Rapid detection, classification and accurate alignment of up to a million or more related protein sequences. Bioinformatics 25, 1869–1875 (2009).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and Python bindings. J. Chem. Inf. Model. 61, 3891–3898 (2021).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Jorgensen, W., Chandrasekhar, J., Madura, J., Impey, R. & Klein, M. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Huang, J. & MacKerell, A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Anandakrishnan, R., Aguilar, B. & Onufriev, A. V. H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 40, W537–W541 (2012).
Guvench, O., Hatcher, E., Venable, R. M., Pastor, R. W. & MacKerell, A. D. CHARMM additive all-atom force field for glycosidic linkages between hexopyranoses. J. Chem. Theory Comput. 5, 2353–2370 (2009).
Tabachnikov, O. & Shoham, Y. Functional characterization of the galactan utilization system of Geobacillus stearothermophilus. FEBS J. 280, 950–964 (2013).
Goubet, F., Jackson, P., Deery, M. J. & Dupree, P. Polysaccharide analysis using carbohydrate gel electrophoresis: a method to study plant cell wall polysaccharides and polysaccharide hydrolases. Anal. Biochem. 300, 53–68 (2002).
Shao, W., Sharma, R., Clausen, M. H. & Scheller, H. V. Microscale thermophoresis as a powerful tool for screening glycosyltransferases involved in cell wall biosynthesis. Plant Methods 16, 99 (2020).
Zhao, J. et al. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 163, 330–336 (2017).
Notredame, C., Desmond, H. G. & Heringa, J. T-Coffee: a novel method for multiple sequence alignments. J. Mol. Biol. 302, 205–217 (2000).
Goodstein, D. M. et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186 (2011).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–d444 (2022).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Acknowledgements
Funding to support B.R.U., P.K.P., V.S.B. and Y.J.B. was provided by the Center for Bioenergy Innovation (CBI), from the US Department of Energy Bioenergy Research Centers supported by the Office of Biological and Environmental Research in the DOE Office of Science. The work conducted by H.V.S., P.D.A., J.H.P. and M.H. at the Joint BioEnergy Institute is supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research under contract no. DE-AC02-05CH11231 between LBNL and the US Department of Energy. The Advanced Light Source is a Department of Energy Office of Science User Facility under Contract No. DE-AC02-05CH11231. The Berkeley Center for Structural Biology is supported in part by the Howard Hughes Medical Institute. The ALS-ENABLE beamlines are supported in part by the National Institutes of Health, National Institute of General Medical Sciences, grant P30 GM124169. Funding for the SIBYLS beamline at the Advanced Light Source was provided in part by the Offices of Science and Biological and Environmental Research, US Department of Energy, under Contract DE-AC02-05BH11231 and NIGMS grant P30 GM124169-01, ALS-ENABLE. Funding for N.K. and R.T. was provided by R35 GM139656 and R01 GM130915. Work by K.W.M., J.-Y.Y. and D.C. was supported by the US National Institutes of Health grants R01 GM130915 and P41GM103390 (to K.W.M.).
Author information
Authors and Affiliations
Contributions
P.K.P., J.H.P., R.T., W.S., V.S.B., N.K., M.H., P.D.A., H.V.S., D.C., J.-Y.Y. and B.R.U. designed and performed experiments, and analysed data. R.T., V.S.B. and N.K. performed computational simulations and machine learning studies. D.C. and J.-Y.Y. expressed proteins. K.W.M. and Y.J.B. designed experiments, analysed and interpreted data, and edited the paper. P.K.P., J.H.P., R.T., V.S.B., N.K., M.H., P.D.A., H.V.S. and B.R.U. wrote the paper. P.D.A., H.V.S. and B.R.U. conceived the project and B.R.U. led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Neighbor-Joining consensus tree of GT92 peptide sequences from plants.
All full-length protein sequences were downloaded from Phytozome v1295 and generated using the software suite Geneious 2019.1. The position of PtGalS1 in the tree is highlighted with a gold star (☆). Plant species are abbreviated as follows: Selaginella moellendorffii (Sm), Solanum lycopersicum (Solyc), Populus trichocarpa (Potri), Physcomitrella patens (Pp), Panicum virgatum (Pavir), Oryza sativa (Os), Glycine max (Glymax), Eucalyptus grandis (EucGr), Daucus carota (DCAR), Citrus sinensis (Csi), Citrus clementina (Ciclev), Carica papaya (Cpap), Brachypodium distachyon (Bradi), and Arabidopsis thaliana (At).
Extended Data Fig. 2
Schematics of the native Populus trichocarpa GalS1 protein (gene loci Potri.005G258900) and the recombinant proteins used in the study.
Extended Data Fig. 3 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and SEC-MALS-SAXS analysis of GalS1, CBM95 and their variants.
SDS page of a, purified GalS1 WT protein and mutant variants. b, purified GalS1-CBM and its variants. 5 µg of protein was loaded in each well. See Extended Data Fig. 2 for construct information. Expression levels of soluble secreted sfGFP fusion proteins in the culture media were monitored by GFP fluorescence, indicated in Extended Data Tables S2 and S3. Each transfection experiment for mutant variants was performed at least once and two different SDS PAGE gels monitoring protein purification were generated. Data presented here are representative of the final purified product used in respective experiments. c, SEC-MALS-SAXS analysis of GalS1. SEC elution profile for the GalS1, along with masses calculated from MALS and radius of gyrations calculated from SAXS-frame collected across the SEC-elution peak. The masses confirm that untagged GalS1 is a dimer in solution (MW SAXS = 110 kDa, MW MALS = 119 kDa). Original uncropped images are provided in the Source Data.
Extended Data Fig. 4 Electron-density map of GalS1.
a, Stereo view of a sample of 2 |Fo | - |Fc | Фcalc electron density. b, Stereo view of Cα trace of the GalS1 as dimer.
Extended Data Fig. 5 Structural alignment of GalS1 with mammalian β-GlcNAc β-1,4-galactosyltransferases (β4GalTs).
The core catalytic ___domain of GalS1 (ivory) is well aligned with other galactosyltransferases (differently colored). GalS1 shows additional N- and C-terminal domains that are hypothesized to be necessary for binding the RG-I backbone to facilitate galactan chain elongation. GalS1 is aligned with a, Bos taurus Btβ4GalT1 (RMSD 4.7 over 104 residues, green);53 b, Homo sapiens Hsβ4GalT7 (RSMD 5.6 over 128 residues, magenta);35 c, Homo sapiens Hsβ4GalT1 (RMSD 4.2 over 104 residues, aqua);54 and d, Drosophila melanogaster Dmβ4GalT7 and (RMSD 5.7 over 136 residues, red)35.
Extended Data Fig. 6 Role of the conserved stem region on oligomerization and activity of GalS1.
Stem region interaction across monomers in dimeric GalS1. b, Dim plot 2D interaction map of residues that interact between stem regions in GalS1 homodimer. c, Comparison of GalT activity and d, AraT activity in WT and GalS1ΔSTEM variant. The values shown are mean values (bar) ± standard deviation (error bars) of a representative experiment for n = 3 technical replicates (red circles) and plotted using GraphPad Prism 9.5.0. e, Change in oligomerization state of GalS1ΔSTEM as compared to GalS1WT determined by SEC-MALS. f, Web logo showing conservation of residues beyond residue 96 in the predicted stem domains of plant GT92 proteins from Salix suchowensis, Populus alba, Populus trichocarpa, Jatropha curcas, Hevea brasiliensis, Ricinus communis, Manihot esculenta, Olea europaea, Carica papaya, Herrania umbratica, Cephalotus follicularis, Eucalyptus grandis, Cucurbita moschata, Camellia sinensis, Mangifera indica, Punica granatum, Pistacia vera, Durio zibethinus, Telopea speciosissima, Vitis riparia, Gossypium hirsutum, Quercus lobata, Quercus suber, Thalictrum thalictroides, Syzygium oleosum, Sesamum indicum, Gossypium australe, Morus notabilis, and Vitis vinifera. The sequence number on the X-axis refers to native GalS1 from Populus trichocarpa and Y-axis is in bits with error bars that indicate an approximate Bayesian 95% confidence interval. Thirty-three (n = 33) species of plants were examined in this experiment.
Extended Data Fig. 7 GT92 family conserved residues in GalS1 predicted to contribute to ligand specificity.
a, Highlighted residues studied in the current work. b, Hypervariable regions (HV), predicted to impart acceptor specificity to GalS1, and core-hydrophobic regions are shown in orange and yellow, respectively.
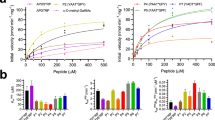
Extended Data Fig. 8 Donor specificity of GalS1 and β-1,4-galactotetraose acceptor dissociation constants (KD) of GalS1 WT and its variants.
a, Sugar-nucleotide specificity of GalS1. Reactions were carried out using 0.1 mM of UDP-substrate with 4 µg of the GalS1 enzyme in 50 mM HEPES, pH 7.5 in the absence of acceptor substrate. The values shown are mean values (bar) ± standard deviation (error bars) of a representative experiment for n = 3 technical replicates (red circles) and plotted using GraphPad Prism 9.5.0. b, Comparison of dissociation constants (KD) of PtGalS1 WT and its variants predicted for β-1,4-galactotetraose acceptor binding by microscale thermophoresis (MST). The values shown are mean values (filled circles) of the KD obtained after using KD fit model in the MO.Affinity Analysis software (NanoTemper Technologies) ± KD confidence (error bars). Error Bars represent standard deviation confidence (SD) values defined by the range where the KD falls with 68% of certainty, each point represents the mean of six sets of measurements (n = 6).
Extended Data Fig. 9 Comparison of galactosyltransferase activity of GalS1 WT and its variants by polysaccharide analysis using carbohydrate gel electrophoresis (PACE).
The results are representative of a single experiment.
Extended Data Fig. 10 Structural similarity between the experientially determined and AlphaFold2 predicted structures of GalS1.
a, Comparison of the X-ray structure of Populus trichocarpa GalS1 (97-495 residues; current study) determined in this work (in magenta) and the computationally predicted AlphaFold2 structure downloaded from the AlphaFold Protein Structure Database (AlphaFold id O22807;96,97 in green) of Arabidopsis thaliana GalS1 (97-496 residues). The root-mean-square deviation (RMSD; using Cα) of the aligned structures is 0.610 Å. b, Comparison of the X-ray structure of Populus trichocarpa GalS1 (97-495 residues; current study) determined in this work (in magenta) with the predicted AlphaFold2 structure of Populus trichocarpa GalS1 (97-495 residues) carried out in house using ColabFold98 (blue). The root-mean-square deviation (RMSD; using Cα) of the aligned structures is 0.593 Å.
Supplementary information
Source Data Extended Data Fig. 3
Unprocessed, uncropped SDS–PAGE gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prabhakar, P.K., Pereira, J.H., Taujale, R. et al. Structural and biochemical insight into a modular β-1,4-galactan synthase in plants. Nat. Plants 9, 486–500 (2023). https://doi.org/10.1038/s41477-023-01358-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01358-4