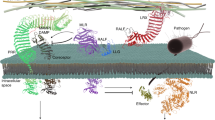
Abstract
Plants deploy intracellular nucleotide-binding leucine-rich repeats (NLRs) to detect pathogen effectors and initiate immune responses. Although the activation mechanism of some plant NLRs forming resistosomes has been elucidated, whether NLR resistosome assembly is regulated to fine-tune immunity remains enigmatic. Here we used an antiviral coiled coil-nucleotide-binding site–leucine rich repeat, Barley Stripe Resistance 1 (BSR1), as a model and demonstrate that BSR1 is phosphorylated. Using a proximity labelling approach, we identified a wall-associated kinase-like protein 20 (WAKL20) which negatively regulates BSR1-mediated immune responses by directly phosphorylating the Ser470 residue in the NB-ARC ___domain of BSR1. Mechanistically, Ser470 phosphorylation results in a steric clash of intramolecular domains of BSR1, thereby compromising BSR1 oligomerization. The phosphorylation site is conserved among multiple plant NLRs and our results show that WAKL20 participates in other NLR-mediated immune responses besides BSR1. Together, our data reveal phosphorylation as a mechanism for modulating plant resistosome assembly, and provide new insight into NLR-mediated plant immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data and materials needed to repeat the work are available. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository83,84 with the dataset identifier PXD047885. Source data are provided with this paper.
References
Kourelis, J. & van der Hoorn, R. A. L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 30, 285–299 (2018).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Dodds, P. N. & Rathjen, J. P. Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548 (2010).
Ngou, B. P. M., Ahn, H. K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Wang, J. et al. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, eaav5870 (2019).
Forderer, A. et al. A wheat resistosome defines common principles of immune receptor channels. Nature 610, 532–539 (2022).
Bi, G. et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell 184, 3528–3541 (2021).
Liu, F. et al. Activation of the helper NRC4 immune receptor forms a hexameric resistosome. Cell 187, 4877–4889 (2024).
Martin, R. et al. Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 370, eabd9993 (2020).
Ma, S. et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 370, eabe3069 (2020).
Jacob, P. et al. Plant “helper” immune receptors are Ca2+-permeable nonselective cation channels. Science 373, 420–425 (2021).
Huang, S. et al. Identification and receptor mechanism of TIR-catalyzed small molecules in plant immunity. Science 377, eabq3297 (2022).
Jia, A. et al. TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity. Science 377, eabq8180 (2022).
Madhuprakash, J. et al. A disease resistance protein triggers oligomerization of its NLR helper into a hexameric resistosome to mediate innate immunity. Sci. Adv. 10, eadr2594 (2024).
Couto, D. & Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552 (2016).
Jones, J. D., Vance, R. E. & Dangl, J. L. Intracellular innate immune surveillance devices in plants and animals. Science 354, aaf6395 (2016).
Ngou, B. P. M., Jones, J. D. & Ding, P. Plant immune networks. Trends Plant Sci. 27, 255–273 (2022).
Zhang, X. et al. Orchestrating ROS regulation: coordinated post-translational modification switches in NADPH oxidases. New Phytol. 245, 510–522 (2024).
Yuan, M., Ngou, B. P. M., Ding, P. & Xin, X. PTI-ETI crosstalk: an integrative view of plant immunity. Curr. Opin. Plant Biol. 62, 102030 (2021).
Liang, X. & Zhou, J. M. Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling. Annu. Rev. Plant Biol. 69, 267–299 (2018).
Tang, D., Wang, G. & Zhou, J. M. Receptor kinases in plant–pathogen interactions: more than pattern recognition. Plant Cell 29, 618–637 (2017).
Meng, X. & Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266 (2013).
Guo, H. et al. Phosphorylation-regulated activation of the Arabidopsis RRS1-R/RPS4 immune receptor complex reveals two distinct effector recognition mechanisms. Cell Host Microbe 27, 769–781 (2020).
Stephens, C., Hammond-Kosack, K. E. & Kanyuka, K. WAKsing plant immunity, waning diseases. J. Exp. Bot. 73, 22–37 (2022).
Rosli, H. G. et al. Transcriptomics-based screen for genes induced by flagellin and repressed by pathogen effectors identifies a cell wall-associated kinase involved in plant immunity. Genome Biol. 14, R139 (2013).
Saintenac, C. et al. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 50, 368–374 (2018).
Hu, K. et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 3, 17009 (2017).
Hurni, S. et al. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl Acad. Sci. USA 112, 8780–8785 (2015).
Zuo, W. et al. A maize wall-associated kinase confers quantitative resistance to head smut. Nat. Genet. 47, 151–157 (2015).
Decreux, A. & Messiaen, J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46, 268–278 (2005).
Brutus, A., Sicilia, F., Macone, A., Cervone, F. & De Lorenzo, G. A ___domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl Acad. Sci. USA 107, 9452–9457 (2010).
Kohorn, B. D. Cell wall-associated kinases and pectin perception. J. Exp. Bot. 67, 489–494 (2015).
Wang, P. et al. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell 32, 3978–4001 (2020).
Cui, Y. et al. Fine mapping of the BSR1 barley stripe mosaic virus resistance gene in the model grass Brachypodium distachyon. PLoS ONE 7, e38333 (2012).
Wu, Q. et al. The CC-NB-LRR protein BSR1 from Brachypodium confers resistance to barley stripe mosaic virus in gramineous plants by recognising TGB1 movement protein. New Phytol. 236, 2233–2248 (2022).
Lee, M. Y. et al. Brachypodium distachyon line Bd3-1 resistance is elicited by the barley stripe mosaic virus triple gene block 1 movement protein. J. Gen. Virol. 93, 2729–2739 (2012).
Zhang, Y. et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 10, 3252 (2019).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Fan, J. et al. Split mCherry as a new red bimolecular fluorescence complementation system for visualizing protein–protein interactions in living cells. Biochem. Biophys. Res. Commun. 367, 47–53 (2008).
Ahn, H. K. et al. Effector-dependent activation and oligomerization of plant NRC class helper NLRs by sensor NLR immune receptors Rpi-amr3 and Rpi-amr1. EMBO J. 42, e111484 (2023).
Contreras, M. P. et al. Sensor NLR immune proteins activate oligomerization of their NRC helpers in response to plant pathogens. EMBO J. 42, e111519 (2023).
Feehan, J. M. et al. Oligomerization of a plant helper NLR requires cell-surface and intracellular immune receptor activation. Proc. Natl Acad. Sci. USA 120, e2210406120 (2023).
Hu, M., Qi, J., Bi, G. & Zhou, J. M. Bacterial effectors induce oligomerization of immune receptor ZAR1 in vivo. Mol. Plant 13, 793–801 (2020).
Wang, Z. et al. Plasma membrane association and resistosome formation of plant helper immune receptors. Proc. Natl Acad. Sci. USA 120, e2222036120 (2023).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Kibby, E. M. et al. Bacterial NLR-related proteins protect against phage. Cell 186, 2410–2424 (2023).
Chou, W., Jha, S., Linhoff, M. W. & Ting, J. P. Y. The NLR gene family: from discovery to present day. Nat. Rev. Immunol. 23, 635–654 (2023).
Tena, G., Boudsocq, M. & Sheen, J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14, 519–529 (2011).
Arthur, J. S. C. & Ley, S. C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013).
Herold, L. et al. Arabidopsis WALL-ASSOCIATED KINASES are not required for oligogalacturonide-induced signaling and immunity. Plant Cell 37, koae317 (2025).
Larkan, N. J. et al. The Brassica napus wall-associated kinase-like (WAKL) gene Rlm9 provides race-specific blackleg resistance. Plant J. 104, 892–900 (2020).
Yue, Z. L. et al. The receptor kinase OsWAK11 monitors cell wall pectin changes to fine-tune brassinosteroid signaling and regulate cell elongation in rice. Curr. Biol. 32, 2454–2466 (2022).
Yuan, J. J. et al. The Arabidopsis receptor-like kinase WAKL4 limits cadmium uptake via phosphorylation and degradation of NRAMP1 transporter. Nat. Commun. 15, 9537 (2024).
Zhang, Q. et al. A maize WAK-SnRK1α2-WRKY module regulates nutrient availability to defend against head smut disease. Mol. Plant 17, 1654–1671 (2024).
Du, D. et al. The TaWAK2-TaNAL1-TaDST pathway regulates leaf width via cytokinin signaling in wheat. Sci. Adv. 10, eadp5541 (2024).
Wang, J. et al. Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 364, eaav5868 (2019).
Orioli, S., Henning Hansen, C. G. & Lindorff-Larsen, K. Transient exposure of a buried phosphorylation site in an autoinhibited protein. Biophys. J. 121, 91–101 (2022).
Henriques, J. & Lindorff-Larsen, K. Protein dynamics enables phosphorylation of buried residues in Cdk2/Cyclin-A-bound p27. Biophys. J. 119, 2010–2018 (2020).
Gong, T., Jiang, W. & Zhou, R. Control of inflammasome activation by phosphorylation. Trends Biochem. Sci. 43, 685–699 (2018).
Kim, J. et al. Rsk-mediated phosphorylation and 14-3-3ε binding of Apaf-1 suppresses cytochrome c-induced apoptosis. EMBO J. 31, 1279–1292 (2012).
McKee, C. M., Fischer, F. A., Bezbradica, J. S. & Coll, R. C. PHOrming the inflammasome: phosphorylation is a critical switch in inflammasome signalling. Biochem. Soc. Trans. 49, 2495–2507 (2021).
Song, N. et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 68, 185–197 (2017).
Zdrzalek, R., Stone, C., De la Concepcion, J. C., Banfield, M. J. & Bentham, A. R. Pathways to engineering plant intracellular NLR immune receptors. Curr. Opin. Plant Biol. 74, 102380 (2023).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Waadt, R. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56, 505–516 (2008).
Walter, M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438 (2004).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Goodin, M. M., Dietzgen, R. G., Schichnes, D., Ruzin, S. & Jackson, A. O. pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–383 (2002).
Gao, Z. et al. Coat proteins of necroviruses target 14-3-3a to subvert MAPKKKα-mediated antiviral immunity in plants. Nat. Commun. 13, 716 (2022).
Gao, Z. et al. Tobacco necrosis virus-AC single coat protein amino acid substitutions determine host-specific systemic infections of Nicotiana benthamiana and soybean. Mol. Plant Microbe Interact. 34, 49–61 (2021).
Zhao, Y. B. et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 8, eabq5108 (2022).
Bai, S. et al. Structure–function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 8, e1002752 (2012).
Wang, X. et al. Hsc70-2 is required for beet black scorch virus infection through interaction with replication and capsid proteins. Sci. Rep. 8, 4526 (2018).
Jiang, Z. et al. The barley stripe mosaic virus γb protein promotes viral cell-to-cell movement by enhancing ATPase-mediated assembly of ribonucleoprotein movement complexes. PLoS Pathog. 16, e1008709 (2020).
Li, Z. et al. Hijacking of the nucleolar protein fibrillarin by TGB1 is required for cell-to-cell movement of barley stripe mosaic virus. Mol. Plant Pathol. 19, 1222–1237 (2018).
Zhang, Q. et al. RETICULON-LIKE PROTEIN B2 is a proviral factor co-opted for the biogenesis of viral replication organelles in plants. Plant Cell 35, 3127–3151 (2023).
Zhang, Y. et al. TurboID-based proximity labeling for in planta identification of protein–protein interaction networks. J. Vis. Exp. 159, e60728 (2020).
Zhang, D. et al. The MAPK-Alfin-like 7 module negatively regulates ROS scavenging genes to promote NLR-mediated immunity. Proc. Natl Acad. Sci. USA 120, e2214750120 (2023).
Zhang, D. et al. Proxitome profiling reveals a conserved SGT1-NSL1 signaling module that activates NLR-mediated immunity. Mol. Plant 17, 1369–1391 (2024).
Wen, Z. et al. DEAD-box RNA helicase RH20 positively regulates RNAi-based antiviral immunity in plants by associating with SGS3/RDR6 bodies. Plant Biotechnol. J. 22, 3295–3311 (2024).
Hu, Y. et al. Phosphorylation of TGB1 by protein kinase CK2 promotes barley stripe mosaic virus movement in monocots and dicots. J. Exp. Bot. 66, 4733–4747 (2015).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Acknowledgements
We thank S. Ouyang at Fujian Normal University for kindly providing the Sr35 and AvrSr35 plasmids; J.-M. Zhou, M. Hu and Q. Shen at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for the MLA10 plasmid and valuable suggestions; L. Wan at the Institute of Plant Physiology and Ecology, Chinese Academy of Sciences for critical reading of this manuscript; Q. Jiang at Sichuan Agricultural University for help in preparation of BSR1-transgenic materials; X.-B. Wang, C. Han, J. Yu, M. Yang, Q. Gao and X. Fang at China Agricultural University for valuable suggestions on this work; Z. Li and Z. Su (China Agricultural University) for technical assistance in mass spectrometry and bioinformatic analyses; Z. Zhang and X. Guo (China Agricultural University) for help in structural modelling. This work was supported by grants from the National Natural Science Foundation of China (grant numbers 32320103003 and 32122070, Y.Z.), the Pinduoduo-China Agricultural University Research Fund (grant number PC2023B02012, Y.Z. and PC2024B01003, W.S.), the Chinese Universities Scientific Fund (grant number 2023TC074, Y.Z.) and the 2115 Talent Development Program of China Agricultural University (Y.Z.).
Author information
Authors and Affiliations
Contributions
Y.Z., C.Z., W.L., D.L., Z.L., S.P.D.-K. and W.S. conceptualized the project. C.Z., W.L., X.Z. and W.S. developed the methodology. C.Z., W.L., X.Z., D.Z. and Z.W. conducted formal analysis. C.Z., W.L., X.Z., Z.W., D.Z., Z.J., Z.G., H.G. and G.B. conducted investigations. Y.Z., C.Z. and D.Z. wrote the original draft. Y.Z. and S.P.D.-K. reviewed and edited the manuscript. Y.Z., D.L. and S.P.D.-K. supervised the project. Y.Z. acquired funding and administered the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Bostjan Kobe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Proxitome profiling of the BSR1 protein.
a, Diagram of the constructs used for the identification of proximal proteins to BSR1. The full-length BSR1 protein and its CC, NB-ARC, and LRR domains are shown on the top. b, Functional validation of TurboID-fused BSR1 protein. Fusion of TurboID to the C-terminus of BSR1 has no discernible effect on the ability of BSR1 to induce cell death. Cell death phenotype under bright light (left) or after trypan blue staining (right) is shown. c, Workflow for the PL analysis in this study. Agrobacterium mixtures containing TurboID fusions, with (+TGB1) or without TGB1 (−TGB1) under the control of the 35S promoter, were co-infiltrated into the leaves of N. benthamiana plants. At 36 hpi, 200 µM biotin was infiltrated into pre-infiltrated leaves. 8 h later, the infiltrated leaves were harvested for subsequent processing as indicated on the panel. Each treatment was performed with three independent biological replicates (n = 3 plants for each technical replicate). The numbers 126 to 131 above the leaf schematic indicate the 6-plex TMT reporter ion masses at m/z 126 to 131. d, e, Immunoblot analysis to confirm the enrichment of biotinylated proteins without (d) and with (e) the addition of TGB1. Infiltrated N. benthamiana leaves were harvested, followed by protein extraction and streptavidin beads enrichment. The enriched products were subjected to immunoblot analysis using the Streptavidin-HRP or antibodies as indicated on the bottom right of corresponding panels. Arrowheads indicate the bands of TurboID fusions. f, A Venn diagram illustrates the overlap among significantly enriched proteins identified in two sets of PL experiments depicted in (c).
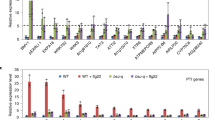
Extended Data Fig. 2 Screening of kinases that phosphorylate BSR1 protein.
a, List of seven kinases selected from the proximity labeling data for subsequent screening. b, BiFC assay to test the interaction between BSR1 and seven candidate kinase proteins. Scale bars, 20 μm. c, Immunoblot analysis to detect the protein expression in the infiltrated leaves shown in (b). The asterisk represents a nonspecific band. d, Analysis of the kinase activity of each protein using the universal kinase substrate myelin basic protein. e, f, Analysis of the phosphorylation of BSR1 by six candidate kinases. Autoradiographic images are shown on the top and the CBB-stained gel used as a loading control are shown below.
Extended Data Fig. 3 HvWAKL20 interacts with and phosphorylate the NB-ARC ___domain of BSR1.
a, BiFC assay to determine the interactions between HvWAKL20 and BSR1 NB-ARC ___domain. YFPc-fused bsr1, a null allele of BSR1, served as a negative control. Scale bars, 20 μm. b, Immunoblot analysis to validate protein expression shown in (a). c, MBP pull-down assay to test the interaction between the HvWAKL20 kinase ___domain (KD) or its variant and BSR1 NB-ARC ___domain. d, e, HvWAKL20 phosphorylates the BSR1 NB-LRR (d) and NB-ARC (e) domains in vitro. NB-ARC and NB-LRR domains were incubated with purified HvWAKL20 kinase ___domain respectively. The kinase-dead mutant HvWAKL20K387E served as the negative control.
Extended Data Fig. 4 Identification of phosphorylation site within the BSR1 by LC-MS/MS.
a, b, In vitro phosphorylation of BSR1 CC and LRR domains by NbWAKL20. The kinase-dead mutant NbWAKL20K375E served as negative control. The autoradiographic signals (left) and the CBB-stained gel used as a loading control (right) are shown. c, Silver-stained SDS-PAGE gel image of BSR1-9×Flag protein immunoprecipitated from N. benthamiana leaf tissues. The band indicated by the arrow was excised for LC-MS/MS analysis. d, CBB-stained SDS-PAGE gel image showing MBP-NB-ARC or MBP-NB-LRR proteins after treatment with NbWAKL20KD with or without the addition of ATP. The band indicated by the arrowheads were excised for LC-MS/MS analysis. e, Summary of the phosphorylation sites within the BSR1 identified by LC-MS/MS. f, In vitro phosphorylation of BSR1 NB-ARC ___domain and its variants by NbWAKL20. The autoradiographic signals (left) and the CBB-stained gel used as a loading control (right) are shown.
Extended Data Fig. 5 Identification of phosphorylation sites within BSR1 proteins driven by its native promoter.
a, Diagram of the BSR1 expression vector under its native promoter with different lengths. b, Immunoblot analysis of BSR1 expression driven by its native promoter. Asterisk represents a nonspecific band. c, Cell death phenotype of transiently expressed BSR1 under its native promoter in the presence of BSMV. Leaf regions infiltrated with Agrobacterium carrying an empty vector (EV) served as the control. d, Silver staining analysis of immunoprecipitation-enriched BSR1 protein expressed under the native promoter. e, Protein sequence coverage of the LC-MS/MS analysis of the enriched BSR1 protein shown in panel d, red letters indicate the peptides that were identified by LC-MS/MS. f, LC-MS/MS spectra demonstrate that Ser470 of BSR1 expressed under its native promoter is phosphorylated in vivo.
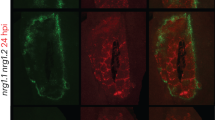
Extended Data Fig. 6 Brachypodium distachyon WAKL20 ortholog (BdWAKL20) interacts with and phosphorylates BSR1.
a, Confocal microscopy analysis revealed the colocalization of BdWAKL20 with BSR1. b, BiFC assay demonstrating the interaction between BdWAKL20 and BSR1. YFPc-fused bsr1, a null allele of BSR1, served as a negative control. The cell wall (dashed lines), the retracted plasma membrane (solid arrowhead), and Hechtian strands (hollow arrowhead) between the plasma membrane and the cell wall after plasmolysis are indicated. Scale bars, 20 μm. c, Immunoblot analysis validating the protein expression shown in b. Asterisk represents a nonspecific band. d, e, BdWAKL20 phosphorylates BSR1 (d) and its NB-ARC ___domain (e) in vitro. Full-length BSR1 and the NB-ARC ___domain were incubated with purified BdWAKL20 kinase ___domain. The kinase-dead mutant BdWAKL20K246E served as the negative control. f, BdWAKL20 predominantly phosphorylates the Ser 470 (S470) site within the NB-ARC ___domain. For (d–f), autoradiographic signals (upper) and the CBB-stained gel used as a loading control (lower) are shown. The experiments were independently repeated twice with similar results.
Extended Data Fig. 7 Structural modeling of BSR1 and MLA10 resistosomes.
Predicted 3D structure of BSR1 (a) and MLA10 (b) using AlphaFold 3. The predicted local distance difference test (pLDDT) scores range from 0 to 100, where pLDDT ≥ 90 indicates residues predicted with very high confidence. Residues with 90 > pLDDT ≥ 70 are classified as confident, while those with 70 > pLDDT ≥ 50 are predicted with low confidence. Values below 50 indicate extremely low confidence, suggesting that accurate prediction may not be possible. The PAE plots for the top two ranked models are shown on the right.
Extended Data Fig. 8 TGB1 effector attenuates HvWAKL20-mediated phosphorylation of BSR1 in a dose-dependent manner.
a, Co-localization of HvWAKL20 and TGB1 on the plasma membrane. The panels on the right depict normalized fluorescence intensities in the GFP (green) and mCherry (red) channels along the dashed lines in the merged images. Scale bars, 10 µm. b, BiFC to determine the interaction between HvWAKL20 and TGB1. PM and Hechtian strands between the PM and the cell wall after plasmolysis are indicated by dotted white lines and triangles, respectively. Scale bars, 20 µm. c, HvWAKL20 physically interacts with TGB1 protein, as indicated by the GST pull-down assay. Purified GST-tagged TGB1 (GST-TGB1) was incubated with the His-tagged kinase ___domain of NbWAKL20 (HvWAKLKD-His) or its kinase-dead variant (HvWAKLK387E-His). His-tagged GFP protein served as the negative control. d, e, In vivo interaction between the HvWAKL20 and the BSR1 NB-ARC ___domain was weakened in the presence of TGB1, as indicated by the LCI assay (d). Luminescence signals was measured by normalizing to the average fluorescence intensity using ImageJ software (e). Error bars represent mean ± SD (n = 4 biologically independent plants). Asterisks indicated the significant difference based on unpaired two-tailed t-test, ****P < 0.0001. f, TGB1 interferes with the binding of HvWAKL20 to BSR1, as indicated by in vitro competitive MBP pull-down assay. g, TGB1 compromised HvWAKL20-mediated phosphorylation of BSR1 NB-LRR ___domain, as indicated by the in vitro phosphorylation assay (upper). The CBB-stained gel served as the loading control (below).
Extended Data Fig. 9 Highly conserved kinase ___domain of putative WAKL20 orthologs in monocot and dicot plants.
Multiple sequence alignment of the kinase domains of putative WAKL20 proteins from various monocot and dicot plant species.
Extended Data Fig. 10 Analysis of the kinase activity of WAKL20 and its variants.
a, Schematic representation of WAKL20 and its variants used in the in vitro phosphorylation assay. SP, signal peptide; TM, transmembrane ___domain. b, Kinase activity of WAKL20 and its variants. c, Kinase activity of WAKL20 or WAKL20Δ26-262 in the presence or absence of BSMV infection. For panels b and c, the in vitro phosphorylation assay was conducted using proteins immunoprecipitated from N. benthamiana leaves transiently expressing WAKL20 or its variants, as indicated above each panel, with 5 μg of universal substrate Myelin basic protein (MBP). EXP. 1–3 represents three independent biological replicates. d and e show the quantification of the Myelin basic protein band intensity from panels b and c, respectively. Error bars represent the mean ± SD (n = 3 biological repeats). Different letters in the chart signify statistically significant differences among different groups, determined through unpaired two-tailed t-test (P < 0.05; see the Source Data file for the exact P values). n.s. = not significant, P > 0.05. No adjustments for multiple comparisons were made for indication of the significance.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Table 1, methods and source data for supplementary figures.
Supplementary Dataset 1–4
Supplementary Dataset 1. Full list of enriched identifications with BSR1-TurboID without decoys and common contaminants. Dataset 2. Full list of enriched identifications with BSR1-TurboID without decoys and common contaminants in the presence of TGB1 effector. Dataset 3. List of significantly enriched BSR1-proximal proteins using Citrine-TurboID as the control. Dataset 4. List of significantly enriched BSR1-proximal proteins using Citrine-TurboID as the control in the presence of TGB1 effector.
Source data
Source Data Figs. 1–7, and Source Data Extended Data Figs. 1–6, 8 and 10
Unprocessed western blots and/or gels, and statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, C., Li, W., Zhang, X. et al. A cell wall-associated kinase phosphorylates NLR immune receptor to negatively regulate resistosome formation. Nat. Plants 11, 561–579 (2025). https://doi.org/10.1038/s41477-025-01949-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-025-01949-3