Abstract
Toll/interleukin-1 receptor (TIR) ___domain proteins are immune signalling components and function as NAD+-cleaving enzymes to activate defence responses. In plants, TIR activation triggers cell death and severely represses growth, especially under osmotic stress, while in animals, it promotes axon degeneration. However, the mechanisms regulating TIR suppression remain unclear. Here we show that TIR NADase activity requires a conserved serine residue spatially close to the catalytic glutamate. The osmotic-stress-activated plant Ca2+-dependent protein kinases (CPKs), the mammalian Ca2+/calmodulin-dependent protein kinase II delta (CAMK2D) and TANK-binding kinase 1 (TBK1) phosphorylate TIR domains at this conserved serine, which blocks TIR NADase activities and functions, thereby maintaining growth in plants and suppressing SARM1 TIR signalling in animals. Our findings define a fundamental molecular mechanism by which phosphorylation at a conserved serine residue inhibits TIR signalling in plants and animals and sustains plant growth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the main text or the supplementary files. The biological materials are available from the corresponding author upon reasonable request. The mass spectrometric data on in vitro and in planta CPK3-mediated phosphorylation of SNC1TIR have been deposited in the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD060014 and PXD060129, respectively. The genes mentioned in this study can be found in the TAIR database (https://www.arabidopsis.org/) or the NCBI database (https://www.ncbi.nlm.nih.gov/) under the following identifiers: AT5G61900 (BON1), AT4G16890 (SNC1), AT1G47370 (RBA1), U27081 (L6), NM_015077 (SARM1), AT4G23650 (CPK3), AT4G09570 (CPK4), AT4G35310 (CPK5), AT2G17290 (CPK6), AT1G35670 (CPK11), AT5G66210 (CPK28), AT2G43790 (MPK6), AT1G51660 (MKK4), AT4G33950 (SnRK2.6), Q13557 (CAMK2D), Q9UQM7 (CAMK2A), Q9UHD2 (TBK1) and O43318 (TAK1). Source data are provided with this paper.
References
Horsefield, S. et al. NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365, 793–799 (2019).
Wan, L. et al. TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365, 799–803 (2019).
Essuman, K. et al. The SARM1 Toll/Interleukin-1 receptor ___domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343 (2017).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015).
Manik, M. K. et al. Cyclic ADP ribose isomers: production, chemical structures, and immune signaling. Science 377, eadc8969 (2022).
Leavitt, A. et al. Viruses inhibit TIR gcADPR signalling to overcome bacterial defence. Nature 611, 326–331 (2022).
Jia, A. L. et al. TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity. Science 377, eabq8180 (2022).
Huang, S. J. et al. Identification and receptor mechanism of TIR-catalyzed small molecules in plant immunity. Science 377, eabq3297 (2022).
Gilley, J. et al. Enrichment of SARM1 alleles encoding variants with constitutively hyperactive NADase in patients with ALS and other motor nerve disorders. eLife 10, e70905 (2021).
Bloom, A. J. et al. Constitutively active SARM1 variants that induce neuropathy are enriched in ALS patients. Mol. Neurodegener. https://doi.org/10.1186/s13024-021-00511-x (2022).
Chen, K. et al. BONZAI proteins control global osmotic stress responses in plants. Curr. Biol. 30, 4815–4825 e4814 (2020).
Kim, T. H. et al. Natural variation in small molecule-induced TIR–NB–LRR signaling induces root growth arrest via EDS1- and PAD4-complexed R protein VICTR in Arabidopsis. Plant Cell 24, 5177–5192 (2012).
Ariga, H. et al. NLR locus-mediated trade-off between abiotic and biotic stress adaptation in Arabidopsis. Nat. Plants 3, 17072 (2017).
Gilley, J., Orsomando, G., Nascimento-Ferreira, I. & Coleman, M. P. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 10, 1974–1981 (2015).
Angeletti, C. et al. SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated by multiple physiologically relevant NAD metabolites. iScience https://doi.org/10.1016/j.isci.2022.103812 (2022).
Figley, M. D. et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD+ ratio to trigger axon degeneration. Neuron https://doi.org/10.1016/j.neuron.2021.02.009 (2021).
Clough, S. J. et al. The Arabidopsis dnd1 ‘defense, no death’ gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl Acad. Sci. USA 97, 9323–9328 (2000).
Balague, C. et al. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15, 365–379 (2003).
Jambunathan, N., Siani, J. M. & McNellis, T. W. A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13, 2225–2240 (2001).
Hua, J., Grisafi, P., Cheng, S. H. & Fink, G. R. Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes Dev. 15, 2263–2272 (2001).
Yang, S. & Hua, J. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16, 1060–1071 (2004).
Li, Y., Pennington, B. O. & Hua, J. Multiple R-like genes are negatively regulated by BON1 and BON3 in Arabidopsis. Mol. Plant Microbe Interact. 22, 840–848 (2009).
Yang, D.-L. et al. Calcium pumps and interacting BON1 protein modulate calcium signature, stomatal closure, and plant immunity. Plant Physiol. 175, 424–437 (2017).
Mehlmer, N. et al. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J. 63, 484–498 (2010).
Boudsocq, M. et al. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464, 418–422 (2010).
Boudsocq, M., Droillard, M.-J., Regad, L. & Laurière, C. Characterization of Arabidopsis calcium-dependent protein kinases: activated or not by calcium? Biochem. J. 447, 291–299 (2012).
Li, Q., Hu, T., Lu, T., Yu, B. & Zhao, Y. Calcium-dependent protein kinases CPK3/4/6/11 and 27 respond to osmotic stress and activate SnRK2s in Arabidopsis. Dev. Cell https://doi.org/10.1016/j.devcel.2024.12.036 (2025).
Le Roux, C. et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 161, 1074–1088 (2015).
Saucet, S. B. et al. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat. Commun. 6, 6338 (2015).
Williams, S. J. et al. Structural basis for assembly and function of a heterodimeric plant immune receptor. Science 344, 299–303 (2014).
Zhu, S. Y. et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19, 3019–3036 (2007).
Mori, I. C. et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4, e327 (2006).
Ding, Y. L. et al. CPK28–NLP7 module integrates cold-induced Ca2+ signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. https://doi.org/10.1126/sciadv.abn7901 (2022).
Ding, C., Wu, Y. J., Dabas, H. & Hammarlund, M. Activation of the CaMKII–Sarm1–ASK1–p38 MAP kinase pathway protects against axon degeneration caused by loss of mitochondria. eLife 11, e73557 (2022).
Fujii, H., Verslues, P. E. & Zhu, J. K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl Acad. Sci. USA 108, 1717–1722 (2011).
Ichimura, K., Mizoguchi, T., Yoshida, R., Yuasa, T. & Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 24, 655–665 (2000).
Ofir, G. et al. Antiviral activity of bacterial TIR domains via immune signalling molecules. Nature 600, 116–120 (2021).
Essuman, K., Milbrandt, J., Dangl, J. L. & Nishimura, M. T. Shared TIR enzymatic functions regulate cell death and immunity across the tree of life. Science 377, eabo0001 (2022).
Yu, H. et al. Activation of a helper NLR by plant and bacterial TIR immune signaling. Science 386, 1413–1420 (2024).
Chen, H. et al. Two interacting transcriptional coactivators cooperatively control plant immune responses. Sci. Adv. 7, eabl7173 (2021).
Tian, L., Lu, J. X. & Li, X. Differential requirement of TIR enzymatic activities in TIR-type immune receptor SNC1-mediated immunity. Plant Physiol. 190, 2094–2098 (2022).
Schulze, S. et al. A role for calcium-dependent protein kinases in differential CO2- and ABA-controlled stomatal closing and low CO2-induced stomatal opening in Arabidopsis. N. Phytol. 229, 2765–2779 (2021).
Freischmidt, A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 (2015).
Cirulli, E. T. et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347, 1436–1441 (2015).
Kenna, K. P. et al. NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 48, 1037–1042 (2016).
Xu, D. C. et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell 174, 1477–1491 (2018).
Ratajczak, J. et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. https://doi.org/10.1038/ncomms13103 (2016).
Jing, T. et al. Copine proteins are required for brassinosteroid signaling in maize and Arabidopsis. Nat. Commun. 15, 2028 (2024).
Wang, Z., Meng, P., Zhang, X., Ren, D. & Yang, S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 67, 1081–1093 (2011).
Monaghan, J. et al. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16, 605–615 (2014).
Wang, J. et al. A regulatory module controlling homeostasis of a plant immune kinase. Mol. Cell 69, 493–504 e496 (2018).
Zhou, J. M. & Zhang, Y. Plant immunity: danger perception and signaling. Cell 181, 978–989 (2020).
Tian, H. et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature 598, 500–503 (2021).
Gerdts, J., Summers, D. W., Milbrandt, J. & DiAntonio, A. Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron 89, 449–460 (2016).
Zhang, Y., Goritschnig, S., Dong, X. & Li, X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15, 2636–2646 (2003).
Gantner, J., Ordon, J., Kretschmer, C., Guerois, R. & Stuttmann, J. An EDS1–SAG101 complex is essential for TNL-mediated immunity in Nicotiana benthamiana. Plant Cell 31, 2456–2474 (2019).
Nishimura, M. T. et al. TIR-only protein RBA1 recognizes a pathogen effector to regulate cell death in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E2053–E2062 (2017).
Wan, L. et al. Crystallization and preliminary X-ray diffraction analyses of the TIR domains of three TIR–NB–LRR proteins that are involved in disease resistance in Arabidopsis thaliana. Acta Crystallogr. F 69, 1275–1280 (2013).
Studier, F. W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005).
Essuman, K. et al. TIR ___domain proteins are an ancient family of NAD+-consuming enzymes. Curr. Biol. 28, 421–430 (2018).
Allen, J. J. et al. A semisynthetic epitope for kinase substrates. Nat. Methods 4, 511–516 (2007).
Acknowledgements
We thank J. L. Dangl and M. T. Nishimura for careful reading and discussion of the manuscript. We thank the members of the Zhao Lab for helpful discussions. We thank X. Xu and S. Wang of the Core Facility Center, CEMPS, for their help in mass spectrometry. We are grateful to M. Li (Zhongshan Hospital, Fudan University) for her contributions to the construction of NRK1- or TBK1/NRK1-HEK293T stable cell lines and the related transfection experiments. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences grant nos. XDB063020102 (to Y.Z.) and XDB27040214 (to L.W.), the Project of Stable Support for Youth Teams in Basic Research Field of the Chinese Academy of Sciences (no. YSBR-119 to Y.Z.), and the Science and Technology Commission of Shanghai Municipality grant no. 23ZR1470700 (to B.Y.). Y.Z. was supported by the Key Laboratory of Plant Carbon Capture and the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences. L.W. was supported by the State Key Laboratory of Plant Trait Design at the Institute of Plant Physiology and Ecology/Center for Excellence in Molecular Plant Sciences.
Author information
Authors and Affiliations
Contributions
Y.Z. and L.W. conceived and designed the research. J.L., S.C., B.Y., Q.L., R.L. and Z.W. performed the experiments. J.L., S.C., B.Y., L.W. and Y.Z. analysed the results. Y.Z., J.L. and L.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Patent applications related to this work have been submitted by Y.Z., L.W., J.L., S.C. and B.Y. The other authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Jonathan Jones and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dominant-negative SNC1 mutations suppress NLR signaling in bon1 mutants.
a, Gene structure of SNC1. The mutations of SNC1 in the insensitive to osmotic stress (ios) mutants are indicated. The ios mutants suppress bon1-7 based on plant growth under osmotic stress. The ios26 (snc1-17, a single amino acid change V518I) and ios102 (snc1-18, a single amino acid change G491S) mutations were distributed on the NB-ARC ___domain, likely affecting SNC1 oligomerization. The ios112 (snc1-20, a nonsense mutation resulting in a premature termination at codon 734), and ios113 (snc1-21, a single amino acid change L647F) mutations were distributed on the LRR ___domain, likely affecting SNC1 autoinhibition. The ios22 (snc1-16, a single amino acid change G1343D) was distributed on the C-terminal jelly-roll/Ig-like ___domain (C-JID). Interestingly, the ios109 (snc1-19, a single amino acid change S609N) mutation was distributed in the linker between the two domains (NL linker), adjacent to the autoimmune mutation of the gain-of-function snc1-1 (a single amino acid change E561K). b,c,e,f, Shoot growth of Col-0, bon1, ios22 (snc1-16) and ios26 (snc1-17) mutants, and bon1 transformed with OE-SNC1G1343D (corresponding to snc1-16 mutation) or CPK3pro::CPK3CA 15 days post-transfer to 1/2 MS medium (e,f) or 1/2 MS medium containing 100 mM mannitol (b,c). d, Morphology of 6-week-old soil-grown bon1 and ios26 (snc1-17), and OE-SNC1V518I (corresponding to snc1-17 mutation) transgenic plants in bon1-1 background. g-i, Free SA levels (g) and PR1 and PR2 expression (h,i) in AEQsig6 (wild type), bon1-7 and ios22, and bon1-7 transformed with OE-SNC1G1343D 5 days post-transfer to 1/2 MS medium containing 0 or 100 mM mannitol. j,k, Protein abundance of SNC1G1343D (j, snc1-16) and SNC1V518I (k, snc1-17) in OE-SNC1G1343D and OE-SNC1V518I transgenic plants in bon1 background was detected by anti-Myc antibody. Actin was used as a loading control. l, Shoot growth of Col-0 (wild-type) and snc1-1 mutant 15 days post-transfer to 1/2 MS medium containing 0 or 100 mM mannitol. Scale bars, 0.5 cm (b,c,e,f), and 2 cm (d). Error bars represent mean ± SD (n = 3 biological replicates) (g-i). Statistical analysis was performed using a two-way ANOVA with Tukey’s test; NS, not significant, P > 0.05, ****P < 0.0001.
Extended Data Fig. 2 Constitutively active CPK3 suppresses NLR signaling in bon1 mutants.
a, CPKs were co-immunoprecipitated with SNC1 in OE-SNC1-Myc transgenic plants treated with 600 mM mannitol. b, Venn diagram represents the overlap of CPKs detected as candidate SNC1 interacting proteins (a) and candidate osmotic stress-responsive proteins from the in vivo phosphoproteomics data from (https://www.pnas.org/doi/10.1073/pnas.1308974110). c, Expression of CPK3, 4, 5, 6, and 11 in Arabidopsis according to the Arabidopsis electronic fluorescent pictograph (eFP) browser. Expression is colored with increasing saturation from light blue to dark blue. d-f, Activation of CPK3 by osmotic stress in seedlings. CPK3 was immunoprecipitated from 7-day-old CPK3pro::CPK3-4Myc transgenic seedlings treated with 0 or 0.6 M mannitol for 30 min, and used for phosphorylation assay with [γ-³²P]ATP (d) or the ATP analog ATPγS (e). Autoradiography exhibits CPK3 phosphorylation (d). The thiophosphate ester groups were detected by anti-thiophosphate ester antibody (e), and relative band intensity was quantified (f). Total CPK3 was detected by anti-Myc antibody. g-i, Levels of free SA (g) and expression of PR1 (h) and PR2 (i) in Col-0, bon1-1, and bon1-1 transformed with CPK3pro::CPK3CA 5 days post-transfer to 1/2 MS medium containing 0 or 100 mM mannitol. j, The structure of CPK3 (top) and Sanger sequencing (bottom) show wild-type CPK3 and mutations on CPK3 in CPK3pro::CPK3CA transgenic plants in a bon1-1 mutant background, with the kinase-dead mutations (K107M and ΔK107). k, In planta kinase activity of CPK3CA-HA and its kinase-dead mutant (K107M) immunoprecipitated from N. benthamiana leaves 48 h post-agroinfiltration. Anti-thiophosphate ester antibody detected thiophosphate ester groups (top). Total CPK3CA and CPK3CA-K107M proteins were detected by an anti-HA antibody. l, Expression of wild-type and mutated CPK3CA-Myc and Actin in Col-0, bon1-1, and CPK3pro::CPK3CA, CPK3pro::CPK3CA-K107M, and CPK3pro::CPK3CA-ΔK107 in bon1-1 background. m, Protein abundance of SNC1-Myc, CPK3CA-Myc, and Tubulin in Col-0 and OE-SNC1-4Myc transgenic plants with or without CPK3pro::CPK3CA in Col-0 background. Error bars represent mean ± SD (n = 3 biological replicates) (g-i). Statistical analysis was performed using a two-way ANOVA with Tukey’s test; ***P = 0.0002, ****P < 0.0001. All experiments except (a, once; b and c, previously published data) were repeated at least three times with similar results.
Extended Data Fig. 3 CPKs interact with and phosphorylate SNC1TIR in vitro and in planta.
a, CPK3-SNC1TIR interaction in a co-immunoprecipitation assay using N. benthamiana eds1 mutant leaves expressing SNC1TIR-Myc, CPK3-YFP, and YFP-YFP. Total proteins were immunoprecipitated with anti-GFP agarose and detected with anti-Myc antibody. *CPK3-YFP; **YFP-YFP; ***SNC1TIR-Myc. b, CPK3-SNC1TIR interaction in split-LUC assays using N. benthamiana eds1 mutant leaves expressing CPK3-nLUC and cLUC-SNC1TIR. c, CPK3CA-SNC1TIR or CPK3CA-K107M-SNC1TIR interaction in N. benthamiana leaves in a co-immunoprecipitation assay. Total proteins were immunoprecipitated with anti-HA beads and detected with an anti-Flag antibody. d,e, Mass spectrometric analysis of GST-SNC1TIR phosphorylation at Ser18 (d) and Ser26 (e) by GST-CPK3. f, Phosphorylation of GST-SNC1TIR by GST-CPK3. Anti-thiophosphate ester antibody detected thiophosphate ester groups (top), anti-GST was used as loading control (bottom). g, Ser26 phosphorylation in SNC1TIR by GST-CPK3, without or with λ-protein phosphatase (λ-PPase). Anti-phospho-Ser26-SNC1TIR antibody (anti-pS26) detected phosphorylation (top), anti-GST was used as loading control (bottom). h-j, In planta Ser26 phosphorylation in SNC1TIR-Flag (h) or full-length SNC1-Flag (i) by CPK3CA-HA. SNC1TIR-Flag or SNC1-Flag was immunoprecipitated from N. benthamiana leaves co-expressing CPK3CA-HA. Anti-pS26 detected phosphorylation (top), and relative band intensity was quantified (j). Anti-Flag and anti-HA antibodies were used as loading controls. Error bars represent mean ± SD (n = 3). Two-sided Student’s t-test, ****P < 0.0001. k, In planta Ser26 phosphorylation in SNC1TIR by CPK3CA was detected by data-independent acquisition (DIA)-based mass spectrometry using immunoprecipitated SNC1TIR from N. benthamiana leaves co-expressing CPK3CA-HA. l, Phosphorylation of SNC1TIR by CPK3-4Myc immunoprecipitated from transgenic seedlings treated with 0.6 M mannitol for 30 min. Anti-thiophosphate ester antibody detected the phosphorylation (top), anti-SNC1 antibody was used as loading control. m-o, Phosphorylation of SNC1TIR by GST-CPK4/6/11 (m), GST-CPK5 (n) and GST-CPK28 (o). Autoradiography (top) and Coomassie staining (bottom) exhibit phosphorylation and loading. p, Phosphorylation of RBA1 and RBA1-S19A by GST-CPK3. Autoradiography exhibits phosphorylation (top). Anti-Flag antibody was used as a loading control (bottom). All experiments except (d,e,k, once) were repeated at least three times with similar results.
Extended Data Fig. 4 MPK6 and SnRK2.6 cannot phosphorylate the TIR ___domain of SNC1.
a,b, Phosphorylation of wild-type SNC1TIR by CPK3, MPK6 or SnRK2.6. GST-CPK3 was used as a positive control. Autoradiography (top) and Coomassie staining (bottom) exhibit phosphorylation and the loading of recombinant MBP-MAPK6, MKK4DD, GST-CPK3, MBP-SnRK2.6 and GST-SNC1TIR (a). The anti-thiophosphate ester antibody was used to detect the phosphorylation of SNC1TIR, CPK3, MAPK6 and SnRK2.6 (b, top panel). The anti-MBP and anti-GST antibodies were used to detect the loading of recombinant GST-SNC1TIR, MBP-MAPK6, MBP-MKK4DD, and MBP-SnRK2.6 (b, middle panel and bottom panel). All experiments were repeated at least three times independently with similar results.
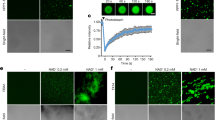
Extended Data Fig. 5 Phosphorylation of TIR represses its NADase activity.
a, Cartoon structures of SNC1TIR (PDB: 5TEC) and hSARM1TIR (PDB: 8D0J) showing catalytic glutamate (orange) and conserved serine (red) as sticks. The distances are shown for the carboxylic acid group of the catalytic glutamate with the hydroxyl group of the conserved serine. b-g, Purified wild-type RBA1 protein, but not the catalytically dead RBA1-E86A mutant, exhibited strong NADase activity. LC-MS/MS results showing that RBA1 could degrade NAD+ (b,c) and produce Nam (d,e) and 2′cADPR (f,g). LC-MS/MS chromatograms (b,d,f) and areas under curves (c,e,g) indicating that NADase activity of RBA1 depends on the catalytic glutamate. The purified RBA1-S19A and RBA1-S19D proteins were also used for in vitro NADase assays (Fig. 2c–f). h,i, LC-MS/MS analysis of the generation of Nam by wild-type RBA1, RBA1-S19A, and RBA1-S19D in vitro. Error bars represent mean ± SEM (n = 3). Two-tailed Student’s t-test (c,e,g), or one-way ANOVA with Tukey’s test (i), NSP = 0.0752, *P = 0.0372, **P = 0.0022 (g) and 0.0074 (i), ***P = 0.0009. RBA1-S19D could not produce Nam, degrade NAD+ (Fig. 2c, d) or produce 2′cADPR (Fig. 2e, f). j, Wild-type and mutated RBA1-Flag proteins purified from insect cells were detected by anti-Flag antibody. The purified RBA1 proteins were used for in vitro NADase assays (Fig. 2c–f, and b–i). k, Wild-type and mutated RBA1-YFP proteins were expressed in N. benthamiana eds1-2 mutant and detected by anti-GFP antibody. The resulting accumulation of 2′cADPR was shown in Fig. 2g. l, RBA1-Flag and RBA1-S19A-Flag were stably expressed in HEK293T cells, with transient expression of CPK3CA-Myc. RBA1-Flag, RBA1-S19A-Flag and CPK3CA-Myc were detected by anti-Flag and anti-Myc antibodies. Actin was detected with anti-actin antibody. The resulting degradation of NAD+ was shown in Fig. 2h. All experiments except (a) were repeated at least three times with similar results.
Extended Data Fig. 6 Phosphomimetic mutations of TIR repress TIR-mediated cell death.
Cell death phenotypes of wild-type and mutated TIRs upon transient expression in N. benthamiana leaves. The cell-death phenotype was visualized under visible light (left panel) or UV (right panel) and imaged 3 days after agroinfiltration inoculation. Different degrees of cell death were defined as the cell death score (0, blue; 1, coral pink; 2, orange). Each color on stacked bars shows the proportions (in numbers) of the corresponding degrees of cell death. a, Expression of SNC1TIR-YFP, non-phosphorylatable SNC1TIR-S18A-YFP, and phospho-mimic SNC1TIR-S18D-YFP. Plant leaves were infiltrated with Agrobacterium tumefaciens bacteria with OD600 at 0.2 (left) or 0.4 (right). b, Expression of SNC1TIR-YFP, non-phosphorylatable SNC1TIR-S18A/S26A (AA)-YFP, phospho-mimic SNC1TIR- S18D/S26D (DD)-YFP, and catalytically dead SNC1TIR-E93A-YFP. c, Expression of AbTIR-YFP, non-phosphorylatable AbTIR-S140A-YFP, phospho-mimic AbTIR-S140D-YFP, and catalytically dead AbTIR-E208A-YFP. All experiments were repeated at least three times with similar results.
Extended Data Fig. 7 Expression of wild-type and mutated TIR proteins and CPKs in planta.
a-c, SNC1TIR-YFP, L6TIR-YFP, RBA1-HA, and CPK3CA-cLUC-Flag proteins were transiently expressed in N. benthamiana leaves and detected by western blot. The anti-GFP antibody was used to detect SNC1TIR-YFP and L6TIR-YFP. The anti-HA antibody was used to detect RBA1-HA. The anti-Flag antibody was used to detect CPK3CA-cLUC-Flag. Cell death phenotypes of wild-type TIRs with or without CPK3CA were shown in Fig. 3b. d,e, SNC1TIR-S26A-Flag, full-length SNC1-Flag and CPK3CA-HA were transiently expressed in N. benthamiana leaves and detected by western blot. The anti-Flag antibody was used to detect SNC1TIR-Flag or full-length SNC1-Flag. The anti-HA antibody was used to detect CPK3CA-HA. Cell death phenotypes of SNC1TIR-S26A and full-length SNC1 with or without CPK3CA were shown in Figs. 3c, d, respectively. f-l, Wild-type and mutated TIR proteins were transiently expressed in N. benthamiana leaves and detected by western blot. Total proteins were extracted from N. benthamiana leaves. Ponceau S staining of RuBisCO was used as a loading control. The anti-GFP antibody was used to detect wild-type and mutated TIR-YFP proteins (f-j,l). The anti-HA antibody was used to detect RBA1-HA (k). SNC1TIR (f-i) related cell death phenotype was shown in Fig. 3e and S6; L6TIR (j) related cell death phenotype was shown in Fig. 3f; RBA1 (k) related cell death phenotype was shown in Fig. 3g; while hSARM1tSAM-TIR (l) related cell death phenotype was shown in Fig. 3h. m, Expression of CPK3CA-Myc and Actin in Col-0, cpk3/4/5/6/11 quintuple mutant, and CPK3pro::CPK3CA transgenic plants in Col-0 background was analyzed by reverse transcription PCR. The corresponding plant growth phenotype was shown in Figs. 3l, m. All experiments were repeated at least three times with similar results.
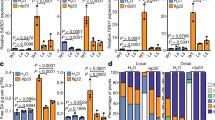
Extended Data Fig. 8 Phosphomimetic mutation of SNC1TIR represses its immune function and maintains plant growth.
a-c, Levels of relative expression of PR1 (a), PR2 (b) and free SA (c) in Col-0 and OE-SNC1-Myc transgenic plants expressing wild-type or S18D/S26D (DD)-mutated SNC1 in Col-0 background five days after transfer to the 1/2 MS medium containing 0 or 100 mM mannitol. d-f, Pst DC3000 triggered plant immunity in Col-0 and OE-SNC1-4Myc transgenic plants expressing wild-type or S18D/S26D (DD)-mutated SNC1 in Col-0 background. Plant leaves were infiltrated with Pst DC3000 bacteria with OD600 at 0.002. Disease symptoms (d), bacterial populations (e), and levels of free SA (f) were monitored 3 days post-infection. Error bars represent mean ± SD (n = 3 experiments) for (a-c), ± SD (n = 4 experiments) in (e), and ± SEM (n = 3 experiments) in (f). Letters denote significant differences (two-way ANOVAs, Tukey’s test; a,b). Asterisks denote significant differences: two-way ANOVA with Tukey’s test (c,f) and one-way ANOVA with Tukey’s test (e), NS P > 0.05, *P < 0.05, ****P < 0.0001. g, Protein abundance of SNC1 and SNC1S18D/S26D (DD) in OE-SNC1-4Myc transgenic plants expressing wild-type or S18D/S26D (DD)-mutated SNC1 in Col-0 background. The anti-Myc antibody was used to detect SNC1 and SNC1S18D/S26D (DD). All experiments were repeated at least three times with similar results.
Extended Data Fig. 9 The cpk3/4/5/6/11 quintuple mutant exhibited shoot growth defects and elevated levels of defense marker gene expression under hyperosmotic stress.
a,b, Shoot growth of Col-0, cpk3-2 mutant (a), and cpk3/4/5/6/11 quintuple mutant (b), 15 days post-transfer to 1/2 MS medium containing 0 mM or 100 mM mannitol. Scale bars, 0.5 cm. c,d, Levels of relative expression of PR1 (c) and PR2 (d) in Col-0 and cpk3/4/5/6/11 quintuple mutant five days after transfer to the 1/2 MS medium containing 0 or 100 mM mannitol. All experiments were repeated at least three times with similar results. Error bars represent means ± SD (n = 3). Asterisks denote significant differences (two-way ANOVA with Tukey’s test, ****P < 0.0001).
Extended Data Fig. 10 Regulation of SARM1TIR by TBK1.
a, Expression of hTBK1-Flag and Actin in N. benthamiana leaves transiently expressing hSARM1TIR-GFP with or without hTBK1-Flag was analyzed by reverse transcription PCR. The anti-GFP antibody was used to detect hSARM1TIR-GFP proteins. b, Human SARM1TIR-GFP-Flag and hSARM1TIR-S567A-GFP-Flag proteins were transiently expressed in the NRK1-HEK293T or TBK1/NRK1-HEK293T stable cell lines and detected by western blot. The anti-Flag antibody was used to detect wild-type and mutated hSARM1TIR-GFP-Flag. The anti-Myc antibody was used to detect TBK1-Myc. The anti-actin was used as a loading control. All experiments were repeated at least three times with similar results.
Supplementary information
Supplementary Information
Supplementary methods.
Supplementary Data 1
IP–MS data for SNC1–Myc under osmotic stress.
Supplementary Data 2
Primers used in this study.
Source data
Source Data Fig. 1
Unprocessed western blots for Fig. 1.
Source Data Figs. 1–4 and Extended Data Figs. 1–3, 5, 6, 8 and 9
Statistical source data for Figs. 1–4 and Extended Data Figs. 1–3, 5, 6, 8 and 9.
Source Data Extended Data Fig. 1
Unprocessed western blots for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Unprocessed western blots and gels for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3.
Source Data Extended Data Fig. 5
Unprocessed western blots for Extended Data Fig. 5.
Source Data Extended Data Fig. 7
Unprocessed western blots and gels for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Unprocessed western blots for Extended Data Fig. 8.
Source Data Extended Data Fig. 10
Unprocessed western blots and gels for Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Chen, S., Yu, B. et al. TIR immune signalling is blocked by phosphorylation to maintain plant growth. Nat. Plants 11, 1193–1204 (2025). https://doi.org/10.1038/s41477-025-02012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-025-02012-x