Abstract
Mitotic spindles are dynamically intertwined with the cytoplasm they assemble in. How the physicochemical properties of the cytoplasm affect spindle architecture and size remains largely unknown. Using quantitative biochemistry in combination with adaptive feedback microscopy, we investigated mitotic cell and spindle morphology during neural differentiation of embryonic stem cells. While tubulin biochemistry and microtubule dynamics remained unchanged, spindles changed their scaling behaviour; in differentiating cells, spindles were considerably smaller than those in equally sized undifferentiated stem cells. Integrating quantitative phase imaging, biophysical perturbations and theory, we found that as cells differentiated, their cytoplasm became more dilute. The concomitant decrease in free tubulin activated CPAP (centrosomal P4.1-associated protein) to enhance the centrosomal nucleation capacity. As a consequence, in differentiating cells, microtubule mass shifted towards spindle poles at the expense of the spindle bulk, explaining the differentiation-associated switch in spindle architecture. This study shows that cell state-specific cytoplasmic density tunes mitotic spindle architecture. Thus, we reveal physical properties of the cytoplasm as a major determinant in organelle size control.
Similar content being viewed by others
Main
The mitotic spindle is a prime example to study subcellular organizing principles and link physical laws to organelle function1. While spindles showcase a staggering morphological diversity, their primary function remains a mechanical one: to precisely partition the genetic material into two daughter cells2. Generally, spindles show two scaling regimes adapting to changes in cell size. In small cells, spindle size scales linearly with cell size and in large cells, the size of the spindle becomes decoupled from cell size and reaches an upper limit. Such key observations of spindle scaling mostly stem from fast reductive divisions in early mouse, frog, fish and worm embryos3,4,5,6,7,8,9,10. However, later in development, cellular differentiation poses a similar challenge: differentiating cells change size, morphology and function and therefore must reorganize and adapt their internal architecture. Changing spindle architecture while maintaining spindle function poses a structural and mechanical challenge to the cell and may require adaptation of the organizing principles11. Whether and how mitotic spindles adjust their morphology in differentiating cells remains largely unknown.
While spindles can function in a variety of cells across orders of magnitude of cell sizes, they all assemble mainly from one building block: αβ-tubulin. Tubulin assembles into dynamic microtubules that self-organize with the help of microtubule-associated proteins (MAPs) and motors into a spindle with a steady-state length. How can more or less identical building blocks assemble spindles with varying sizes and architectures? Our current understanding is that small spindles modulate microtubule dynamics to adjust spindle size, whereas large spindles require additional microtubule nucleation9,12,13. Spindle microtubule nucleation and dynamics can be modulated in many ways, by changes in tubulin biochemistry14, activity of MAPs and motors8,15,16,17, by the presence of a limiting component18,19 or changes in the biochemical composition of the cytoplasm6,9,20. In the context of differentiation, adjustments to spindle architecture have been described21,22. Whether these architectural adjustments occur via modified microtubule dynamics or spatial regulation of microtubule nucleation is currently unknown. We therefore lack a systematic understanding how microscopic processes collectively give rise to a mesoscale spindle that is adaptive to the differentiating cellular context.
It is increasingly appreciated that spindles are dynamically intertwined with the cytoplasm they assemble in. However, mechanisms of how cytoplasmic properties affect spindle assembly and function are only starting to emerge. For example, it has been shown that cytoplasmic viscosity affects the rates of microtubule polymerization and depolymerization23, spindle function24 and spindle positioning25,26. The mixing of the nucleoplasm and cytoplasm upon mitotic entry affects free tubulin concentration globally and consequently microtubule dynamics27. At the same time, local enrichment of tubulin by organelle-exclusion zones28,29 or at centrosomes30,31 spatially supports microtubule nucleation and growth. In addition, the mitotic cytoplasm softens32 and is fluidized by microtubule polymerization dynamics33. Both changes are thought to maintain diffusivity through the heterogenous metaphase cytoplasm. Although differentiation-mediated changes in cytoplasmic properties have been observed34,35,36,37,38,39,40, we do not know how these changes directly affect spindle assembly and scaling.
Here, we use a cell culture model of neural differentiation to study changes in spindle architecture, while cells change size, morphology and function. Studying neural spindle scaling was motivated by the observation that neurodevelopment is particularly susceptible to mutations in spindle genes, and by pathological evidence that links spindle morphology and mitotic susceptibility22,41,42,43. To do so, we established a noninvasive, long-term image acquisition and analysis workflow that allowed us to image more than 4,000 cells at single-cell resolution throughout neural differentiation. We find that spindle size subscales with cell volume; in early-differentiated cells spindles are 24% smaller when compared with spindles in equally sized undifferentiated stem cells, but still follow a similar functional dependence on cell size. Despite this difference in size, tubulin biochemistry and microtubule dynamics remain unchanged. We show that cytoplasmic dilution and a drop in tubulin concentration increase centrosomal nucleation capacity. This is dependent on centrosomal P4.1-associated protein (CPAP), a centrosomal regulator44,45,46. As a consequence, microtubule growth is redistributed to the spindle poles, resulting in a shift in spindle architecture. Consistently, differentiation-independent cytoplasmic dilution or the specific inhibition of the CPAP–tubulin interaction in undifferentiated embryonic stem cells (ESCs) phenocopied spindle architecture and size characteristic of early-differentiated cells. Our data are consistent with a theory in which microtubule number scales with cell volume and the inhibition of a centrosomal regulator determines the distribution between astral and bulk microtubules. Ultimately, our study links cell state-specific cytoplasmic material properties to spindle architecture and size. More generally, this work shows how local intracellular environments can exert control over organelle morphology and scaling.
Results
Spindle size subscales with cell volume upon differentiation
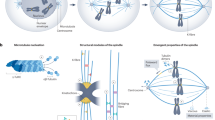
Mechanistic insights into spindle scaling stem primarily from fast, reductive cell divisions during early animal development3,4,8,9,13. It thus remains largely unknown whether differentiating cells tune spindle size relative to cell size and if so, whether the observed physicochemical mechanisms apply to this cellular context. This question is particularly relevant in neurally differentiating cells where spindle defects have documented pathological implications41. An experimentally accessible model is the differentiation of neurons from mouse ESCs in adherent cell culture47. To quantitatively study spindle scaling, we differentiated ESCs that stably expressed tubulin::GFP towards the neural lineage (Fig. 1a). This allowed us to image cells from pluripotency until terminal differentiation at single-cell resolution on a single substrate and in large numbers. We found that within 48–72 h of neural induction, the differentiating cells downregulated the expression of the pluripotency marker OCT-4 and started expressing nestin (Fig. 1a–d) and PAX6 (Extended Data Fig. 1a), consistent with previous reports47,48. Notably, we observed the formation of neural rosettes (Supplementary Video 1), which showed hallmarks of neuroepithelial physiology in terms of marker expression, cell polarity and interkinetic nuclear migration49,50 (Fig. 1a, Extended Data Fig. 1b–g and Supplementary Video 2). We concluded that this system represented a suitable model to study spindle morphology in a differentiation context.
a, Immunofluorescence staining of mouse ESCs driven towards neural fates in adherent monolayer. Every 24 h, a replicate culture was co-stained for OCT-488 (yellow, top row) and nestin (grey, top row) or stained for βIII-tubulin (grey, bottom row) (left). Neural rosette after 6 days of differentiation stained with Hoechst (blue) and against nestin (grey) (right). All images are maximum projections. Scale bars, 50 µm. Illustration showing the differentiation of ESCs towards neural progenitors (bottom). b, Percentage of OCT-4-positive cells (as in a), covering the first 5 days of neural differentiation (day 1, n = 418 cells; day 2, n = 1,316 cells; day 3, n = 1,199 cells; day 4, n = 2,666 cells; day 5, n = 3,976 cells from one experiment). c, As in b but showing percentage of nestin-positive cells (same experiment as b). d, Immunoblots probing for differentiation markers on a series of cell lysates (n = 1 experiment), covering 5 days of neural differentiation. α-Tubulin as a loading control. e, Automated microscopy setup. ESCs were either kept undifferentiated (‘ESCs’) or driven towards neural differentiation (‘DIF’). Adaptive feedback microscopy pipeline for live-cell imaging with high-content confocal series of metaphase cells expressing tubulin::GFP and chromatin stained with SiR-DNA. Scale bar, 50 µm. f, Confocal raw high-resolution data of a metaphase cell (n = 9 experiments) as described above. Tubulin::GFP (inverted grey) and SiR-DNA (blue). Scale bar, 5 µm. g, 3D-rendered metaphase spindles (grey) after automated volumetric segmentation and morphometry using the Fiji plugin Spindle3D51 and the pixel-classification tool Ilastik52. Chromosomes are shown in blue. The cell volume is illustrated as a cartoon for clarity. h, Spindle volumes scale with cell volumes during differentiation. Each data point represents an individual cell (ESCs n = 1,084 (yellow) and DIF n = 2,920 (blue)). Big circles represent the mean of each differentiation time bin. Error bars show the s.d. Data are pooled from nine independent experiments. rs: Spearman’s correlation, P < 1 × 10−100. i, In equally sized cells, spindle volume subscales in DIF (blue) when compared with ESCs (yellow). n (ESCs, DIF) = 5, 208; 57, 1,051; 280, 1,017; 445, 543; 238, 91; 59, 10 cells binned from 1,000 µm3 through 3,500 µm3 in 500-µm3 bins, with the same experiments as in h. White lines inside boxes denote medians, boxes show interquartile ranges, whiskers show minima and maxima. d, Cohen’s d (effect size). Welch’s t-test (two-sided) per bin. ****P < 0.0001; **P < 0.01; *P < 0.05.
Using this model system, we designed an experimental and analytical methodology to track individual cell families through differentiation while simultaneously detecting and quantifying spindle morphology. First, to overcome the challenge of generating sufficient quantitative three-dimensional (3D) imaging data of metaphase cells with minimal phototoxicity, we developed an adaptive feedback microscopy pipeline for live-cell imaging (Fig. 1e and Methods). Throughout the differentiation process, the fully autonomous microscope located metaphase cells within the larger population and launched high-content recordings exclusively of cells of interest (Fig. 1f). Next, using Spindle3D51 and Ilastik52, we extracted 3D morphometric parameters, including spindle volume, width, pole-to-pole length, cell volume and cell surface area from 1,084 undifferentiated and 2,920 differentiating mitotic cells (Fig. 1g,h and Extended Data Fig. 2a–d). To obtain the volumetric relationship between spindle size and cell size, we pixel-classified, segmented and validated cell volume through cytoplasmic tubulin::GFP. To segment spindles in 3D, the Spindle3D pipeline robustly finds a critical signal threshold value above which tubulin voxels are incorporated into the spindle bulk mask (Extended Data Fig. 2a). Thus, spindle volume serves as a proxy for spindle microtubule mass. Within the differentiating population, the average volume of mitotic cells decreased by 30% when compared with undifferentiated ESCs (VESC = 2,719 ± 567 µm3 and VDIF = 1,942 ± 424 µm3; Fig. 1h). This was independent of cell confluency and medium conditions (Extended Data Fig. 2e,f). Spindle volume decreased and scaled with cell volume (rs = 0.8; Fig. 1h). However, spindles in the early-differentiating cells (up to 5 days of differentiation) occupied significantly less cell volume than spindles in undifferentiated ESCs (Extended Data Fig. 2g). This became even more apparent when we binned cell volumes: in cells with comparable cell volume, the median spindle volumes were up to 24% smaller in cells undergoing differentiation (Fig. 1i). This subscaling behaviour was independent of cell geometry or microtubule density within the spindle (Extended Data Fig. 2h–j). Thus, spindle size is not specified solely by cell volume. Taken together, we established a noninvasive, long-term image acquisition and analysis workflow, which allowed us to quantify cell and spindle morphologies over many generations to show that spindle volume subscales in early-differentiating cells. This meant that cell volume cannot be the only determinant of spindle size. Rather, our findings implied a cell state-specific spindle scaling mechanism that was responsive to the biochemical or physical changes imposed by cellular differentiation.
Spindle architecture switches upon differentiation
Cells can change spindle size by changing the balance between microtubule nucleation, growth and turnover, which is known to alter microtubule polymer mass and spindle volume9,53,54. To test whether spindles in neurally differentiating cells change their size because of altered microtubule dynamics, we measured spindle microtubule turnover via fluorescence recovery after photobleaching (FRAP) measurements (Fig. 2a,b). We found the half-time of microtubule recovery in both spindle types to be comparable: 11.1 ± 4.6 s in stem cells and 9.7 s ± 5.8 s in differentiating cells (Fig. 2c). To measure microtubule growth velocities, we used the plus end-tracking protein EB1::tdTomato (Fig. 2d). We acquired time-lapse recordings of undifferentiated ESCs and early-differentiated cells and tracked growing microtubule plus ends (Supplementary Video 3). We found growth velocity to be independent of spindle size and mitotic cell volume (Extended Data Fig. 3a) and identical between the differentiation states (vp_ESC = 0.26 ± 0.034 µm s−1 and vp_DIF = 0.26 ± 0.031 µm s−1; Fig. 2e). Consistently, the cellular levels of the major microtubule polymerases CKAP5, which belongs to the TOG/XMAP215 family55, and CKAP2 (ref. 56) remained comparable (Extended Data Fig. 3b–e). Thus, despite the difference in spindle size, the microtubule growth dynamics and turnover remained unchanged.
a, FRAP (n = 2 experiments) of tubulin::GFP turnover in spindles. Selected frames pre-bleach and post-bleach are shown. Scale bar, 5 µm. b, Normalized FRAP recovery curves from a, lines show the mean (ESCs n = 31 cells, DIF n = 31 cells pooled from two independent experiments), bands show the s.d. c, Recovery half-times of tubulin::GFP derived from b, large circles show means, error bars indicate s.d., small circles show individual cells. Welch’s t-test (two-sided), P = 0.32. d, Mitotic cells expressing EB1::tdTomato imaged live in 0.4-s intervals (left) to determine growth speed and distribution of growing microtubules before and after differentiation (right). e, Average growth speed of EB1::tdTomato-labelled microtubules. Data points show individual cells (ESCs n = 92 cells, DIF n = 75 cells pooled from 6 independent experiments). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show minima and maxima. Welch’s t-test (two-sided), P = 0.41. f, EB1::tdTomato videos (20 s) were sum-projected (left). Half-spindle sum intensity profiles were drawn and subdivided into spindle poles (normalized distance 0–0.5) and central spindle (0.5–1) (right). g, Percentage of summed up EB1::tdTomato signal at the spindle pole (normalized half-spindle distance 0–0.5). Data points show individual cells (ESCs n = 158 cells, DIF n = 98 cells pooled from six independent experiments). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show minima and maxima. Welch’s t-test (two-sided) P = 1.9 × 10−7. h, Total number of EB1 comets per unit cell volume. Data points show ratio in individual cells (ESCs n = 65, DIF n = 47 from six independent experiments). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show minima and maxima. Welch’s t-test (two-sided), P = 0.47. i, Ratio of astral and spindle bulk EB1 comets. Data points show individual cells (ESCs n = 71 cells, DIF n = 48 cells pooled from six independent experiments). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show minima and maxima. Welch’s t-test (two-sided), P = 1.1 × 10−7. j, Max-projected micrographs showing immunostained microtubules (grey). Chromatin counterstained by Hoechst (blue). Dotted lines show cell boundaries. Scale bar, 5 µm. aMTs, astral microtubules. Bottom row, zoomed-in details (Scale bar, 2 µm). k, Differentiating cells increase their number of astral microtubules (as determined in f). Each data point represents a single cell (ESCs n = 115 cells, DIF n = 122 cells pooled from three independent experiments), large circles denote medians in each cell volume bin, error bars show interquartile ranges. ****P < 0.0001; NS, not significant, P > 0.05.
However, we found a considerable switch in spindle architecture: at 48 h of differentiation, microtubules increasingly grew from spindle poles but less in the spindle bulk (Fig. 2f,g and Extended Data Fig. 3a). This led to a shift in the ratio of astral to spindle bulk microtubules at a constant number of microtubules per cell volume (Fig. 2h,i). To complement the live data, we additionally visualized microtubule populations via 3D immunofluorescence (Fig. 2j), which confirmed that the number of astral microtubules was significantly higher in early-differentiated cells when compared with undifferentiated ESCs of equal cell volumes (Fig. 2k). Notably, in ESCs the number of astral microtubules seemed to saturate at a critical cell volume above ~3,000 µm3 (Fig. 2k). Together, these data indicated that the observed cell state-specific spindle scaling resulted from a redistribution of microtubules towards the asters away from the spindle bulk.
The switch in spindle architecture is analogous to observations in the developing mouse neocortex, where early neurogenic spindles displayed pronounced astral microtubules22. In this system, TPX2, a MAP that stabilizes microtubules and stimulates augmin-mediated microtubule nucleation57,58,59, has been identified as a main contributor of spindle morphology switches. In our system, however, neither cellular TPX2 nor augmin levels, nor their localization on spindles changed between undifferentiated ESCs and early-differentiated cells (Extended Data Fig. 4). Thus, we concluded that the switch in spindle architecture was not a consequence of diminished TPX2- or augmin-loading upon differentiation. Alternatively, microtubule number could be modulated by microtubule severing60,61,62. While total cellular concentrations of spastin and katanin p60 and p80 did not or only slightly change between stem cells and differentiating cells, we found katanin p80 to be enriched on spindle poles of stem cells (Extended Data Fig. 5a–g). However, while katanin knockdown did produce aberrant spindle phenotypes with buckled microtubules within the bulk63 (Extended Data Fig. 5h–k), it had no effect on spindle scaling (Extended Data Fig. 5l,m), ruling out microtubule severing as the main factor of spindle subscaling in our system.
Taken together, these data suggested that differentiating cells changed their spindle architecture and size by redistributing microtubule growth to the spindle poles. This resulted in smaller spindles relative to cell size when comparing differentiating and undifferentiated cells.
Centrosomes superscale in early-differentiated cells
The apparent increase in nucleation capacity at spindle poles could simply be a result of increased centrosome size64. Centrosomes are the major microtubule nucleating centres in mammalian cells and important for mitotic spindle assembly, asymmetric cell division, and cell polarity65,66. Therefore, we directly visualized and measured centrosomes by staining γ-tubulin (Fig. 3a) and components of the pericentriolar material (PCM), that is CDK5RAP2, CEP192 and pericentrin (Extended Data Fig. 6a–c). We found that centrosomes superscaled upon differentiation, where the relative volume occupied by centrosomal proteins increased by approximately 1.4-fold in early-differentiated cells when compared with the undifferentiated stem cells (Fig. 3b). Centrosome volume scaled with cell volume for both cell states (Fig. 3c), as has been observed in early Caenorhabditis elegans and Zebrafish embryogenesis4,67,68. However, centrosomes of undifferentiated ESCs became independent of cell size and approached an upper limit in cells with volumes above ~3,000 µm3 (Fig. 3c and Extended Data Fig. 6d–f). Indeed, in early-differentiated cells, more γ-tubulin was recruited to the centrosomes when compared with stem cells (Fig. 3d), while overall γ-tubulin levels did not change (Fig. 3e). The relative increase in centrosome size paralleled the increase in nucleation capacity and astral microtubule number in early-differentiated cells (Fig. 2k). This suggested a direct relation between centrosome volume and nucleation capacity, as has been reported previously64.
a, Confocal micrographs (maximum projected, representative of n = 6 experiments) of fixed ESCs or DIF at metaphase. Immunostained γ-tubulin signal (inverted grayscale) (top), immunostained γ-tubulin (yellow), tubulin::GFP (grey) and chromatin (Hoechst, blue) (bottom). Dotted lines indicate cell boundaries. Scale bar, 5 µm. b, Fold change in centrosome occupancy (centrosome volume:cell volume) in DIF relative to ESCs, comparing four centrosomal markers. Data points represent individual cells, boxes show interquartile ranges, vertical lines show medians and whiskers show the minima and maxima. n = 188, 182; 113, 100; 56, 44; 89, 73 cells (ESCs, DIF) stained for γ-tubulin, CDK5RAP2, CEP192 and pericentrin, each from six, three, one and three independent experiments, respectively. Significances against ESCs were tested with Welch’s t-tests (two-sided; γ-tubulin, P = 1.3 × 10−24; CDK5RAP2, P = 2.4 × 10−19; CEP192, P = 2.1 × 10−15; pericentrin, P = 1.3 × 10−24). c, Centrosome volume (γ-tubulin) scales with cell volume. Each data point represents a single cell (ESCs n = 188 cells and DIF n = 182 cells pooled from six independent experiments), large circles denote medians in each cell volume bin (bin size = 500 µm3), error bars show the interquartile ranges. Statistics per cell volume bin by Welch’s t-test (two-sided; 2,000–2,500 µm3, P = 0.01; 2,500–3,000 µm3, P = 1.8 × 10−3). d, γ-Tubulin localization (top). Centrosomal to bulk γ-tubulin mass ratio in immunostained cells (see a) (bottom). Data points represent individual cells (sample sizes as in c), boxes show interquartile ranges, vertical lines show medians and whiskers show the minima and maxima. Welch’s t-test (two-sided) P = 1.1 × 10−5. e, Top: representative western blots against γ-tubulin in ESCs or DIF. GAPDH as loading control. γ-Tubulin (normalized to GAPDH), relative fold change (bottom). Bars show mean (n = 3 biological replicates), error bars show s.d., circles show replicates. Welch’s t-test (two-sided), P = 0.28. f, Cell cycle lengths determined by tracking of individual cell families. Visual representation of tracking data of an exemplary adaptive feedback recording (Fig. 1) of ESCs. g, Cell cycle length changes upon differentiation89,90 Intermitotic times from time-lapse recordings (Fig. 1) of ESCs (yellow) or DIF (blue). Data points represent individual cells (n = 2,614 and 2,230 from ESCs and DIF, respectively, pooled from nine independent replicates). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show the minima and maxima. Welch’s t-test (two-sided), P = 1.2 × 10−55. h, As spindles subscale, centrosomes superscale upon differentiation. Comparing spindle bulk scaling to cell volume (ESCs n = 1,084 cells and DIF n = 2,920 cells pooled from nine independent experiments) and centrosome scaling to cell volume (data as in c) between ESCs and DIF. Circles show means, error bars show 95% confidence intervals. ****P < 0.0001, NS, not significant, P > 0.05.
Commonly, to prepare for mitosis, microtubule remodelling starts with a dramatic increase in microtubule nucleation at the centrosomes driven by the recruitment and local activation of γ-TuRC64,69. Our long-term imaging strategy allowed us to track individual cells across several generations (Fig. 3f). This showed that cells classified as early-differentiated cells had on average divided three to four times (Fig. 3g) and thus dynamically remodelled their PCM. Taken together, these data suggested that the relative increase in centrosome size shifts the microtubule nucleation capacities towards the spindle poles at the expense of the spindle bulk (Fig. 3h) leading to smaller spindles with larger asters in early-differentiated cells.
Constant tubulin biochemistry between the differentiation states
Another way of changing spindle architecture in differentiating cells could be through fundamental changes in tubulin biochemistry. Therefore, we measured tubulin isoform composition, tubulin post-translational modifications (PTMs) and tubulin levels. To determine tubulin isoforms and PTMs, we purified tubulin from either ESCs or early-differentiated cells70. Intact protein mass spectrometry revealed no significant changes in isoform composition between day 0 and 4 of differentiation (Fig. 4a,b). Considerable βIII-tubulin expression (as neuronal marker) started only with terminal differentiation later than day 4 (Fig. 1a) as reported previously47. Similarly, there were no differences between the PTM patterns in ESCs and early-differentiated cells (Fig. 4c). Together, this showed that neither tubulin isoform composition nor tubulin PTMs change during early differentiation. Next, we measured cellular tubulin levels by quantitative immunoblots (Fig. 4d and Methods). For each time point during differentiation, tubulin constituted approximately 1.5% of total cellular protein mass (Fig. 4e). From these data we concluded that changes in spindle morphology were not driven by tubulin biochemistry, and thus other spindle scaling mechanisms must be operating.
a, Deconvoluted mass spectra of tubulins purified from ESCs (left) or DIF (right). Average masses of the most abundant signals and corresponding tubulin isoforms, βV (MW = 49,670.3 Da, UniProt P99024), βIVb (MW = 49,830.5 Da, UniProt P68372), βIIb (MW = 49,952.6 Da, UniProt Q9CWF2), αIb (MW = 50,151.1 Da, UniProt P05213). b, Mean relative abundances of the four most dominant isoforms purified from ESCs versus 96 h DIF (n = 3 biological replicates) measured by intact protein mass spectrometry. Error bars show s.e.m. Circles show replicates. Welch’s t-test (two-sided), αIb: P = 0.68, βIIb: P = 0.40, βIVb: P = 0.79, βV: P = 0.50. c, Tubulin PTMs are comparable between differentiation states. Western blots using whole-cell lysates and purified tubulin (n = 2 biological replicates). Affinity-purified Bos taurus (Bt) brain tubulin loaded as positive control. Poly-Glu, poly-glutamylation; K40-Ac, tubulin lysine 40 acetylation; Detyr, detyrosinated tubulin. d, Representative tubulin western blot of three differentiation time points and whole-cell lysates (n = 3 biological replicates) (top). For downstream calibration, defined masses of purified tubulin were blotted onto the same membrane (three technical replicates per batch of lysates). Coomassie-stained whole protein content on a replica gel (bottom). e, Tubulin consistently represents 1.5% of the cellular protein mass. Relative tubulin content in total cellular protein mass in whole-cell lysates of ECSs (0 h) versus 48 h or 96 h DIF cells. Bars show mean ± s.e.m. of n = 3 biological replicates. Circles show mean ± s.e.m. of three technical replicates per experiment. One-way analysis of variance (ANOVA), F-statistic = 0.1263, P = 0.88. NS, not significant, P > 0.05.
The cytoplasm is diluted in early-differentiating cells
Differentiation-induced changes in cell function have been correlated with reductions in cellular mass density in a variety of cellular systems34,35,36,37,38,39. To test whether in our system cells change their mass density (defined as dry mass/volume; Methods)29 during differentiation, we measured subcellular distributions of refractive indices using correlative fluorescence and optical diffraction tomography71. Notably, we found that cellular mass density in early-differentiated cells was reduced by 10% relative to the undifferentiated ESCs (ρESC = 140 ± 12 mg ml−1 versus ρDIF = 125 ± 11 mg ml−1, Fig. 5a,b). This differentiation-associated dilution of the cytoplasm was observed across all cell volumes (Extended Data Fig. 7). Consistently, we measured a reduction in total cellular (spindle and cytoplasmic) tubulin::GFP fluorescence using live-cell imaging (Fig. 5c). This indicated a drop of total tubulin concentration concomitant with cytoplasmic dilution. While the reduction in total tubulin reduced spindle volume, it did not lead to a reduction in microtubule density within the spindle bulk (Extended Data Figs. 2j and 3a). Notably, both the observed decrease in cellular mass density as well as changes in spindle scaling were independent of culturing and differentiation conditions (Fig. 5d,e). Taken together, these data imply that changes in cellular mass density could have consequences for spindle architecture and scaling during differentiation.
a, Average (Avg) z-projections of 3D refractive index (RI) maps derived from optical diffraction tomography of mitotic ESCs or DIF (top). Colour-coded according to RI. Maximum-projected epi-fluorescent micrographs showing the tubulin::GFP signal (bottom). Dotted lines show cell boundaries. Representative images from n = 3 experiments. Scale bars, 5 µm. b, Cellular mass density decreases upon differentiation. 3D cellular mass densities of mitotic ESCs versus DIF. Data points show individual cells (ESCs n = 60 cells and DIF n = 70 cells pooled from three independent experiments). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show the minima and maxima. Welch’s t-test (two-sided), P = 2.2 × 10−10. c, Average tubulin::GFP signal (3D total cell) decreases during differentiation. Data from long-term automated imaging (Fig. 1). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show the minima and maxima. Data points show individual cells (ESCs n = 1,084 cells and DIF n = 2,920 cells pooled from nine independent experiments). Welch’s t-test (two-sided), P = 1.2 × 10−28. d, As in b but showing cellular mass density of DIF from 2i + LIF ESCs cultures (ESCs n = 52 cells and DIF n = 52 cells pooled from two independent experiments). Welch’s t-test (two-sided), P = 1.3 × 10−9. e, Spindle volume subscaling is differentiation intrinsic. Comparing spindle bulk volume scaling to cell volume in DIF originating from 2i + LIF ESCs cultures or from FBS + LIF ESCs cultures, as determined by confocal live-cell imaging (left). Data points show individual cells (ESCs (2i + LIF) n = 215, ESCs (FBS + LIF) n = 181, DIF (from 2i + LIF) n = 98, DIF (from FBS + LIF) n = 215, cells pooled from five independent experiments). Large circles represent medians in 500 µm3 cell volume bins. Error bars show interquartile ranges. Zoomed-in detail (right). ****P < 0.0001.
Cytoplasmic dilution shifts spindle architecture by increasing centrosomal nucleation capacity
The above data suggested that tuning cellular mass density could contribute to changes in spindle volume. If cytoplasmic dilution altered spindle morphology universally, we would expect a differentiation-independent drop in cellular mass density to affect spindle size in undifferentiated ESCs. To test this, we diluted the cytoplasm of stem cells by lowering the osmolality of the culturing medium (Fig. 6a,b). As expected, a decrease in osmolality led to an increase in cell volume and a reduction in cellular mass density (ρISO = 118 ± 10 mg ml−1 versus ρHYPO = 112 ± 9 mg ml−1; Fig. 6c,d). This drop in cellular mass density led to an increase in γ-tubulin at the centrosomes (Fig. 6e,f), consequently an increase in centrosomal EB1 concentration (Fig. 6g) and higher numbers of astral microtubules (Fig. 6h,i). Taken together, these data confirmed that a reduction in cellular mass density is sufficient to increase the centrosome’s nucleation capacity at the expense of the spindle bulk (Fig. 6j), resulting in a reduced spindle volume with constant microtubule density. Indeed, we were able to gradually adjust spindle scaling and architecture in stem cells by modulating cellular mass densities (Extended Data Figs. 8a–f). In the same manner, we rescued spindle subscaling in differentiating cells by concentrating the cytoplasm by hyperosmotic challenges (Extended Data Fig. 8g–j). These data imply that the spindle assembly mechanism is intertwined with the physical properties of the cytoplasm. But how is this implemented at the molecular level?
a, Osmotic perturbation of ESCs (i) and liberating CPAP from its inhibitory binding to tubulin in ESCs (ii). b, Average (Avg) z-projections of 3D RI maps derived from optical diffraction tomography (ODT) imaging of mitotic ESCs after adding isotonic medium (Iso, 337 mOsmol kg−1) or 25% ultrapure water (Hypo, 250 mOsmol kg−1) (top). Colour-coded according to RI. Maximum-projected epi-fluorescent micrographs showing tubulin::GFP signal (bottom). Dotted lines show cell boundaries. Scale bars, 5 µm. c, ODT-derived cell volumes after hypo-osmotic treatment of ESCs. Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show the minima and maxima. Data points show individual cells (n isosmotic: 107, n hypo-osmotic: 96 cells pooled from four independent experiments). Welch’s t-test (two-sided), P = 0.02. d, As c but showing mitotic cellular mass density. P = 6.2 × 10−6. e, Maximum-projected confocal micrographs showing immunostained γ-tubulin signals (yellow, or inverted grey (cropped images), respectively) in fixed ESCs, tubulin::GFP in grey, chromatin in blue. Cells after hypo-osmotic challenge (‘Hypo’) versus control (‘Iso’) (top), cells after CCB02 treatment versus control (dimethylsulfoxide ‘DMSO’) (bottom). Scale bars, 5 µm. f, Fraction of total γ-tubulin signals residing at centrosomes, comparing iso- versus hypo-osmotically treated ESCs (n = 71 and 60 cells from iso- and hypo-osmotically treated ESCs, respectively, pooled from two independent experiments, normalized to isosmotic control) and comparing DMSO- versus CCB02-treated ESCs (n = 109 and 121 cells from DMSO- and CCB02-treated ESCs, respectively, pooled from three independent experiments, normalized to DMSO control) and comparing ESCs (n = 188 cells) with DIF (n = 182 cells) (pooled from six independent experiments (Fig. 3b), normalized to ESCs). Boxes show interquartile ranges, black lines inside boxes denote medians, whiskers show the minima and maxima. Welch’s t-test (two-sided), Iso versus Hypo, P = 2.7 × 10−4; DMSO versus CCB02, P = 1.4 × 10−5; ESCs versus DIF, P = 2.9 × 10−8. g, Left: Sum-projected videos (20 s) of mitotic ESCs expressing EB1::tdTomato, after iso- or hypo-osmotic treatment (top), or after treatment with CCB02 or DMSO (bottom). Rectangle indicates region for sum intensity profiles, subdivided into spindle pole and central spindle. Boxplots as in f but showing fraction of EB1::tdTomato sum at spindle poles (right). Iso- (n = 53 cells) or hypo-osmotically treated (n = 67 cells) ESCs (pooled from n = 2 independent experiments), and of ESCs after CCB02 treatment (n = 40 cells) (versus DMSO control, n = 49 cells) (pooled from two independent experiments) and ESCs (n = 158 cells) or DIF (n = 98 cells) (same as Fig. 2g, pooled from six independent experiments). Iso versus Hypo, P = 7.2 × 10−4; DMSO versus CCB02, P = 8.7 × 10−4; ESCs versus DIF, P = 2.0 × 10−7. h, Confocal micrographs (max-projected) of metaphase control (‘Iso’) ESCs or after hypo-osmotic treatment (‘Hypo’) (top row) or CCB02- or DMSO-treated ESCs (bottom row), stained with anti-tubulin antibodies (grey). Chromatin in blue. Dotted lines show cell boundaries. Scale bar, 5 µm. i, Boxplots as in f but showing number of astral microtubules, comparing iso- (n = 37 cells) versus hypo-osmotically treated ESCs (n = 37 cells) (pooled from two independent experiments, normalized to isosmotic control), and comparing DMSO- (n = 54 cells) versus CCB02-treated (n = 70 cells) ESCs (pooled from two independent experiments, normalized to DMSO control), and comparing ESCs (n = 115 cells) with DIF (n = 122 cells) (pooled from three independent experiments (same data as Fig. 2k), normalized to ESCs). Iso versus Hypo, P = 1.1 × 10−7; DMSO versus CCB02, P = 1.1 × 10−6; ESCs versus DIF, P = 2.5 × 10−12. j, Boxplots as in f but showing percentage of cell volume occupied by spindle, comparing iso- (n = 67 cells) versus hypo-osmotically treated ESCs (n = 71 cells) (pooled from n = 2 experiments), and comparing DMSO- (n = 136 cells) versus CCB02-treated (n = 119 cells) ESCs (pooled from three independent experiments), and comparing ESCs (n = 181 cells) with DIF (n = 119 cells) (pooled from five independent experiments (data as Fig. 5e)). Iso versus Hypo, P = 1.3 × 10−5; DMSO versus CCB02, P = 3.8 × 10−20; ESCs versus DIF, P = 2.3 × 10−6. *P < 0.05; ***P < 0.001; ****P < 0.0001.
So far, there is no established link between cytoplasmic mass density and centrosome function. However, it has been reported that centrosomes can increase their nucleation capacity when free cytoplasmic tubulin decreases. This is because of the inhibitory interaction of soluble tubulin with the centrosomal protein CPAP72. We speculated that the observed decrease in free tubulin liberated CPAP, which in turn increased PCM recruitment and centrosomal microtubule nucleation. To mimic a decrease in free tubulin in stem cells, we liberated CPAP from tubulin using a small molecule inhibitor (CCB02 (ref. 73); Fig. 6a). This should result in an increase in astral microtubule number and a decrease in spindle bulk without changing cell size. Indeed, upon inhibitor treatment, spindles in undifferentiated ESCs partitioned more γ-tubulin to the centrosomes (Fig. 6e,f), which showed an increase in nucleation capacity as evidenced by a higher centrosomal EB1 concentration (Fig. 6g) and astral microtubule number (Fig. 6h,i), leading to a reduction in spindle volume in equally sized cells (Fig. 6j). Thus, we propose that CPAP is a molecular determinant of centrosomal nucleation capacity that is responsive to cytoplasmic dilution. Together, our physical perturbation via cytoplasmic dilution and biochemical perturbation using a small molecule inhibitor allowed us to phenocopy the spindle architecture characteristic of early-differentiated cell states in undifferentiated stem cells.
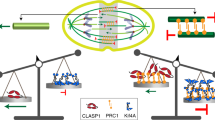
Notably, our data are consistent with quantitative models of spindle scaling9,18,53. In the Supplementary Note, we provide a theoretical model that allows us to link cell volume, spindle volume and cytoplasmic density to estimate the number of astral microtubules (also Supplementary Table 1). In our model, through the inhibition of a centrosomal regulator by free tubulin, the astral microtubule number has a Michaelis–Menten dependence on cell volume and is proportional to the concentration of free CPAP. This model is able to reproduce our experimental observations in stem cells and differentiating cells (Fig. 7a). Of note, this shows that spindle architecture can change considerably while total microtubule number does not. To ensure spindle size regulation during differentiation, this separation allows fine-tuning of spindle architecture without altering gross microtubule dynamics. Thus, by combining our experimental data and theory, we propose that the fundamental physical property of the cytoplasm (its mass density) provides a mechanism for regulating spindle architecture and size during differentiation.
a, For both cell states (ESCs, yellow; DIF, blue), number of astral microtubules is estimated from spindle volume data (experimental data as Fig. 1h and Supplementary Note Fig. 3) and grouped by cell volume bins of 500 µm3. Large circles depict averages for each cell volume bin (modelled data) and are fitted to equation (6), error bars show s.d. Solid curves represent fit curves. Dashed lines depict predicted saturation values for astral microtubule number. b, Upon differentiating of ESCs, a reduction in cellular mass density leads to enlargement of the pericentriolar material (PCM), increasing centrosomal nucleation capacity and redistribution of microtubule mass away from the spindle bulk towards the asters.
Discussion
The remarkable capacity of the spindle to scale with cell size has been observed across eukaryotes with spindle lengths ranging from 2 µm (yeast) to >50 µm (frog oocytes). Spindles showcase a large range in size not only between species but also within a single organism74. Yet, how spindles adapt to changes in cell size, shape and cytoplasmic properties as cells undergo differentiation was unclear. Here, we systematically quantify changes in spindle morphology and size during differentiation. Our data are consistent with a spindle scaling mechanism in which a reduction in cellular mass density leads to a relative increase in centrosome size in early-differentiated cells. As a consequence, microtubule nucleation shifts towards the spindle poles in these cells, promoting the generation of astral microtubules at the expense of the spindle bulk (Fig. 7b). This mechanism explains how a fundamental physical property of the cell (mass density) can lead to changes in the spindle’s nucleation profile and consequently spindle architecture.
Spindle scaling mechanisms
Based on the current knowledge of spindle assembly, a number of biophysical mechanisms has been established that explain the regulation of spindle size and the coupling to cell size to achieve spindle scaling. In the limiting component (volume-sensing) model, the finite availability of critical components has been implicated in the regulation of organelle size. In the context of spindle assembly, limiting components can be structural18,19 or regulatory8,13. A special case of component limitation is the cell surface-sensing mechanism, where the active cytoplasmic concentration of a spindle assembly factor is regulated through the sequestration of importin-α to the membrane6,9,20. Both the volume-sensing and surface-sensing scaling models describe physical mechanisms where the coupling of spindle size to cell size is inherently self-correcting. Here, we propose that another physical property of the cell is essential to spindle size regulation. We propose that changes in cellular mass density explain the cell state-specific scaling phenotypes in a differentiating system (Fig. 7). Indeed, a relationship between decreasing mass density and progressing differentiation has been proposed in many systems, including chondrocytes, keratinocytes, myeloid precursors and neurons34,35,36,37,38,39. Still, it is not immediately evident why and how neurally differentiating cells would change their mass density, in particular before terminal differentiation. One idea is that single-cell properties affect the overall mechanical properties of the developing tissue and this, in turn, influences differentiation75,76,77. This, however, was not the scope of this study and will therefore be part of future investigations.
In contrast to early stages of development, which typically are transcriptionally silent78,79, differentiation is not. Why would a physical mechanism for spindle scaling be in place? Cell-state transitions are often asynchronous within a tissue80,81,82. Thus, programmed scaling mechanisms based only on developmental timing or cytoplasmic composition would not robustly couple spindle size to cell size, potentially leading to errors in spindle positioning and chromosome segregation. This might be particularly relevant in neural development, a process that is notoriously sensitive to mutations in spindle and centrosome genes42,43.
We suggest CPAP as a molecular determinant of centrosomal nucleation capacity that is responsive to cytoplasmic dilution. Mutations in the CPAP gene link centrosomal defects to primary autosomal recessive microcephaly, a disorder characterized by severely reduced brain size and cognitive disability83. Consistently, mitotic spindle orientation defects have been described in cells depleted of CPAP84,85. While a direct link between microcephaly and spindle orientation defects remains to be definitely established, our data provide an explanation how interfering with CPAP function can have consequences for spindle architecture. Moreover, our findings are consistent with studies in neural stem cells of the embryonic developing mouse neocortex. Here, metaphase spindles displayed differences in their architecture from early to late neurogenic stages. At early neurogenic stages (comparable to the early-differentiated cells in our system), spindles displayed long astral microtubules and a reduced microtubule density near the chromosomes22. Together, these observations suggest that centrosome function via CPAP and resulting switches in spindle architecture could be a potential source for major developmental defects.
Coordination of microtubule nucleation pathways
Mitotic spindle assembly mainly relies on two pathways that generate microtubules: centrosome- and chromatin-driven microtubule nucleation. Both pathways depend on the recruitment of γ-tubulin, either to the centrosome or to pre-assembled microtubules. The current understanding is that in very large cells, spindle scaling is mainly achieved via microtubule nucleation to create sufficient amounts of polymer mass, while in small cells regulating microtubule dynamics can be sufficient to scale spindle size9. Indeed, the dominance of nucleation pathways has been shown to shift in the course of mitosis86 or even gradually over the course of development6,10. This cooperation of pathways is thought to provide robustness and mitotic fidelity87, but as we show here, it can also result in the construction of spindles with differing architectures, providing the necessary plasticity to adapt spindles to evolving cellular environments.
Methods
Quantification and statistical analysis
In each figure legend, details about the quantifications are provided, including the number of analysed cells and spindles measured (n). The effect size (Cohen’s d) was calculated by: d = (mean_of_ESCs − mean_of_DIFs)/s where s is the pooled s.d. of the parameter. No statistical methods were used to pre-determine sample sizes. No randomization was performed, as experiments were conducted with independent cell culture populations. Data collection and analysis were not performed blind to the conditions of the experiments. No data points were excluded from the analyses.
Data analysis was performed using the pandas library91 and the NumPy library92 in Jupyter Notebooks (using Python v.3.7.1). Data distribution was assumed to be normal, but this was not formally tested. Statistical tests were performed using the SciPy library (Welch’s t-test for unequal variances, Wilcoxon signed-rank test)93 or the Statsmodels package (ANOVA)94. Spearman’s correlation coefficients were determined using the SciPy stats.spearman package. Minimum-maximum normalization (EB1 half-spindle profiles) was performed using the Scikit-learn library95.
Stem cell culture and differentiation
R1/E mouse ESCs stably transfected with bacterial artificial chromosomes harbouring the eGFP-fused coding region of human β5-tubulin and its regulatory sequences for native expression levels (a gift from the Hyman laboratory)96 were cultured in FBS + LIF (16% FBS (Gibco), nonessential amino acids (Gibco), 50 µM β-mercaptoethanol, pen–strep (Invitrogen), 103 U ml−1 recombinant mouse LIF (Sigma-Aldrich) in high-glucose DMEM supplemented with l-Gln, pyruvate). After thawing from N2 storage, ESCs were passaged every 48 h. Tissue culture dishes (100 × 25 mm) were coated with 0.1% gelatin. The cells were washed with pre-warmed 1× PBS (pH 7.4) and detached from the vessel (Accutase (Invitrogen) at room temperature (RT)). Detachment was stopped by addition of FBS + LIF medium. Cells were centrifuged for 2 min at 200g. Cells were replated at a density of 25,000 cells per cm2 and incubated at 37 °C, 95% humidity and 5% CO2.
Alternatively, ESCs were cultured using 2i + LIF medium (N2 in DMEM/F12 (Gibco) mixed 50:50 with B27 (200×, without vitamin A, Gibco) in neurobasal medium (Gibco), supplemented with l-Gln and 50 µM β-mercaptoethanol, pen–strep (Invitrogen), 3 µM CHIR99021 and 1 µM PD0325901) prepared from N2 supplement (100×) made in-house (0.6 mg ml−1 progesterone (Sigma-Aldrich), 1.6 mg ml−1 putrescine (Sigma-Aldrich), 10 mg ml−1 apo-transferrin (Sigma-Aldrich), 3 µM sodium selenite (Sigma-Aldrich), 2.5 mg ml−1 insulin (Sigma-Aldrich) and 0.5% bovine albumin fraction V (Gibco) in DMEM/F12 (Gibco)). The cells were passaged as described above but using 2i + LIF instead of FBS + LIF and at a reduced cell seeding density of 15,000 cells per cm2.
For neural differentiation, ESCs were taken up in N2B27 medium (N2 (see above) in DMEM/F12 (Gibco) mixed 50:50 with B27 (200×, plus vitamin A, Gibco) in neurobasal medium (Gibco), supplemented with l-Gln and 50 µM β-mercaptoethanol, pen–strep (Invitrogen), 500 nM retinoic acid (Roth)) and seeded onto laminin-511 (L511, 2.5 µg ml−1 in 1× PBS (containing Ca2+ and Mg2+)) coated dishes or imaging wells at a density of 19,000 cells per cm2 and incubated at 37 °C, 95% humidity, 5% CO2. For differentiation times longer than 48 h, cells were passaged after 48 h and replated at a density of 200,000 cells per cm2.
Cells were routinely tested for Mycoplasma contamination using a commercial detection kit.
RNAi
For siRNA transfection, ESCs were seeded at a seeding number of 3,500 cells per cm2 on L511-coated dishes. After 24 h, the cells were transfected using the Lipofectamine 3000 kit (Thermo) and 20 nM of siRNAs (katnb1 5′-GGACUACAGGAGAUAUCAAtt-3′ or scrambled 5′-UUCUCCGAACGUGUCACGUtt-3′) according to the manufacturer’s instructions. After 48 h of knockdown, the cells were either fixed, immunostained and imaged or lysed and prepared for western blotting.
Live-cell confocal microscopy
For single time point imaging of mitotic cells, cells were seeded and differentiated as described above in L511-coated imaging plates (polymer bottom, Ibidi). Cells were imaged using a Nikon spinning-disk confocal equipped with an incubation chamber (37 °C, 95% humidity, 5% CO2), an Andor Revolution SD System (CSU-X), an iXon3 DU-888 Ultra EMCCD camera and a ×60 Plan Apo oil objective (numerical aperture (NA) 1.40), or on a Nikon Crest X-Light V3 spinning-disk confocal equipped with an incubation chamber (37 °C, 95% humidity, 5% CO2) and CFI Plan Apo VC ×60 water objective (NA 1.2).
Confocal series (z-range of 23 µm at a step size of 0.3 µm) were recorded using a multi-wavelength filter and the 488 nm and 640 nm laser lines at 5% laser power and 200-ms exposure time.
Adaptive feedback microscopy
Datasets were recorded on two laser-scanning confocal microscopes, the Zeiss LSM 800 and LSM 900, equipped with custom-built, temperature, CO2 and humidity-controlled incubation chambers (electronic and mechanical workshops, European Molecular Biology Laboratory (EMBL) Heidelberg). A C-Apochromat ×40 water objective (NA 1.2) was used. Signals were detected using GaAsP photomultiplier tubes. The objective was equipped with a custom-built automated water immersion system (electronic and mechanical workshops, EMBL Heidelberg).
The pipeline automatically locates mitotic cells and images them in 3D with high resolution. The pipeline consists of four image acquisition tasks and three image analysis tasks as described below. For settings, see Supplementary Table 2. The pipeline was driven by Zeiss ZEN Blue macros and an analysis protocol in Fiji97 using the AutoMicTools package.
-
(1)
Low-zoom autofocus. For each position in each imaging cycle, the focal plane was re-determined by using a reflection signal from coverslip surface. A fast XZ scan was performed using the piezo drive and the reflection signal was calculated by online image analysis and fed back to the microscope to trigger the acquisition of the low-zoom population image in focus.
-
(2)
Low-zoom population image. Two-channel (DNA, GFP) overview images of cells at predefined positions were acquired by recording 2 × 2-tile scans with a 10% overlap stitched in the ZEN Blue software. The stitched image was processed to identify positions of mitotic cells.
-
(3)
High-zoom autofocus. After receiving the xy coordinates of the positive hits, the pipeline determined the central focal plane for each mitotic cell for (4). For that purpose, a stack at low lateral resolution, high zoom factor and extended z-range was automatically acquired for each cell in question. The online image analysis in AutoMicTools identified the mask of the tubulin::GFP-labelled spindle and found its brightest z-section to correct lateral and axial position of a high-resolution acquisition.
-
(4)
High-zoom single-cell image. The positive hits were recorded in two channels with high spatial resolution.
For one imaging cycle (interval of 10 min), the microscope moved to the first predefined xy position and recorded the low-zoom autofocus image (1) that was analysed using the AutoMicTools plugin in Fiji. The correct focal z plane was sent back to the microscope that subsequently performed task (2). In the dedicated AutoMicTools online image analysis task the GFP channel in the overview raw image was maximum projected. Positions of mitotic cells were identified using the Multi-Template Matching plugin98 where we provided a template for object detection of tubulin::GFP-labelled spindles. The list with the xy coordinates was fed back to the microscope to proceed with tasks (3) and (4). Once completed, the cycle started anew at the next predefined position.
Quantification of cell cycle duration
Cell families were traced using the manual mode in TrackMate99 in the max-projected and median (sigma = 2)-filtered time-lapse videos generated via adaptive feedback microscopy. The time interval between two track division events was read out as the cell cycle duration.
Quantification of cell and spindle morphology
Cell volumes and cell surface areas were calculated via MorphoLibJ100 in Fiji after pixel-classifying and segmenting cells in 3D based on tubulin::GFP signal using Ilastik52 or using the Volume Manager Fiji plugin (Dresden, https://sites.imagej.net/SCF-MPI-CBG/). Spindle volume, length and width were determined using Spindle3D (v.0.80)51 after excluding all voxels outside the cell binary masks. In brief, Spindle3D finds a tubulin threshold by subdividing the tubulin voxels that are in contact with the chromatin mask into two populations, the cytoplasmic pool and the spindle pool. Thus, spindle volume is a proxy for spindle tubulin mass. Other geometrical parameters such as spindle length and width are based on the spindle volume mask. For clarity, microscopic images shown in the figures only show voxels within the cell binary masks.
Quantification of spindle tubulin turnover via fluorescence recovery after photobleaching
Tubulin::GFP signals in live spindles were bleached in Leica LAS software using a Leica TCS SP8 (Leica Microsystems) laser-scanning confocal system equipped with an incubation chamber (37 °C, 95% humidity, 5% CO2) and a ×63 Plan Apo glycerol objective (NA 1.30) and an Argon laser (set to 10%). A bleaching region of interest (ROI) of 4.4 × 1 µm spanning the spindle width at the half-distance between equator and pole was defined. Before and after bleaching, cells were imaged using the 488 nm laser (6.84 %), a photomultiplier tube with a gain of 760 V, in a field of view of 512 × 512, a zoom factor of 5.00 and a scanning speed of 400. One acquisition encompassed three frames pre-bleach at 1 s per frame, 1 s of bleach (488 nm at 100%) and 35 frames post-bleach at 1 s per frame. Signals within the bleached ROI were corrected for regular photobleaching during capture using a control ROI within the other half of the spindle, and min–max normalized. To determine recovery half-times, the corrected data were fit to an exponential recovery function A × (1 − e(−t/ tau)) + C using the scipy.optimize library. Half-times were calculated by t1/2 = tau × ln 2.
Quantification of microtubule growth velocity and EB1 comet numbers
ESCs were transiently transfected following manufacturer’s instructions (Lipofectamine 3000, Thermo) with plasmids carrying the EB1::tdTomato coding sequence under a CMV promoter (Addgene 50825). After overnight transfection, cells were plated onto L511-coated wells of a 24-well polymer bottom imaging plate (Ibidi) at a seeding density of 19,000 cells per cm2, and either maintained in FBS + LIF medium, or N2B27 medium for neural differentiation.
After 48 h, cells were imaged on a Nikon spinning-disk confocal equipped with an incubation chamber (37 °C, 95% humidity, 5% CO2), a Andor Revolution SD System (CSU-X), an iXon3 DU-888 Ultra EMCCD camera and a ×100 Plan Apo oil objective (NA 1.45). A confocal series of the tubulin::GFP signal was recorded (step size of 0.75 μm), using a multi-band filter and the 488 nm laser at 5% power and 200 ms of excitation duration.
Fluorescent EB1 comets were imaged in the most central position within the spindle (400 ms frames for 1 min) using a single-band filter in combination with the 561 nm laser at 5% and 300 ms of excitation duration.
For automated single-particle tracking using the Fiji plugin TrackMate99, the recordings were processed using a median filter (sigma = 1 μm) and a rolling-ball background subtraction (radius of 1 μm). In TrackMate, particles were detected using the Laplacian of Gaussian algorithm (subpixel localization, estimated blob diameter 0.5 μm, no median filter and threshold of 100–130). To generate tracks, the LAP tracker algorithm was used with the following settings: frame linking max distance 0.3 μm, gap closing not allowed, track splitting not allowed, track merging not allowed. Tracks were filtered by duration (2–4 s), median track quality (200–600) and track start and end within the video (frames 0–30).
Comet numbers were determined manually. For each recording, the numbers of spindle bulk EB1 comets and astral EB1 comets were determined in frame number 1, 20 and 40 and averaged. Last, the EB1::tdTomato signals were sum-projected in Fiji. Using the Fiji line tool at an averaging of 15 pixels, half-spindle profiles were drawn starting from one pole to the spindle equator. Projections were only included if the centre of the spindle and at least one pole was clearly in focus. For each profile, the x axis and the y axis were normalized using the min_max_scaler.fit_transform function in the Scikit-learn library95.
Quantification of pericentriolar material and spindle-localized proteins
Cells were chemically fixed using 3.2% PFA (in 1× PBS) for 10 min or fixed with ice-cold methanol for 4 min at −20 °C. After three washes with 1× PBS and 10 min quenching with 0.1 M glycine at RT (PFA-fixation only), the cells were treated with BSA blocking buffer (3% BSA/0.5% TX-100 in 1× PBS) for 1 h at RT. Primary antibody incubation was performed in BSA blocking buffer for 1 h at RT (CDK5RAP5 (rabbit, Sigma 06-1398), 1:500; CEP192 (rabbit, Proteintech 18832-1-AP), 1:500; pericentrin (rabbit, Abcam 4448), 1:500; γ-tubulin (mouse, Sigma T6557), 1:100; katanin p60 (rabbit, Proteintech 17560-1-AP), 1:125; katanin p80 (rabbit, Proteintech 14969-1-AP), 1:250, HAUS6 (rabbit, a gift from L. Pelletier), 1:200, TPX2 (mouse, a gift from A. Bird), 1:500, CKAP2 (mouse, Proteintech 25486-1-AP) and 1:200, KIF2C/MCAK (rabbit, Abcam ab71706), 1:500). Secondary antibody incubation was performed for 1 h at RT using goat anti-mouse (Thermo A-21235) or anti-rabbit (Thermo A-21244) Alexa Fluor 647 secondary antibodies diluted 1:1,000 in BSA blocking buffer.
Cells were imaged on a Nikon spinning-disk confocal microscope at RT, equipped with an Andor Revolution SD System (CSU-X), an iXon3 DU-888 Ultra EMCCD camera and a ×100 Plan Apo oil objective (NA 1.45) or a ×60 Plan Apo oil objective (NA 1.40). Confocal series were recorded using a z-step size of 0.3 µm. Using single-band filters, stacks of the individual channels were recorded serially (405 nm, 5% laser power; 488 nm, 18% laser power; and 640 nm, 5% laser power). Excitation duration per slice was 200 ms. Cells and spindles were segmented as described above.
The spindle binary mask was used to collect fluorescent signals of spindle-localized proteins (HAUS6, TPX2, CKAP2, KIF2C/MCAK, γ-tubulin). For 3D segmentation of the PCM, the PCM signal was thresholded using the maximum entropy algorithm in Fiji, considering the histogram from all optical sections. This method was robust because all noncell voxels were already eliminated from the confocal stack. Volumes of the centrosomes were calculated from the centrosome binary mask using the Analyze Regions 3D function in MorphoLibJ100. The average cellular fluorescence was determined using the Fiji 3D Suite101 and the cell binary mask. Analogously, this was repeated for the average signal in the centrosome binary mask/the spindle binary mask. The average signals from the centrosome mask and the spindle mask, respectively, were normalized using the cell average signal.
Quantification of astral microtubules
For this work, we define astral microtubules as the subpopulation of mitotic microtubules that originate at the centrosomes and are not incorporated into the spindle bulk. To visualize astral microtubules, cells were fixed and stained with anti-tubulin antibodies. Cells were chemically fixed for 10 min (3.2% paraformaldehyde/0.1% glutaraldehyde in 1× PBS), washed three times with 1× PBS, quenched with 0.1% NaBH4 in 1× PBS for 7 min at RT and for 10 min with 100 mM glycine in 1× PBS and blocked (3% BSA / 0.5% TX-100 in 1× PBS) for 1 h at RT. The cells were incubated for 1 h at RT with BSA blocking buffer supplemented with anti-α-tubulin antibodies (DM1a (Sigma T-6199, mouse origin), diluted 1:1,000 and Yol1/34 (Bio-Rad MCA78G, rat origin), diluted 1:1,000). After three washes with 1× PBS, cells were incubated with secondary antibodies (goat anti-mouse Alexa Fluor 647 (Thermo A-21235) 1:1,000 in blocking buffer and goat anti-rat Alexa Fluor 647 (Thermo A-21247) 1:1,000 in blocking buffer). After DNA staining with Hoechst 33342 (1:10,000 in 1× PBS) for 5 min at RT, cells were washed three times with 1× PBS.
Cells were imaged on a Nikon spinning-disk confocal microscope at RT. Confocal series were recorded using a z-step size of 0.3 µm. Using single-band filters, stacks of the individual channels were recorded serially (405 nm, 5% power; 488 nm, 18% power; and 640 nm, 5% power). Excitation duration per slice was 200 ms.
The stained microtubule signals were maximum projected in Fiji. The individual astral microtubules were traced using the free-hand selection tool.
Western blotting and tubulin quantification
Whole-cell lysates were prepared from adherent ESCs throughout differentiation. The lysis of cells from different stages (24–120 h) of differentiation was synchronized by staggering the differentiation onset. Using the differentiation procedures as described above, cells were seeded onto L511-coated six-well plates, three replica wells per differentiation day. For the ‘0 h’ time point, the ESCs were seeded at a density of 19,000 cells per cm2 and incubated in FBS + LIF for 48 h.
Plates were placed on ice and cells were incubated with cold, protease inhibitor-supplemented RIPA lysis buffer (150 mM NaCl/50 mM Tris-HCl (pH 8.0)/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS in ddH2O) and scraped off. Lysates were collected and incubated for 15 min on ice. The lysates were sonicated in a water bath sonicator three times for 2 s each, with 1-min recovery periods on ice between the pulses. The lysates were incubated another 15 min on ice before centrifuging at 13,000g for 5 min at 4 °C. Protein concentrations of supernatants were determined using a bicinchoninic acid (BCA) assay (Thermo).
Lysates were diluted 1:4 with SDS sample buffer (4×) (200 mM Tris-Cl (pH 6.8), 400 mM dithiothreitol, 8% SDS, 0.4% bromophenol blue and 40% glycerol) and heated for 5 min at 95 °C. Sample volumes corresponding to 4 µg, 2 µg and 1 µg of total protein, alongside defined masses (25 ng, 50 ng and 100 ng) of tubulin purified from ESCs, were loaded onto NuPAGE 4–12% Bis-Tris protein gels (Thermo) and mounted in gel running chambers filled with 1× NuPAGE SDS running buffer (Thermo). A replicate gel (with adjusted masses of tubulin controls of 100 ng, 200 ng and 400 ng) was loaded in parallel for Coomassie staining of total protein for normalization102.
The original gel was blotted onto a nitrocellulose membrane using 1× NuPAGE transfer buffer and a wet transfer protocol in an ice-cold transfer chamber for 1 h at 40 V. The membrane was blocked in TBS blocking buffer (LI-COR) for 1 h at RT. Staining with anti-α-tubulin antibodies (DM1a (Sigma T-6199), 1:5,000) was carried out in TBS blocking buffer supplemented with 0.2% Tween-20 overnight at 4 °C. Next, the membranes were washed three times using TBS-T and then incubated with secondary antibodies (goat anti-mouse Alexa Fluor 800 (Thermo A-32730), 1:5,000) for 1 h at RT. Finally, the membranes were washed again three times in TBS-T. Bands were fluorescently detected on an Odyssey XF system (LI-COR) with a 2-min integration time. The signals were quantified using Fiji’s rectangular selection tool. First, the sum signals of the tubulin standards were measured to generate a calibration curve. The sum signals of the lysate lanes were determined (using the rectangular selection tool) and calibrated using the calibration curve.
For normalization, the replicate gel was stained using an InstantBlue Coomassie protein stain (Abcam) according to the manufacturer’s instructions. Coomassie can be used as a sensitive fluorescent in-gel protein stain103. Protein was fluorescently detected in a ChemiDoc system (Bio-Rad) using 700 nm excitation light. The raw total protein reference image was de-noised in Fiji using a median filter (sigma = 3 pixels). The background was subtracted using the Fiji rolling-ball algorithm at a radius of 50 pixels. For each of the lanes including the tubulin calibration lanes, the raw pixel sum was measured using the rectangular selection tool.
Tubulin purification
Tubulin was purified as previously described70,104. In brief, cultures of undifferentiated ESCs and early-differentiated ESCs were collected and centrifuged for 5 min at 300g at 4 °C. Lysis was performed on ice using a Dounce homogenizer and supernatants were loaded onto a TOG column and tubulin was eluted using high salt.
Intact protein analysis by liquid chromatography mass spectrometry
Intact protein masses were determined by liquid chromatography mass spectrometry (LC–MS) as described previously105. In brief, samples were analysed using the Ultimate 3000 liquid chromatography system connected to a Q Exactive HF mass spectrometer via the ion max source with HESI-II probe (Thermo). The proteins were desalted and concentrated by injection on a reversed-phase cartridge (MSPac DS-10, 2.1 × 10 mm, Thermo). Full MS spectra were acquired using the following parameters: intact protein mode on, mass range m/z 600–2500, resolution 15,000, AGC target 3 × 106, microscans 5 and maximum injection time 200 ms. The software tool UniDec was used for data processing and deconvolution of protein masses106. First, an averaged spectrum was generated from each measurement followed by spectral deconvolution using the default settings except for the following adjustments: charge range 38–65, mass range 45–55 kDa, sample mass every 1 Da and peak FWHM 0.1.
Osmotic challenge
For hypo-osmotic challenges, the growth medium was diluted down to 80%, 75% or 50% of its original concentration with ultrapure DNase/RNase-free distilled water (Invitrogen). As isosmotic control, full growth medium was added. To allow spindle recovery, live-cell imaging or chemical fixation was performed after 2.5 h post-treatment. Media osmolalities were measured using freezing point osmometry via the Osmomat 3000 basic (Gonotec).
For hyperosmotic challenge, 50 mM or 100 mM sorbitol to the differentiation medium. For isosmotic control, the corresponding volume of regular differentiation medium was added. Before imaging, the cells were left for 1 h to recover.
Quantification of cellular mass density
Cellular mass density was measured using optical diffraction tomography as described by Biswas et al.29. In brief, a coherent laser beam was split into two using a beam splitter. While one beam was used as a reference beam, the sample was illuminated with the other using a ×60 water-dipping objective lens. Using a dual-axis galvanometer mirror, the sample was illuminated from 150 incident angles. After passing through the sample, the scattered light was gathered by a ×60 objective. At the image plane, the sample beam interfered with the reference beam to generate spatially modulated holograms. These were recorded by a charge-coupled device camera. To generate 3D tomograms, the holograms were reconstructed using a custom-written MATLAB script (github.com/OpticalDiffractionTomography)107. In Fiji, the cells were 3D segmented as described above, using the refractive index (RI) intensities in the tomograms. The cell average RI value (corresponding to the average RI value of all voxels within the 3D cell mask) was determined using the Fiji 3D Suite101. For translating the raw RI value into mass density (in mg ml−1), we applied:
The RI of the medium (RImedium) was determined for each experiment using an Abbe refractometer (Arcada ABBE-2WAJ).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided alongside the paper. Supporting data are available in the BioStudies database108 (http://www.ebi.ac.uk/biostudies) under accession code S-BIAD1680. Mass spectrometry data that support the findings of this study have been deposited to the ProteomeXchange Consortium under accession code PXD061228. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Cell lines are available from S.R. under a material transfer agreement with the Max Planck Society. Source data are provided with this paper.
Code availability
Source code, usage and installation guidelines for the implemented adaptive feedback microscopy pipeline is available at https://git.embl.de/grp-almf/automictools-zenblue-spindle-kletter. Analytical codes used for this study are deposited under https://github.com/TobiasKletter/Scaling.
References
Thompson, D. W. On Growth and Form (Cambridge Univ. Press, 1992).
Valdez, V. A., Neahring, L., Petry, S. & Dumont, S. Mechanisms underlying spindle assembly and robustness. Nat. Rev. Mol. Cell Biol. 24, 523–542 (2023).
Wühr, M. et al. Evidence for an upper limit to mitotic spindle length. Curr. Biol. 18, 1256–1261 (2008).
Greenan, G. et al. Centrosome size sets mitotic spindle length in Caenorhabditis elegans embryos. Curr. Biol. 20, 353–358 (2010).
Courtois, A., Schuh, M., Ellenberg, J. & Hiiragi, T. The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J. Cell Biol. 198, 357–370 (2012).
Wilbur, J. D. & Heald, R. Mitotic spindle scaling during Xenopus development by kif2a and importin α. Elife 2013, 2–4 (2013).
Farhadifar, R. et al. Scaling, selection, and evolutionary dynamics of the mitotic spindle. Curr. Biol. 25, 732–740 (2015).
Milunović-Jevtić, A., Jevtić, P., Levy, D. L. & Gatlin, J. C. In vivo mitotic spindle scaling can be modulated by changing the levels of a single protein: the microtubule polymerase XMAP215. Mol. Biol. Cell 29, 1311–1317 (2018).
Rieckhoff, E. M. et al. Spindle scaling is governed by cell boundary regulation of microtubule nucleation. Curr. Biol. 30, 4973–4983.e10 (2020).
Kiyomitsu, A. et al. Ran-GTP assembles a specialized spindle structure for accurate chromosome segregation in medaka early embryos. Nat. Commun. 15, 981 (2024).
Kletter, T., Biswas, A. & Reber, S. Engineering metaphase spindles: construction site and building blocks. Curr. Opin. Cell Biol. 79, 102143 (2022).
Decker, F., Oriola, D., Dalton, B. & Brugués, J. Autocatalytic microtubule nucleation determines the size and mass of Xenopus laevis egg extract spindles. eLife 7, e31149 (2018).
Lacroix, B. et al. Microtubule dynamics scale with cell size to set spindle length and assembly timing. Dev. Cell 45, 496–511.e6 (2018).
Hirst, W. G., Biswas, A., Mahalingan, K. K. & Reber, S. Differences in intrinsic tubulin dynamic properties contribute to spindle length control in Xenopus species. Curr. Biol. 30, 2184–2190.e5 (2020).
Loughlin, R., Wilbur, J. D., McNally, F. J., Nédélec, F. J. & Heald, R. Katanin contributes to interspecies spindle length scaling in xenopus. Cell 147, 1397–1407 (2011).
Helmke, K. J. & Heald, R. TPX2 levels modulate meiotic spindle size and architecture in Xenopus egg extracts. J. Cell Biol. 206, 385–393 (2014).
Miller, K. E., Session, A. M. & Heald, R. Kif2a scales meiotic spindle size in Hymenochirus boettgeri. Curr. Biol. 29, 3720–3727.e5 (2019).
Good, M. C., Vahey, M. D., Skandarajah, A., Fletcher, D. A. & Heald, R. Cytoplasmic volume modulates spindle size during embryogenesis. Science 342, 856–860 (2013).
Hazel, J. et al. Changes in cytoplasmic volume are sufficient to drive spindle scaling. Science 342, 853–856 (2013).
Brownlee, C. & Heald, R. Importin a partitioning to the plasma membrane regulates intracellular scaling. Cell 176, 805–815.e8 (2019).
Mora-Bermúdez, F., Matsuzaki, F. & Huttner, W. B. Specific polar subpopulations of astral microtubules control spindle orientation and symmetric neural stem cell division. Elife 3, 1–31 (2014).
Vargas-Hurtado, D. et al. Differences in mitotic spindle architecture in mammalian neural stem cells influence mitotic accuracy during brain development. Curr. Biol. 29, 2993–3005.e9 (2019).
Molines, A. T. et al. Physical properties of the cytoplasm modulate the rates of microtubule polymerization and depolymerization. Dev. Cell 57, 466–479.e6 (2022).
Shimamoto, Y., Maeda, Y. T., Ishiwata, S., Libchaber, A. J. & Kapoor, T. M. Insights into the micromechanical properties of the metaphase spindle. Cell 145, 1062–1074 (2011).
Bai, L. & Mitchison, T. J. Spring-like behavior of cytoplasm holds the mitotic spindle in place. Proc. Natl Acad. Sci. USA 119, e2203036119 (2022).
Xie, J. et al. Contribution of cytoplasm viscoelastic properties to mitotic spindle positioning. Proc. Natl Acad. Sci. USA 119, e2115593119 (2022).
Mchedlishvili, N., Matthews, H. K., Corrigan, A. & Baum, B. Two-step interphase microtubule disassembly aids spindle morphogenesis. BMC Biol. 16, 14 (2018).
Schweizer, N., Pawar, N., Weiss, M. & Maiato, H. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. J. Cell Biol. 210, 695–704 (2015).
Biswas, A., Kim, K., Cojoc, G., Guck, J. & Reber, S. The Xenopus spindle is as dense as the surrounding cytoplasm. Dev. Cell 56, 967–975.e5 (2021).
Woodruff, J. B. et al. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077.e10 (2017).
Baumgart, J. et al. Soluble tubulin is significantly enriched at mitotic centrosomes. J. Cell Biol. 218, 3977–3985 (2019).
Hurst, S., Vos, B. E., Brandt, M. & Betz, T. Intracellular softening and increased viscoelastic fluidity during division. Nat. Phys. 17, 1270–1276 (2021).
Carlini, L., Brittingham, G. P., Holt, L. J. & Kapoor, T. M. Microtubules enhance mesoscale effective diffusivity in the crowded metaphase cytoplasm. Dev. Cell 54, 574–582.e4 (2020).
Maric, D., Maric, I. & Barker, J. L. Buoyant density gradient fractionation and flow cytometric analysis of embryonic rat cortical neurons and progenitor cells. Methods 16, 247–259 (1998).
Jackson, C. W., Steward, S. A., Brown, L. K. & Look, A. T. Inverse relationship between megakaryocyte buoyant density and maturity. Br. J. Haematol. 64, 33–43 (1986).
Sasai, Y., Hachisuka, H., Mori, O. & Nomura, H. Separation of keratinocytes by density gradient centrifugation for DNA cytofluorometry. Histochemistry 80, 133–136 (1984).
Chalut, K. J., Ekpenyong, A. E., Clegg, W. L., Melhuish, I. C. & Guck, J. Quantifying cellular differentiation by physical phenotype using digital holographic microscopy. Integr. Biol. 4, 280 (2012).
Cooper, K. L. et al. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495, 375–378 (2013).
Neurohr, G. E. & Amon, A. Relevance and regulation of cell density. Trends Cell Biol. 30, 213–225 (2020).
Bergert, M. et al. Cell surface mechanics gate embryonic stem cell differentiation. Cell Stem Cell 28, 209–216.e4 (2021).
Woods, C. G. & Basto, R. Microcephaly. Curr. Biol. 24, R1109–R1111 (2014).
Nano, M. & Basto, R. The Janus soul of centrosomes: a paradoxical role in disease? Chromosome Res. 24, 127–144 (2016).
Viais, R. et al. Augmin deficiency in neural stem cells causes p53-dependent apoptosis and aborts brain development. eLife 10, e67989 (2021).
Zheng, X. et al. Conserved TCP ___domain of Sas-4/CPAP is essential for pericentriolar material tethering during centrosome biogenesis. Proc. Natl Acad. Sci. USA 111, E354–E363 (2014).
Ramani, A. et al. Plk1/Polo phosphorylates Sas-4 at the onset of mitosis for an efficient recruitment of pericentriolar material to centrosomes. Cell Rep. 25, 3618–3630.e6 (2018).
Varadarajan, R. & Rusan, N. M. Bridging centrioles and PCM in proper space and time. Essays Biochem. 62, 793–801 (2018).
Ying, Q. L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003).
Wongpaiboonwattana, W. & Stavridis, M. P. Neural differentiation of mouse embryonic stem cells in serum-free monolayer culture. J. Vis. Exp. 2015, 45–49 (2015).
Taverna, E., Götz, M. & Huttner, W. B. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502 (2014).
Miotto, M. et al. Collective behavior and self-organization in neural rosette morphogenesis. Front. Cell Dev. Biol. 11, 134091 (2023).
Kletter, T. et al. Volumetric morphometry reveals spindle width as the best predictor of mammalian spindle scaling. J. Cell Biol. 221, 202106170 (2021).
Berg, S. et al. Ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Reber, S. B. et al. XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat. Cell Biol. 15, 1116–1122 (2013).
Brugués, J. & Needleman, D. Physical basis of spindle self-organization. Proc. Natl Acad. Sci. USA 111, 18496–18500 (2014).
Brouhard, G. J. et al. XMAP215 is a processive microtubule polymerase. Cell 132, 79–88 (2008).
McAlear, T. S. & Bechstedt, S. The mitotic spindle protein CKAP2 potently increases formation and stability of microtubules. eLife 11, e72202 (2022).
Gruss, O. J. et al. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell 104, 83–93 (2001).
Roostalu, J., Cade, N. I. & Surrey, T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434 (2015).
Alfaro-Aco, R., Thawani, A. & Petry, S. Biochemical reconstitution of branching microtubule nucleation. Elife 9, 1–16 (2020).
Srayko, M., O’Toole, E. T., Hyman, A. A. & Müller-Reichert, T. Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis. Curr. Biol. 16, 1944–1949 (2006).
McNally, K., Audhya, A., Oegema, K. & McNally, F. J. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 175, 881–891 (2006).
Loughlin, R., Heald, R. & Nédélec, F. A computational model predicts Xenopus meiotic spindle organization. J. Cell Biol. 191, 1239–1249 (2010).
Guerreiro, A. et al. Wdr62 localizes katanin at spindle poles to ensure synchronous chromosome segregation. J. Cell Biol. 220, e202007171 (2021).
Piehl, M., Tulu, U. S., Wadsworth, P. & Cassimeris, L. Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc. Natl Acad. Sci. USA 101, 1584–1588 (2004).
Woodruff, J. B., Wueseke, O. & Hyman, A. A. Pericentriolar material structure and dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130459 (2014).
Gomes Pereira, S., Dias Louro, M. A. & Bettencourt-Dias, M. Biophysical and quantitative principles of centrosome biogenesis and structure. Annu. Rev. Cell Dev. Biol. 37, 43–63 (2021).
Decker, M. et al. Limiting amounts of centrosome material set centrosome size in C. elegans embryos. Curr. Biol. 21, 1259–1267 (2011).
Rathbun, L. I. et al. PLK1- and PLK4-mediated asymmetric mitotic centrosome size and positioning in the early zebrafish embryo. Curr. Biol. 30, 4519–4527.e3 (2020).
Liu, P. et al. Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 578, 467–471 (2020).
Reusch, S., Biswas, A., Hirst, W. G. & Reber, S. Affinity purification of label-free tubulins from Xenopus egg extracts. STAR Protoc. 1, 100151 (2020).
Kim, K. & Guck, J. The relative densities of cytoplasm and nuclear compartments are robust against strong perturbation. Biophys. J. 119, 1946–1957 (2020).
Gopalakrishnan, J. et al. Tubulin nucleotide status controls Sas-4-dependent pericentriolar material recruitment. Nat. Cell Biol. 14, 865–873 (2012).
Mariappan, A. et al. Inhibition of CPAP–tubulin interaction prevents proliferation of centrosome‐amplified cancer cells. EMBO J. 38, e99876 (2019).
Crowder, M. E. et al. A comparative analysis of spindle morphometrics across metazoans. Curr. Biol. 25, 1542–1550 (2015).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Guo, M. et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl Acad. Sci. USA 114, E8618–E8627 (2017).
Rafelski, S. M. & Theriot, J. A. Establishing a conceptual framework for holistic cell states and state transitions. Cell 187, 2633–2651 (2024).
Newport, J. & Kirschner, M. A major developmental transition in early xenopus embryos: II. control of the onset of transcription. Cell 30, 687–696 (1982).
Peshkin, L. et al. On the relationship of protein and mRNA dynamics in vertebrate embryonic development. Dev. Cell 35, 383–394 (2015).
Cuomo, A. S. E. et al. Single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun. 11, 810 (2020).
Ochocka, N. et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun. 12, 1151 (2021).
Mulas, C., Chaigne, A., Smith, A. & Chalut, K. J. Cell state transitions: definitions and challenges. Development 148, dev199950 (2021).
Al-Dosari, M. S., Shaheen, R., Colak, D. & Alkuraya, F. S. Novel CENPJ mutation causes Seckel syndrome. J. Med. Genet. 47, 411–414 (2010).
Kitagawa, D. et al. Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. J. Cell Sci. 124, 3884–3893 (2011).
An, H.-L., Kuo, H.-C. & Tang, T. K. Modeling human primary microcephaly with hiPSC-derived brain organoids carrying CPAP-E1235V disease-associated mutant protein. Front. Cell Dev. Biol. 10, 830432 (2022).
David, A. F. et al. Augmin accumulation on long-lived microtubules drives amplification and kinetochore-directed growth. J. Cell Biol. 218, 2150–2168 (2019).
Khodjakov, A. & Rieder, C. L. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 153, 237–242 (2001).
Nichols, J. et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 (1998).
Arai, Y. et al. Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat. Commun. 2, 154 (2011).
Waisman, A. et al. Cell cycle dynamics of mouse embryonic stem cells in the ground state and during transition to formative pluripotency. Sci. Rep. 9, 8051 (2019).
Reback, J. et al. pandas-dev/pandas: Pandas 1.1.3. Zenodo https://doi.org/10.5281/zenodo.4067057 (2020).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Seabold, S. & Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python http://statsmodels.sourceforge.net/ (2010).
Pedregosa, F. et al. Scikit-Learn: Machine Learning in Python. vol. 12 2825–2830 http://scikit-learn.sourceforge.net (2011).
Poser, I. et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat. Methods 5, 409–415 (2008).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Thomas, L. S. V. & Gehrig, J. Multi-template matching: a versatile tool for object-localization in microscopy images. BMC Bioinform. 21, 44 (2020).
Tinevez, J.-Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Legland, D., Arganda-Carreras, I. & Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32, 3532–3534 (2016).
Ollion, J., Cochennec, J., Loll, F., Escudé, C. & Boudier, T. TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841 (2013).
Eaton, S. L. et al. Total protein analysis as a reliable loading control for quantitative fluorescent western blotting. PLoS ONE 8, e72457 (2013).
Butt, R. H. & Coorssen, J. R. Coomassie blue as a near-infrared fluorescent stain: a systematic comparison with sypro ruby for In-gel protein detection. Mol. Cell. Proteom. 12, 3834–3850 (2013).
Widlund, P. O. et al. One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Mol. Biol. Cell 23, 4393–4401 (2012).
Hirst, W. G. et al. Purification of functional Plasmodium falciparum tubulin allows for the identification of parasite-specific microtubule inhibitors. Curr. Biol. 32, 919–926.e6 (2022).
Marty, M. T. et al. Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem. 87, 4370–4376 (2015).
Kim, K. et al. Diffraction optical tomography using a quantitative phase imaging unit. Opt. Lett. 39, 6935–6938 (2014).
Sarkans, U. et al. The BioStudies database-one stop shop for all data supporting a life sciences study. Nucleic Acids Res. 46, D1266–D1270 (2018).
Ton, C. C. T. et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell 67, 1059–1074 (1991).
Frederiksen, K. & McKay, R. D. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J. Neurosci. 8, 1144–1151 (1988).
Guillemot, F. et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75, 463–476 (1993).
Aaku-Saraste, E., Hellwig, A. & Huttner, W. B. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure—remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol. 180, 664–679 (1996).
Arnold, S. J. et al. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 22, 2479–2484 (2008).
Camargo Ortega, G. et al. The centrosome protein AKNA regulates neurogenesis via microtubule organization. Nature 567, 113–117 (2019).
Jiang, K. et al. Microtubule minus-end regulation at spindle poles by an ASPM–katanin complex. Nat. Cell Biol. 19, 480–492 (2017).
Acknowledgements
The authors thank all past and current members of the Zaburdaev and Reber laboratories. We thank staff at the Advanced Medical Bioimaging Core Facility (Charité, Berlin), the Advanced Light Microscopy Facility at the EMBL and Zeiss for imaging support. The authors acknowledge the support of i3S Scientific Platform Advanced Light Microscopy, a site of PPBI Euro-Bioimaging Node. For mass spectrometry, we acknowledge the assistance of staff at the Core Facility BioSupraMol supported by the Deutsche Forschungsgemeinschaft. We thank H. Maiato and laboratory for their support. We thank J. Gopalakrishnan for sharing reagents. We thank R. Basto, E. Taverna and F. Mora Bermudez for helpful discussions and advice on neural differentiation and M. Taylor, V. Štimac and M. Mendoza for critical comments on the manuscript. For funding, the authors acknowledge the Max Planck Society (to O.M. and S.R.), the Horizon 2020 Framework Programme of the European Union (iNEXT grant 653706, project PID 3503 to S.R.) and the Deutsche Forschungsgemeinschaft Project-ID 528483508 – FIP 12 (to S.R.).
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
T.K. and S.R. conceived and designed the study. T.K. performed all experiments including data and image analysis. S.R. contributed to method development. A.B. supported ODT setup development and quantitative phase imaging. B.N. supported long-term live-cell imaging and RNAi experiments. A.H. designed the adaptive microscopy feedback pipeline. B.K. performed and interpreted the mass-spectrometric analyses. O.M. and V.Z. conceptualized and wrote the Supplementary Note. T.K. and S.R. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Nicolas Minc and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Marker expression and tissue architecture confirm successful differentiation of ESCs into neural rosettes.
a. Top: Confocal data showing immunostaining of PAX6109 in fixed ESCs (“Day 0”) and during 5 days of neural differentiation. Bottom: Nuclei stained by Hoechst. Scale bar: 50 µm. b. Immunofluorescent detection of nestin110 in ESCs-derived rosette cells after 5 days of differentiation. Inlets show nuclei stained by Hoechst. Scale bars: 20 µm. c. As in (b) but detecting MASH1 (also known as ASCL1), a proneural transcription factor111. d. As in (b) but detecting ZO-1, marking tight junctions in the rosette centre112. e. As in (b) but detecting PAX6, uniformly expressed in the rosette bulk. f. As in (b) but detecting TBR2113, sporadically expressed by cells in the rosette periphery. g. As in (b) but detecting AKNA, a centrosomal protein in neural stem and progenitor cells. AKNA+ centrosomes strictly localize to the “apical” pole during interphase114. Lookup table of this panel is inverted in comparison with other panels for better visibility. All stainings (a-g) were performed for N = 2 independent experiments.
Extended Data Fig. 2 Spindle size subscaling in differentiating embryonic stem cells (ESCs) is independent of confluency and cell geometry.
a. Volumetric cell analysis, based on training pixel-classifier models in Ilastik52 to distinguish mitotic cytoplasm via tubulin::GFP. Spindle volume masks were generated via Spindle3D51, based on adaptive thresholding of tubulin::GFP. b. Spindle length after binning data into cell volume bins (bin size 500 µm3). Numbers inside the bins show respective n of cells (data sets as in Fig. 1h, n = 5, 208; 57, 1051; 280, 1017; 445, 543; 238, 91; 59, 10 cells for ESCs, DIF conditions binned from 1000 µm3 through 3500 µm3 in 500 µm3 bins, pooled from 9 independent replicates). Boxes denote interquartile ranges, horizontal lines inside boxes show medians, whiskers indicate minima and maxima. Welch’s t-test (two-sided). c. Same as c) but showing spindle width. d. Spindle aspect ratio (spindle length / spindle width). Boxes denote interquartile ranges, horizontal lines inside boxes show medians, whiskers indicate minima and maxima. Data points show individual cells (ESCs n = 1,084 cells, DIF n = 2,920 cells pooled from 9 independent experiments, see Fig. 1h). Welch’s t-test (two-sided), P = 9.3 x 10-45. e. Mitotic cell volume is independent of the local cell confluency. Single data points represent individual cells (ESCs n = 110 cells (yellow) and DIF n = 151 cells (blue) from 3 experiments). rs: Spearman’s correlation coefficient (PESCs = 0.07, PDIF = 0.21). f. Osmolality of the ESCs culturing medium and of the differentiation medium. g. Cell volume occupied by the spindle in ESCs (yellow) and DIF (blue) per differentiation time bin. The circles show the means, error bars show the standard deviation. Welch’s t-test (two-sided). n: data points in each bin. h. Mitotic cells in both differentiation states are spherical. Each data point represents an individual cell (ESCs n = 1,084 (yellow) and DIF n = 2,920 (blue), data pooled from 9 independent experiments). Big circles represent the median of each cell volume bin, the error bars show the interquartile range. The dotted line represents the behaviour of a perfect sphere. i. In cells with comparable cell surface area : cell volume ratios, spindle volume subscales in DIF (blue) when compared with ESCs (yellow). Boxes show interquartile ranges, white lines inside boxes denote medians, whiskers show minima and maxima. n = 395, 176; 697, 1647; 67, 1080; 445, 543; 238, 91; 59, 10 cells for ESCs, DIF conditions binned from 0.3 µm-1 through 0.45 µm-1 in 0.05 µm-1 bins, pooled from 9 independent replicates. d: Cohen’s d. Welch’s t-test (two-sided) for data in each bin. j. Top: Maximum-projected confocal images of mitotic ESCs or DIF (tubulin::GFP in grey) acquired during our automated live-cell imaging protocol (Fig. 1e). Bottom: tubulin::GFP-based intensity profiles of a cross-section between spindle equator and pole in maximum projected confocal images (top, dotted white lines). For consistency, cells were randomly drawn from a pool of cells within the 2500-3000 µm3 cell volume bin (ESCs n = 75 cells (yellow) and DIF n = 75 cells (blue) pooled from 6 independent experiments). Lines denote the means, shaded areas denote the 95% confidence intervals. *: P < 0.05, ***: P < 0.001, ****: P < 0.0001, n.s.: not significant, P > 0.05. d: Cohen’s d.
Extended Data Fig. 3 Microtubule growth speed is independent of spindle length and cell volume.
a. Average single-cell microtubule growth velocities (EB1::tdTomato comets) as a (i) function of spindle length and (ii) cell volume in ESCs (n = 91 cells) or DIF (n = 72 cells). Average single-cell number of EB1::tdTomato spindle bulk comets as a function of (iii) spindle length and (iv) cell volume in ESCs (n = 65 cells) or DIF (n = 47 cells). Average single-cell number of total EB1::tdTomato comets as a function (v) of spindle length and (vi) cell volume in ESCs (n = 65 cells) or DIF cells (n = 47 cells). Data from pooled from 6 independent experiments. Small circles show averages of individual cells, large circles represent medians of binned data, error bars represent interquartile ranges. b. Cellular levels of CKAP2 after 48 h of differentiation relative to ESCs, probed by western blotting and normalized to GAPDH (N = 3, each time loading 2 independent batches of protein extracts). Bars show mean, errors show standard deviation. Welch’s t-test (two-sided), P = 0.99. c. Confocal micrographs (maximum projected, from N = 2 experiments) of methanol-fixed ESCs or DIF at metaphase. Top: Immunostained CKAP2 signal, bottom: tubulin::GFP (grey) and chromatin counterstained by Hoechst (blue). Dotted lines indicate cell boundaries. Scale bar: 5 µm. d. Fluorescent CKAP2 signal on spindle (normalized by total cell CKAP2) summed, as a function of spindle volume. Data points represent single cells (ESCs n = 23 cells and DIF n = 21 cells, data from 2 independent experiments.), large circles denote medians in each bin, the error bars show interquartile ranges. Note that spindle volumes in general are smaller because of methanol fixation. e. Cellular levels of CKAP5 after 48 h of differentiation relative to ESCs, probed by western blotting and normalized to tubulin (N = 4 biological replicates). Bars show mean, errors show the standard deviation, circles show replicates. Welch’s t-test (two-sided), P = 0.36. f. As in b) but showing microtubule depolymerase KIF2C/MCAK. P = 3.7 x 10-3. g. As in c) but showing staining for KIF2C/MCAK (N = 2 experiments). h. As in d) but showing KIF2C/MCAK summed spindle signals (ESCs n = 52 cells and DIF n = 74 cells, pooled from 2 independent experiments).
Extended Data Fig. 4 Augmin and TPX2 spindle localization is comparable between the differentiation states.
a. Cellular levels of augmin (subunit HAUS6) after 48 h of differentiation relative to ESCs, probed by western blotting and normalized to tubulin (N = 3 biological replicates). Bars show the mean, errors show the standard deviation, circles show replicates. Welch’s t-test (two-sided), P = 0.57. b. Confocal micrographs (maximum projected, N = 2 experiments) of fixed ESCs or DIF at metaphase. Top: Immunostained HAUS6 signal, bottom: tubulin::GFP (grey) and chromatin counterstained by Hoechst (blue). Dotted lines indicate the cell boundaries. Scale bar: 5 µm. c. Fluorescent HAUS6 signal on spindle (normalized by total cell HAUS6) as a function of spindle volume. Each data point represents a single cell (ESCs n = 159 cells and DIF n = 158 cells pooled from 2 independent experiments), large circles denote medians in each cell volume bin, error bars show interquartile ranges. d. As in a) but showing TPX2 levels (N = 3 biological replicates). P = 0.92. e. As in b) but showing TPX2 immunofluorescence (N = 3 experiments). f. As in c) but showing TPX2 immunofluorescence (ESCs n = 105 cells and DIF n = 141 cells pooled from 3 independent experiments). n.s.: not significant, P > 0.05.
Extended Data Fig. 5 Spindle scaling in ESCs is independent of microtubule severing.
a. Cellular levels of spastin after 48 h of differentiation relative to ESCs, probed by western blotting and normalized to tubulin (N = 2, each time loading 2 independent batches of protein extracts). Bars show mean, errors show the standard deviation, circles show replicates. b. Cellular levels of katanin (subunit p80) after 48 h of differentiation relative to ESCs, probed by western blotting and normalized to tubulin (N = 3 biological replicates). Bars show mean, errors show standard deviation, circles show replicates. Welch’s t-test (two-sided), P = 0.03. c. Confocal micrographs (maximum projected, N = 3 experiments) of fixed ESCs or DIF at metaphase. Top: Tubulin::GFP signal, centre: chromatin (Hoechst), bottom: immunostained katanin p60. Dotted lines indicate cell boundaries. Scale bar: 5 µm. d. As in (c) but staining katanin p80 (N = 3 experiments). e. Average katanin p60 signal at spindle poles. Data points show individual cells (ESCs n = 37 cells and DIF n = 38 cells pooled from 3 independent experiments), boxes denote the interquartile ranges, horizontal lines inside the boxes denote the medians, whiskers show the minimum and maximum. Welch’s t-test (two-sided), P = 0.02. f. As in (e) but showing katanin p80 (ESCs n = 156 cells and DIF n = 168 cells pooled from 3 independent experiments). Welch’s t-test (two-sided), P = 1.2 x 10-8. g. Increased katanin p80 mass in ESCs is independent of spindle volume. Data points show individual cells (ESCs n = 148 cells and DIF n = 155 cells pooled from 3 independent experiments). Large circles show the medians of spindle volume bins (bin size = 100 µm3), error bars show the interquartile ranges. h. Representative western blots (from N = 3 experiments) showing katanin p80 knockdown (and katanin p60 co-depletion, as has been described previously115) in ESCs using siRNAs (Katnb1) next to control using scrambled siRNAs (Scrmbl). GAPDH as loading control. i. Confocal micrographs (maximum projected, N = 3 experiments) of fixed ESCs after katanin p80 knockdown (Katnb1) or control (Scrmbl). Top: tubulin::GFP, centre: γ-tubulin immunostaining as spindle pole reference, bottom: immunostained katanin p80. Dotted lines indicate cell boundaries. Scale bar: 5 µm. j. As in f) but showing Katnb1 siRNA-treated ESCs and control ESCs (Scrmbl) (Katnb1 n = 210 cells and Scrmbl n = 154 cells pooled from 3 independent experiments). Welch’s t-test (two-sided), P = 5.5 x 10-49. k. Left: Representative micrographs (maximum projected, N = 3 experiments) of 4 spindle phenotypes in the RNAi experiment. Right: Percentages of the 4 phenotypes in population after Katnb1 siRNA treatment vs. control (Scrmbl). l. Fraction of tubulin::GFP partitioned to spindle bulk. Data points show individual cells (Katnb1 n = 210 cells and Scrmbl n = 154 cells from 3 independent experiments), boxes show interquartile ranges, horizontal bars show medians, whiskers show minima and maxima. Welch’s t-test (two-sided), P = 0.01. m. As in l) but showing spindle volume as a percentage of cell volume. P = 0.2. *: P < 0.05, ****: P < 0.0001, n.s.: not significant, P > 0.05.
Extended Data Fig. 6 The pericentriolar material (PCM) superscales upon differentiation.
a. Confocal micrographs (maximum projected, representative from ESCs n = 113 cells and DIF n = 100 cells pooled from 3 independent experiments) of fixed ESCs or DIF at metaphase. Top: Immunostained CDK5RAP2 signal (inverted grayscale), bottom: immunostained CDK5RAP2 (yellow), tubulin::GFP (grey) and chromatin (Hoechst, blue). Dotted lines indicate cell boundaries. Scale bar: 5 µm. b. As in a) but using CEP192 (representative from ESCs n = 56 cells and DIF n = 44 cells from 1 experiment). c. As in a) but using pericentrin (representative from ESCs n = 89 cells and DIF n = 73 cells pooled from 3 experiments). d. Scaling relationship between cell volume and centrosome volume based on CDK5RAP2. Data points represent single cells (ESCs n = 113 cells and DIF n = 100 cells pooled from 3 independent experiments), large circles denote medians per cell volume bin, the error bars show interquartile ranges. e. As in d) but based on CEP192 (ESCs n = 56 cells and DIF n = 44 cells pooled from 1 experiment). f. As in d) but based on pericentrin (ESCs n = 89 cells and DIF n = 73 cells pooled from 3 experiments).
Extended Data Fig. 7 The cytoplasm in differentiating cells is diluted across all cell sizes.
Scaling relationship between cell volume and cellular mass density. Data points represent single cells (ESCs n = 61 cells, DIF n = 71 cells from 3 independent experiments, same data as in Fig. 5), the large circles denote the medians in each cell volume bin, the error bars show the interquartile ranges.
Extended Data Fig. 8 Biophysical perturbation of cellular mass density modulates spindle scaling.
a. Hypoosmotic treatment of ESCs for live optical diffraction tomography (ODT) or confocal imaging. b. ODT-derived cellular mass densities after hypoosmotic treatment (left: 80/20, right: 75/25, % medium/H2O) of ESCs. Boxes show interquartile ranges, horizontal lines show medians, whiskers show minima and maxima. Data points show individual cells (80/20: n isosmotic: 52 cells, n hypoosmotic: 51 cells pooled from 3 independent experiments, 75/25: n isosmotic: 107 cells, n hypoosmotic: 96 cells pooled from 4 independent experiments). Welch’s t-test (two-sided), Iso vs. Hypo (80%): P = 0.02; Iso vs. Hypo (75%): P = 6.2 x 10-6. c. Confocal live-cell imaging (central slices) of control ESCs (isosmotic treatment) versus increasing hypoosmotic challenges. Tubulin::GFP in grey, chromatin in blue. Dotted lines show cell boundaries. Scale bar: 5 µm. d. Cell volume-normalized spindle volumes during hypoosmotic challenge of ESCs, fold change relative to control ESCs (Iso). Boxes show interquartile ranges, horizontal lines show medians, whiskers show minima and maxima. Data points show individual cells (n isosmotic: 175 cells, n Hypo (80%): 97 cells, n Hypo (75%): 71 cells, and n Hypo (50%): 37 cells from 5 independent experiments). Welch’s ANOVA and two-sided Games-Howell post-hoc testing, Iso vs. Hypo (80%): P = 0.37; Iso vs. Hypo (75%): P = 1.3 x 10-7; Iso vs. Hypo (50%): P = 1.7 x 10-13. e. Data from (d) re-analysed, showing spindle volumes within cell volume bins (n = 24, 17, 2, 0; 69, 49, 15, 7; 63, 25, 32, 20; 15, 6, 16, 8; 4, 0, 6, 2 for Iso, Hypo (80%), Hypo (70%), and Hypo (50%) conditions, respectively, binned from 2,000 µm3 through 4,000 µm3 in 500 µm3 bins). Circles show means, error bars show standard deviation. Horizontal grey lines indicate mean value of the control ESCs (Iso). f. Boxplots and data as (d) but re-analysed showing spindle aspect ratios (length/width). Welch’s ANOVA and two-sided Games-Howell post-hoc testing, Iso vs. Hypo (80%): P = 0.15; Iso vs. Hypo (75%): P = 2.9 x 10-9; Iso vs. Hypo (50%): P < 1.0 x 10-100. g. Hyperosmotic treatment of DIF for live optical diffraction tomography (ODT) or confocal imaging. h. ODT-derived cellular mass densities after hyperosmotic treatment (left: +50 mM Sorbitol, right: +100 mM Sorbitol) of DIF, untreated ESCs as additional control. Boxes show the interquartile ranges, horizontal lines show medians, whiskers show minima and maxima. Data points show individual cells (+50 mM Sorbitol: n ESCs: 12 cells, n isosmotic DIF: 13 cells, n hyperosmotic DIF: 13 cells pooled from 1 independent experiment, +100 nM Sorbitol: n isosmotic DIF: 15 cells, n hyperosmotic: 15 cells from 1 independent experiment) Welch’s ANOVA and two-sided Games-Howell post-hoc testing, ESCs vs. DIFIso: P = 6.3 x 10-3; ESCs vs. DIF50mM Sorbitol: P = 0.60; ESCs vs. DIF100mM Sorbitol: P = 0.04. i. Cell volume-normalized spindle volumes during hyperosmotic challenge of DIF (fold change relative to ESCs). Boxes show interquartile ranges, horizontal lines show medians, whiskers show minima and maxima. Data points show individual cells (n ESCs: 58 cells, n isosmotic DIF: 91 cells, n hyperosmotic DIF (50 mM): 100, n hyperosmotic DIF (100 mM): 22 pooled from 2 independent experiments). Welch’s ANOVA and two-sided Games-Howell post-hoc testing, ESCs vs. DIFIso: P = 5.3 x 10-4; ESCs vs. DIF50mM Sorbitol: P = 0.99; ESCs vs. DIF100mM Sorbitol: P = 0.90. j. Composite chart showing the cell volume-normalized spindle volumes (fold changes relative to controls) as a function of the mean cellular mass density (as fold change relative to controls). Circles show means, error bars show standard error of the mean. Data merged from this figure and Fig. 6 (n ESCs: 181 cells and n DIF: 119 cells pooled from 5 independent experiments; n ESCs+Iso: 175, n ESCs+Hypo80%: 97, n ESCs+Hypo75%: 71, n ESCs+Hypo50%: 37 pooled from 5 independent experiments; n DIF+Iso: 91 cells, n DIF+50mM: 100 cells, n DIF+100mM: 22 cells pooled from 2 experiments). *: P < 0.05, **: P < 0.01, ***: P < 0.001, ****: P < 0.0001, n.s.: not significant, P > 0.05.
Supplementary information
Supplementary Information
Supplementary Note and Figs. 1–3.
Supplementary Video 1
Emergence of neural rosettes in adherent differentiation culture. Confocal time-lapse recording of ESCs expressing tubulin::GFP (grey) undergoing neural differentiation, chromatin stained using SiR-DNA (blue). In later time points, cells self-organize to form rosette-like tissue architectures. Scale bar, 25 µm, time stamps are hh:mm (15 min per frame, total of 112.25 h).
Supplementary Video 2
Interkinetic nuclear migration in neural rosettes. Confocal time-lapse recording of rosette cells (tubulin::GFP in grey, chromatin stained using SiR-DNA, blue). Mitoses of rosette cells exclusively take place in the centre of the rosette (corresponding to the ‘apical’ side), whereas the nuclei migrate to the periphery of the rosette, a phenomenon called interkinetic nuclear migration. Scale bar, 25 µm, time stamps are hh:mm (5 min per frame).
Supplementary Video 3
Visualizing growing microtubules in live mitotic cells. Confocal time-lapse recording (single z plane) of a mitotic undifferentiated embryonic stem cell expressing EB1::tdTomato (grey). EB1 labels growing microtubules plus ends. Scale bar, 5 µm, 0.4 s per frame.
Supplementary Video 4
Increased EB1 localization at the centrosomes upon differentiation. Confocal time-lapse recordings (single z planes) of mitotic early-differentiated cell (left) and an undifferentiated embryonic stem cell (right) expressing EB1::tdTomato (grey). The EB1 signals are cumulative per frame. The global histograms of the individual videos are normalized to the last frame. Scale bar, 5 µm, 0.4 s per frame.
Supplementary Table 1
Supplementary Table 1: Key measurements of cell and spindle morphometrics. Overview summarizing key measurements (mean ± s.d.) and calculations in undifferentiated ESCs (ESCs) versus early-differentiated cells (DIF), as well as in cells during hypo-osmotic treatment, or treatment with the small molecule inhibitor CCB02. Vbin compares ESCs versus DIF with comparable cell volumes (2,000–2,500 µm3). Iso, isotonic control; DMSO, control for CCB02; ND, not determined; γTub, γ-tubulin. Supplementary Table 2: Summary of the microscope settings of the imaging tasks that were used to run the adaptive feedback microscopy pipeline.
Supplementary Data 1
Source data for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 1
Unprocessed blots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed blots.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 4
Unprocessed blots.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed blots.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 4
Unprocessed blots.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 5
Unprocessed blots.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kletter, T., Muñoz, O., Reusch, S. et al. Cell state-specific cytoplasmic density controls spindle architecture and scaling. Nat Cell Biol 27, 959–971 (2025). https://doi.org/10.1038/s41556-025-01678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-025-01678-x
This article is cited by
-
Cytoplasmic control of spindle architecture
Nature Cell Biology (2025)