Abstract
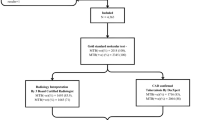
Two in every five patients with active tuberculosis (TB) remain undiagnosed or unreported. Therefore community-based, active case-finding strategies require urgent implementation. However, whether point-of-care (POC), portable battery-operated, molecular diagnostic tools deployed at a community level, compared with conventionally used POC smear microscopy, can shorten time-to-treatment initiation, thus potentially curtailing transmission, remains unclear. To clarify this issue, we performed an open-label, randomized controlled trial in periurban informal settlements of Cape Town, South Africa, where we TB symptom screened 5,274 individuals using a community-based scalable mobile clinic. Some 584 individuals with HIV infection or symptoms of TB underwent targeted diagnostic screening and were randomized (1:1) to same-day smear microscopy (n = 296) or on-site DNA-based molecular diagnosis (n = 288; GeneXpert). The primary aim was to compare time to TB treatment initiation between the arms. Secondary aims included feasibility and detection of probably infectious people. Of participants who underwent targeted screening, 9.9% (58 of 584) had culture-confirmed TB. Time-to-treatment initiation occurred significantly earlier in the Xpert versus the smear-microscopy arm (8 versus 41 d, P = 0.002). However, overall, Xpert detected only 52% of individuals with culture-positive TB. Notably, Xpert detected almost all of the probably infectious patients compared with smear microscopy (94.1% versus 23.5%, P = <0.001). Xpert was associated with a shorter median time to treatment of probably infectious patients (7 versus 24 d, P = 0.02) and a greater proportion of infectious patients were on treatment at 60 d compared with the probably noninfectious patients (76.5% versus 38.2%, P < 0.01). Overall, a greater proportion of POC Xpert-positive participants were on treatment at 60 d compared with all culture-positive participants (100% versus 46.5%, P < 0.01). These findings challenge the traditional paradigm of a passive case-finding, public health strategy and argues for the implementation of portable DNA-based diagnosis with linkage to care as a community-oriented, transmission-interruption strategy. The study was registered with the South African National Clinical Trials Registry (application ID 4367; DOH-27-0317-5367) and ClinicalTrials.gov (NCT03168945).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Individual participant data will be made available to researchers who provide a protocol that is approved by their respective human research ethics committee. All protocols will be reviewed and approved by the XACT consortium trial steering committee up to 5 years after publication. A data sharing agreement will need to be concluded between the representatives of the requesting institution and the University of Cape Town Lung Institute. Data sharing requests should be directed to [email protected].
References
World Health Organization. Global Tuberculosis Report (WHO, 2020).
World Health Organization. Systematic Screening for Active Tuberculosis: Principles and recommendations (WHO, 2013).
Madhi, S. A. et al. COVID-19 lockdowns in low- and middle-income countries: success against COVID-19 at the price of greater costs. S. Afr. Med. J. 110, 724–726 (2020).
Dheda, K. et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 10, 603–622 (2022).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. S2213-2600, 30079-6 (2017).
Marks, G. B. et al. Community-wide screening for tuberculosis in a high-prevalence setting. N. Engl. J. Med. 381, 1347–1357 (2019).
Azman, A. S., Golub, J. E. & Dowdy, D. W. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 12, 216 (2014).
Sun, A. Y. et al. Modeling the impact of alternative strategies for rapid molecular diagnosis of tuberculosis in Southeast Asia. Am. J. Epidemiol. 178, 1740–1749 (2013).
Balcha, T. T., Skogmar, S., Sturegård, E., Björkman, P. & Winqvist, N. Outcome of tuberculosis treatment in HIV-positive adults diagnosed through active versus passive case-finding. Glob. Health Action 8, 27048 (2015).
Kempker, R. R. et al. High yield of active tuberculosis case finding among HIV-infected patients using Xpert MTB/RIF testing. Open Forum Infect. Dis. 6, ofz233 (2019).
Rivera, V. R. et al. Diagnostic yield of active case finding for tuberculosis and HIV at the household level in slums in Haiti. Int. J. Tuberc. Lung Dis. 21, 1140–1146 (2017).
Lorent, N. et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLoS ONE 9, e92754 (2014).
Chen, C. et al. Community-based active case finding for tuberculosis in rural western China: a cross-sectional study. Int. J. Tuberc. Lung Dis. 21, 1134–1139 (2017).
Corbett, E. L. et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 376, 1244–1253 (2010).
Morishita, F. et al. Bringing state-of-the-art diagnostics to vulnerable populations: the use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS ONE 12, e0171310 (2017).
Calligaro, G. L. et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect. Dis. 17, 441–450 (2017).
Choun, K. et al. Performance of algorithms for tuberculosis active case finding in underserved high-prevalence settings in Cambodia: a cross-sectional study. Glob. Health Action 12, 1646024 (2019).
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 2: screening—systematic screening for tuberculosis disease (WHO, 2021).
Burke, R. M. et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health 6, e283–e299 (2021).
World Health Organization, Regional Office for South-East Asia. Optimizing active case-finding for tuberculosis: implementation lessons from South-East Asia (WHO, 2021).
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: diagnosis—rapid diagnostics for tuberculosis detection, 2021 update (WHO, 2021).
Davis, J. L., Cattamanchi, A., Cuevas, L. E., Hopewell, P. C. & Steingart, K. R. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 147–154 (2013).
Meyer, A. J. et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS ONE 12, e0180572 (2017).
Nalugwa, T. et al. Readiness to implement on-site molecular testing for tuberculosis in community health centers in Uganda. Implement. Sci. Commun. 3, 9 (2022).
Cordeiro-Santos, M. et al. Feasibility of GeneXpert((R)) Edge for tuberculosis diagnosis in difficult-to-reach populations: preliminary results of a proof-of-concept study. Am. J. Trop. Med. Hyg. 103, 1065–1066 (2020).
Khanal, S. et al. Yield of intensified tuberculosis case-finding activities using Xpert® MTB/RIF among risk groups in Nepal. Public Health Action 6, 136–141 (2016).
Lawn, S. D. et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin. Infect. Dis. 54, 1071–1079 (2012).
Grzybowski, S., Barnett, G. D. & Styblo, K. Contacts of cases of active pulmonary tuberculosis. Bull. Int. Union Tuberc. 50, 90–106 (1975).
van Geuns, H. A., Meijer, J. & Styblo, K. Results of contact examination in Rotterdam, 1967–1969. Bull. Int. Union Tuberc. 50, 107–121 (1975).
Behr, M. A. et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353, 444–449 (1999).
Tostmann, A. et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin. Infect. Dis. 47, 1135–1142 (2008).
Theron, G. et al. Bacterial and host determinants of cough aerosol culture positivity in patients with drug-resistant versus drug-susceptible tuberculosis. Nat. Med. 26, 1435–1443 (2020).
Fennelly, K. P. et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 169, 604–609 (2004).
Jones-Lopez, E. C. et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am. J. Respir. Crit. Care Med. 187, 1007–1015 (2013).
Menberu, M. A. Performance of the WHO 2011 TB symptom screening algorithm for pulmonary TB diagnosis among HIV-infected patients in Gondar University Referral Hospital, Ethiopia. Int. J. Microbiol. 2016, 9058109 (2016).
Kranzer, K. et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int. J. Tuberc. Lung. Dis. 17, 432–446 (2013).
Esmail, A., Tomasicchio, M., Meldau, R., Makambwa, E. & Dheda, K. Comparison of Xpert MTB/RIF (G4) and Xpert Ultra, including trace readouts, for the diagnosis of pulmonary tuberculosis in a TB and HIV endemic setting. Int. J. Infect. Dis. 95, 246–252 (2020).
Liang, F., Zhang, S., Wang, Q. & Li, W. Treatment effects measured by restricted mean survival time in trials of immune checkpoint inhibitors for cancer. Ann. Oncol. 29, 1320–1324 (2018).
Uno, H. et al. Adding a new analytical procedure with clinical interpretation in the tool box of survival analysis. Ann. Oncol. 29, 1092–1094 (2018).
Perego, C. et al. Utility of restricted mean survival time analysis for heart failure clinical trial evaluation and interpretation. JACC Heart Fail. 8, 973–983 (2020).
Peter, J. G., Theron, G., Singh, N., Singh, A. & Dheda, K. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur. Respir. J. 43, 185–194 (2014).
Acuna-Villaorduna, C. et al. Cough-aerosol cultures of Mycobacterium tuberculosis in the prediction of outcomes after exposure. A household contact study in Brazil. PLoS ONE 13, e0206384 (2018).
Jones-Lopez, E. C. et al. Cough aerosols of Mycobacterium tuberculosis in the prediction of incident tuberculosis disease in household contacts. Clin. Infect. Dis. 63, 10–20 (2016).
Acknowledgements
This work was funded by the National Institutes of Health (grant no. CRDF-OISE-16-62105) and the South African Medical Research Council (grant no. RFA-EMU-02-2017). K.D.’s lab also acknowledges funding from the European and Developing Countries Clinical Trials Partnership (grant nos. TMA-2015SF-1043 and TMA-1051-TESAII), UK Medical Research Council (grant no. MR/S03563X/1) and the Wellcome Trust (grant no. MR/S027777/1). A.E. acknowledges funding from EDCTP (grant no. TMA-2015CDF-1052). The funders have not influenced the results of this trial in any way. We are indebted to the participants who took part in the this study. We thank the Health Directorate of the City of Cape Town for providing access to appropriate health care facilities. We further acknowledge the assistance of clinical staff at each of the TB clinics, the University of Cape Town Research Ethics Committee and community leaders and the University of Cape Town Lung Institute TB community advisory board for enabling this study. Lessons learned from patients during the conduct of the XACT-1 study were implemented in the design of the present study to facilitate acceptance of the XACT-2 active case finding model.
Author information
Authors and Affiliations
Contributions
K.D., A.E., P.R. and G.C. conceived the trial. K.D. was the principal investigator. K.D., A.E., P.R., S.O., M.T., A.P., R.M., E.M., R. W., M. K. and L.M. designed and performed the experiments. K.D., A.E., S.M., P.R., S.O., M.T. and A.P. analyzed the data and wrote the paper. All authors critically reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Gavin Churchyard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cough Aerosol Sampling System (CASS) used to measure culturable M. tuberculosis in cough aerosol droplets as a surrogate for infectiousness.
The CASS system consisting of a six-stage Anderson cascade impactor (ACI) showing the median expected droplet diameter (µm) for each stage (Panel A).Patient sitting in a negative pressure cubical while coughing into a mouth piece that is attached to the ACI (Panel B).Culture plate that enables CFUs from individual aerosol droplets to be isolated (Panel C) The ACI was horizontally contained in a 10 litre autoclavable chamber (panel D).
Extended Data Fig. 2 Overview of the recruitment activities and procedures for the XACT-II study.
* A vast majority (235/288; 81.6%) of the participants in the Xpert arm of the study received their results on the same-day,thus, enabling ‘immediate’ (that is, same-visit) referral for TB treatment initiation during the same interaction while only a small proportion (42/296; 14.2%) of participants in the smear arm received their results in the same-day.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, one figure, background information and summary table describing the contents of the XACT ACF model.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmail, A., Randall, P., Oelofse, S. et al. Comparison of two diagnostic intervention packages for community-based active case finding for tuberculosis: an open-label randomized controlled trial. Nat Med 29, 1009–1016 (2023). https://doi.org/10.1038/s41591-023-02247-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02247-1
This article is cited by
-
Finding the missed millions: innovations to bring tuberculosis diagnosis closer to key populations
BMC Global and Public Health (2024)
-
Multidrug-resistant tuberculosis
Nature Reviews Disease Primers (2024)