Abstract
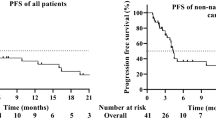
Immunotherapy combined with chemotherapy regimen has been shown to be effective in recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). However, due to the small number of patients, its efficacy remains controversial in Asian populations, particularly in mainland China. Here a randomized, double-blind phase 3 trial evaluated the efficacy and safety of finotonlimab (SCT-I10A), a programmed cell death 1 (PD-1) monoclonal antibody, combined with cisplatin plus 5-fluorouracil (C5F) for the first-line treatment of R/M HNSCC. Eligible patients (n = 370) were randomly 2:1 assigned to receive finotonlimab plus C5F (n = 247) or placebo plus C5F (n = 123). The primary endpoint was overall survival (OS). In the finotonlimab plus C5F group, OS was 14.1 months (95% confidence interval (CI) 11.1–16.4), compared with 10.5 months (95% CI 8.1–11.8) in the placebo plus C5F group. The hazard ratio was 0.73 (95% CI 0.57–0.95, P = 0.0165), meeting the predefined superiority criteria for the primary endpoint. Finotonlimab plus C5F showed significant OS superiority compared with C5F alone and acceptable safety profile with R/M HNSCC, supporting its use as a first-line treatment option for R/M HNSCC. These results validate the efficacy and safety of the combination of finotonlimab and C5F in Asian patients with R/M HNSCC. ClinicalTrials.gov identifier: NCT04146402.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
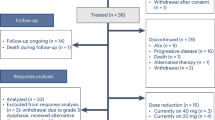
The supporting data for most figures and tables can be found directly within them, while the subgroup analysis data are available in Supplementary Information. Patient-related information cannot be disclosed due to confidentiality agreements. The trial protocol and statistical analysis plan (SAP) will be made available in Supplementary Information. For inquiries regarding access to clinical study documents, please direct your email to the corresponding author with detailed proposals. Requests will be promptly reviewed by the primary investigator and the sponsor to ascertain whether they are subject to any confidentiality obligations. We aim to respond to all requests within 8 weeks. Source data are provided with this paper.
Code availability
Analyses were carried out using commercially available software (SAS version 9.4, SAS Institute Inc.) in accordance with SAP guidelines.
References
Gormley, M., Creaney, G., Schache, A., Ingarfield, K. & Conway, D. I. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br. Dent. J. 233, 780–786 (2022).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Xia, C. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. 135, 584–590 (2022).
Leemans, C. R., Snijders, P. J. F. & Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282 (2018).
Braakhuis, B. J., Brakenhoff, R. H. & Leemans, C. R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: biological risk factors. Ann. Oncol. 23, x173–x177 (2012).
Chow, L. Q. M. Head and neck cancer. N. Engl. J. Med. 382, 60–72 (2020).
Kish, J. et al. Clinical trial of cisplatin and 5-FU infusion as initial treatment for advanced squamous cell carcinoma of the head and neck. Cancer Treat. Rep. 66, 471–474 (1982).
Guo, Y. et al. First-line treatment with chemotherapy plus cetuximab in Chinese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: efficacy and safety results of the randomised, phase III CHANGE-2 trial. Eur. J. Cancer 156, 35–45 (2021).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
First-line combination therapy in metastatic or unresectable, recurrent HNSCC. Keytruda https://www.keytrudahcp.com/efficacy/hnscc-first-line-combination-therapy/ (2019).
Wang, R. et al. Antitumor activity of pegylated human interferon β as monotherapy or in combination with immune checkpoint inhibitors via tumor growth inhibition and dendritic cell activation. Cell. Immunol. 393-394, 104782 (2023).
Bai, M. et al. Phase Ib study of anti-EGFR antibody (SCT200) in combination with anti-PD-1 antibody (SCT-I10A) for patients with RAS/BRAF wild-type metastatic colorectal cancer. Cancer Biol. Med. https://doi.org/10.20892/j.issn.2095-3941.2023.0301 (2023).
Viens, L. J. et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 65, 661–666 (2016).
Ngamphaiboon, N. K. et al. Phase III KEYNOTE-048 study of first-line pembrolizumab for recurrent/metastatic head and neck squamous cell carcinoma: Asia vs non-Asia subgroup analysis. Ann. Oncol. 30, ix97–ix106 (2019). (suppl_9).
Sun, J. M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398, 759–771 (2021).
Pinato, D. J. J. et al. Anti-drug antibodies related to CTLA-4, PD-1, or PD-L1 inhibitors across tumour types: a systematic review. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.e14600 (2023).
Acknowledgements
We express our appreciation for the exceptional dedication and contributions of N. Zhang and X. Liu for the operation and management and D. Liu and J. Yi for the statistical analyses. Medical writing of this manuscript was provided by C. Liu and Y. Chen. We thank H. Zhu (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College) for editorial assistance in the writing of this manuscript. This study was sponsored by Sinocelltech Ltd., Beijing, China, who contributed to trial design, data analyses and preparation of the manuscript. This study was funded by the National Key Research and Development Program of China (2023YFC3042100) and the National Science and Technology Major Projects for Key New Drug Development of China (2019ZX09732-001 and 2017ZX09304015). The funding award has been granted to the study itself and not to any individual author or researcher.
Author information
Authors and Affiliations
Contributions
Y. Shi was the principal investigator, contributing to trial design, supervision, coordination, patient enrollment, patient management, manuscript writing and revising. W. Guo, W.W., Yunteng Wu, M.F., X.H., P.H., Q.Z., P.D., X.Z., H.P., C.H., X.C., Shurong Zhang, Z.C., X.L., Y.D., S.Q., S.J., Songnan Zhang, L.G., Y. Sun, L.W., Y.L., H.W., G.L., Z.F., J.S., H.J., Y.B., J. Cui., Y.Z., W.C., X.J., L.Z., Q.C., D.X., Yunong Wu, J. Cao., R.W., G.H. and L.P. were investigators in this clinical trial, and contributed to patient enrollment and patient management. L.X. contributed to the experimental design, overall management and communication. W. Gai, Y. Wang and Y. Su contributed to the trial design, medical monitoring and supervision of the trial. All authors had access to all data in the trial and were ultimately responsible for the decision to submit the manuscript for publication. The authors were responsible for the accuracy and completeness of the data and the fidelity of the trial to the protocol. All authors read and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
L.X., W. Gai, Y. Wang and Y. Su are employees of Sinocelltech Ltd., Beijing, China. L.X. and W.G. hold potential stock option interests in the company. The other authors, who are investigators involved in this study, declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Alain Algazi, Charlotte Zuur and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Table 1, Protocol and Statistical analysis plan.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Y., Guo, W., Wang, W. et al. Finotonlimab with chemotherapy in recurrent or metastatic head and neck cancer: a randomized phase 3 trial. Nat Med 30, 2568–2575 (2024). https://doi.org/10.1038/s41591-024-03110-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03110-7