Abstract
A spinal cord injury (SCI) disrupts the neuronal projections from the brain to the region of the spinal cord that produces walking, leading to various degrees of paralysis. Here, we aimed to identify brain regions that steer the recovery of walking after incomplete SCI and that could be targeted to augment this recovery. To uncover these regions, we constructed a space–time brain-wide atlas of transcriptionally active and spinal cord-projecting neurons underlying the recovery of walking after incomplete SCI. Unexpectedly, interrogation of this atlas nominated the lateral hypothalamus (LH). We demonstrate that glutamatergic neurons located in the LH (LHVglut2) contribute to the recovery of walking after incomplete SCI and that augmenting their activity improves walking. We translated this discovery into a deep brain stimulation therapy of the LH (DBSLH) that immediately augmented walking in mice and rats with SCI and durably increased recovery through the reorganization of residual lumbar-terminating projections from brainstem neurons. A pilot clinical study showed that DBSLH immediately improved walking in two participants with incomplete SCI and, in conjunction with rehabilitation, mediated functional recovery that persisted when DBSLH was turned off. There were no serious adverse events related to DBSLH. These results highlight the potential of targeting specific brain regions to maximize the engagement of spinal cord-projecting neurons in the recovery of neurological functions after SCI. Further trials must establish the safety and efficacy profile of DBSLH, including potential changes in body weight, psychological status, hormonal profiles and autonomic functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data underpinning this publication have been deposited in Zenodo at https://doi.org/10.5281/zenodo.10628681 (ref. 92) and are publicly available under a Creative Commons Attribution 4.0 International license.
References
Courtine, G. & Sofroniew, M. V. Spinal cord repair: advances in biology and technology. Nat. Med. 25, 898–908 (2019).
Anderson, M. A. et al. Natural and targeted circuit reorganization after spinal cord injury. Nat. Neurosci. 25, 1584–1596 (2022).
Arber, S. & Costa, R. M. Connecting neuronal circuits for movement. Science 360, 1403–1404 (2018).
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012).
Courtine, G. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 14, 69–74 (2008).
Bachmann, L. C. et al. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci. Transl. Med. 5, 208ra146 (2013).
Bonizzato, M. et al. Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury. Nat. Commun. 12, 1925 (2021).
Bonizzato, M. & Martinez, M. An intracortical neuroprosthesis immediately alleviates walking deficits and improves recovery of leg control after spinal cord injury. Sci. Transl. Med. 13, eabb4422 (2021).
Denk, W., Briggman, K. L. & Helmstaedter, M. Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat. Rev. Neurosci. 13, 351–358 (2012).
Tomer, R., Ye, L., Hsueh, B. & Deisseroth, K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 9, 1682–1697 (2014).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Epp, J. R. et al. Optimization of CLARITY for clearing whole-brain and other intact organs. eNeuro 2, ENEURO.0022-15.2015 (2015).
Murray, E. et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514 (2015).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 13, 859–867 (2016).
Friedli, L. et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci. Transl. Med. 7, 302ra134 (2015).
Liu, Y. et al. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron 95, 817–833 (2017).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Goubran, M. et al. Multimodal image registration and connectivity analysis for integration of connectomic data from microscopy to MRI. Nat. Commun. 10, 5504 (2019).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Wickersham, I. R., Sullivan, H. A. & Seung, H. S. Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nat. Protoc. 5, 595–606 (2010).
Voigt, F. F. et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat. Methods 16, 1105–1108 (2019).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016).
Saper, C. B., Chou, T. C. & Scammell, T. E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731 (2001).
Margules, D. L. & Olds, J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 135, 374–375 (1962).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Sinnamon, H. M. & Stopford, C. K. Locomotion elicited by lateral hypothalamic stimulation in the anesthetized rat does not require the dorsal midbrain. Brain Res. 402, 78–86 (1987).
Sinnamon, H. M. Locomotor stepping elicited by electrical stimulation of the hypothalamus persists after lesion of descending fibers of passage. Physiol. Behav. 48, 261–266 (1990).
Sinnamon, H. M., Lee, S. H., Adams, D. B. & Stopford, C. K. Locomotor stepping elicited by electrical stimulation of the lateral hypothalamus requires an ipsilateral descending pathway. Physiol. Behav. 33, 209–215 (1984).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Scheff, S. W., Rabchevsky, A. G., Fugaccia, I., Main, J. A. & Lumpp, J. E.Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 (2003).
Asboth, L. et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 21, 576–588 (2018).
Wang, Z. et al. Brain-wide analysis of the supraspinal connectome reveals anatomical correlates to functional recovery after spinal injury. eLife 11, e76254 (2022).
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Tervo, D. G. R. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Lüscher, C. & Pollak, P. Optogenetically inspired deep brain stimulation: linking basic with clinical research. Swiss Med. Wkly. 146, w14278 (2016).
Chow, B. Y. & Boyden, E. S. Optogenetics and translational medicine. Sci. Transl. Med. 5, 177ps5 (2013).
Deisseroth, K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol. Psychiatry 71, 1030–1032 (2012).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Dominici, N. et al. Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nat. Med. 18, 1142–1147 (2012).
Kathe, C. et al. The neurons that restore walking after paralysis. Nature 611, 540–547 (2022).
Squair, J. W. et al. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science 381, 1338–1345 (2023).
Skinnider, M. A. et al. Single-cell and spatial atlases of spinal cord injury in the Tabulae Paralytica. Nature 631, 150–163 (2024).
Whiting, D. M. et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J. Neurosurg. 119, 56–63 (2013).
Rupp, R. et al. International Standards for Neurological Classification of Spinal Cord Injury: revised 2019. Top. Spinal Cord Inj. Rehabil. 27, 1–22 (2021).
Murphy, J. P. & Gellhorn, E. The influence of hypothalamic stimulation on cortically induced movements and on action potentials of the cortex. J. Neurophysiol. 8, 341–364 (1945).
Lorach, H. et al. Walking naturally after spinal cord injury using a brain–spine interface. Nature 618, 126–133 (2023).
Rowald, A. et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 28, 260–271 (2022).
Capogrosso, M. et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288 (2016).
Wagner, F. B. et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71 (2018).
Squair, J. W. et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 590, 308–314 (2021).
Kathe, C. et al. Wireless closed-loop optogenetics across the entire dorsoventral spinal cord in mice. Nat. Biotechnol. 40, 198–208 (2022).
Takeoka, A., Vollenweider, I., Courtine, G. & Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159, 1626–1639 (2014).
Caggiano, V. et al. Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460 (2018).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Lee, E. et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci. Rep. 6, 18631 (2016).
Capelli, P., Pivetta, C., Esposito, M. S. & Arber, S. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377 (2017).
Wenger, N. et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 22, 138–145 (2016).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Bria, A. & Iannello, G. TeraStitcher—a tool for fast automatic 3D-stitching of teravoxel-sized microscopy images. BMC Bioinformatics 13, 316 (2012).
Sotocinal, S. G. et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 55 (2011).
Elgendi, M., Norton, I., Brearley, M., Abbott, D. & Schuurmans, D. Systolic peak detection in acceleration photoplethysmograms measured from emergency responders in tropical conditions. PLoS ONE 8, e76585 (2013).
Lutti, A., Thomas, D. L., Hutton, C. & Weiskopf, N. High-resolution functional MRI at 3 T: 3D/2D echo-planar imaging with optimized physiological noise correction. Magn. Reson. Med. 69, 1657–1664 (2013).
Weiskopf, N. et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Front. Neurosci. 7, 95 (2013).
Hutton, C. et al. Image distortion correction in fMRI: a quantitative evaluation. NeuroImage 16, 217–240 (2002).
Draganski, B. et al. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). NeuroImage 55, 1423–1434 (2011).
Lutti, A., Dick, F., Sereno, M. I. & Weiskopf, N. Using high-resolution quantitative mapping of R1 as an index of cortical myelination. NeuroImage 93, 176–188 (2014).
Lutti, A. et al. Robust and fast whole brain mapping of the RF transmit field B1 at 7T. PLoS ONE 7, e32379 (2012).
Neudorfer, C. et al. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci. Data 7, 305 (2020).
Ashburner, J. & Friston, K. J. Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation. NeuroImage 55, 954–967 (2011).
Tournier, J.-D. et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. NeuroImage 142, 394–406 (2016).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Horn, A. & Kühn, A. A. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. NeuroImage 107, 127–135 (2015).
Horn, A. et al. Lead-DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage 184, 293–316 (2019).
Friston, K. J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1994).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54, 2033–2044 (2011).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 54, 313–327 (2011).
Schönecker, T., Kupsch, A., Kühn, A. A., Schneider, G.-H. & Hoffmann, K.-T. Automated optimization of subcortical cerebral MR imaging–atlas coregistration for improved postoperative electrode localization in deep brain stimulation. AJNR Am. J. Neuroradiol. 30, 1914–1921 (2009).
Husch, A., Petersen, M. V., Gemmar, P., Goncalves, J. & Hertel, F. PaCER—a fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. NeuroImage Clin. 17, 80–89 (2018).
Treu, S. et al. Deep brain stimulation: imaging on a group level. NeuroImage 219, 117018 (2020).
Horn, A. et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 82, 67–78 (2017).
Middlebrooks, E. H. et al. Neuroimaging advances in deep brain stimulation: review of indications, anatomy, and brain connectomics. AJNR Am. J. Neuroradiol. 41, 1558–1568 (2020).
Jackson, A. B. et al. Outcome measures for gait and ambulation in the spinal cord injury population. J. Spinal Cord Med. 31, 487–499 (2008).
Courtine, G., Barraud, Q. & Squair, J. Datasets related to the manuscript ‘Hypothalamic deep brain stimulation augments walking after spinal cord injury’. Zenodo https://doi.org/10.5281/zenodo.10628681 (2024).
Acknowledgements
This study was supported by ONWARD Medical, the Swiss National Science Foundation (Ambizione fellowship to J.W.S., PZ00P3_208988) and subsidy to G.C. (310030_185214 and 310030_215668), Canadian Institutes of Health Research (Research Fellowship Award to N.C.), European Research Council (ERC-2015-CoG HOW2WALKAGAIN 682999; Marie Skłodowska-Curie individual fellowship 842578 to J.W.S.), H2020-MSCA-COFUND-2016 EPFL Fellows program (no. 665667 to C.K.), Human Frontiers in Science Program long-term fellowship (LT001278/2017-L to C.K.) and the Swiss National Supercomputing Center (CSCS). We are grateful to B. Schneider and S. Arber for providing viral vectors, as well as to the Advanced Lightsheet Imaging Center (ALICe) at the Wyss Center for Bio and Neuroengineering, Geneva.
Author information
Authors and Affiliations
Contributions
V.A., N.D.J. and L.B.-F. contributed equally to this work. N.C., N.D.J., L. Baud, T.H.H. and C.K. performed the animal experiments. J.W.S. and M.A.S. performed computational analysis. N.C., N.D.J., K.G. and Q.B. performed the anatomical experiments. N.C., Q.B. and K.G. performed clearing protocols. M. Goubran developed and shared MIRACL. N.C., J.W.S., Q.B., I.D., A. Leonhartsberger, K.S., M. Gautier, K.G., L. Batti, S.P., K.A.B., A. Laskaratos and M.H. performed image acquisition, processing and analysis. J.R. and F.M. prepared illustrations. N.C., J.W.S., V.A., I.D., A. Leonhartsberger, K.S. and M.A.A. analyzed the experimental data. L.B.-F., V.A., N.H., N.I., C.V., S.C., R.D., L.A. and J.B. performed human experiments. N.I. and L.B.-F. conducted rehabilitation sessions. L.B.-F., N.H., C.V., N.B., L.A. and R.D. analyzed the human data. L.B.-F., L.A., A.W. and L.D. managed regulatory affairs. J.B. and V.A. performed neurosurgical interventions. B.D. performed the fMRI experiments and analyzed the data. G.C. and J.B. conceived and supervised the study. G.C. and J.B. secured funding. G.C. wrote the paper with J.B., N.C., J.W.S., Q.B. and L.A., and all authors contributed to its editing.
Corresponding authors
Ethics declarations
Competing interests
G.C., J.B., N.C., J.W.S., R.D., L.A. and L.B.-F. hold various patents in relation to the present work. G.C. is a consultant of ONWARD Medical. G.C. and J.B. are minority shareholders of ONWARD Medical, a company with partial relationships with the presented work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of whole brain activity after recovery after incomplete SCI.
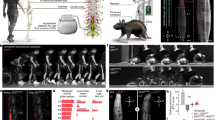
a, Overview of experimental approach for lateral hemisection SCI in mice. Mice were trained to run continuously on a treadmill for 2 weeks prior to injury. Uninjured mice and mice after 1 week (acute) and 8 weeks (chronic) after injury then ran on the treadmill to assess recovery of function. b, Chronophotography of walking captured in uninjured mice and at 1 week (acute) and 8 weeks (chronic) after a lateral hemisection SCI. Ipsilesional (left) leg visualized (mirrored image presented). c, Walking was quantified using principal component analysis applied to gait parameters calculated from kinematic recordings. In this denoised space, each dot represents a mouse (n ≥ 10 gait cycles per mouse, n = 3 mice per group). Larger dots represent the mean of each experimental group. The first principal component (PC1) distinguished gaits of mice without SCI from mice with SCI. Walking scores were thus quantified as the scores on PC1. Analysis of factor loadings on PC1 revealed that the percentage of paw dragging, step height, and endpoint velocity were the parameters that showed high correlation with PC1. Bars report the mean values of these gait parameters. Statistics are provided in Supplementary Data 1. d, To examine whole brain activity, mice (n = 3 mice per group) ran on a treadmill for 45 minutes after which they were perfused 1 hour after the task to allow expression of cFos. iDISCO+ was utilized due to its reliable whole brain immunolabeling methodology14. To visualize cFos (nuclear) staining, a custom-built COLM (CLARITY-optimized lightsheet microscope)10 was used at 4x magnification to ensure adequate resolution of nuclei. Whole mouse brain registration to the Allen Brain Atlas was performed using MIRACL19,20. Raw autofluorescence channel optical sections with demonstration of registration of the Allen Mouse Brain Atlas. Representative visualization of raw data demonstrating labeled cFos-positive cells. This was followed by automated segmentation via the Arivis Vision 4D “Blob Finder” function. Bar plot demonstrating cFos expression in intact mice in areas known to be involved in locomotion with representative optical sections through those regions demonstrating cFos labeling. Representative chronophotography of uninjured mouse walking across the runway shown (mirrored image). MOp5: primary motor area, layer 5; MLR: mesencephalic locomotor region; CUN: cuneiform nucleus; PPN: pedunculopontine nucleus; MARN: magnocellular reticular nucleus. e, 3D representations of all brains used in the analysis of cFos activity. Mice left in the homecage without any treadmill locomotion are also provided for reference.
Extended Data Fig. 2 Characterization of spinal cord-projecting neurons across the whole brain during recovery from SCI.
a, Uninjured mice, and mice 1 (acute) and 8 weeks (chronic) (n = 3 mice per group) after left lateral hemisection at T10 were examined. G-deleted rabies was injected into the left (ipsilesional) lumbar spinal cord. Representative injection site of the left lumbar spinal cord demonstrating laterality of the injection. Demonstration of the whole brain clearing and imaging pipeline for mice injected with rabies. CLARITY was utilized due to its ability to preserve endogenous fluorescence, followed by imaging with a custom-built mesoSPIM lightsheet microscope22. Whole mouse brain registration was performed using MIRACL19,20. Optical sections demonstrating registration of the mouse Allen Brain Atlas on raw CLARITY data of a mouse brain. Representative visualization of labeled neurons with subsequent semi-automated segmentation of neurons using the Arivis Vision 4D three-dimensional analysis software. b, 3D visualization of whole brain labeling of all neurons with direct connections to the lumbar spinal cord. Representative whole brain of uninjured animal with optical sections highlighting labeling in key regions of the brain. c, 3D representations of all brains used in the analysis of whole brain connectivity with time after SCI including uninjured mice and mice 1 week and 8 weeks after injury. The lateral hypothalamus is highlighted with optical sections.
Extended Data Fig. 3 Quality control and differential analysis of whole brain transcriptional activity and anatomical connectivity.
a, Number of counts per region throughout the brain with transcriptional activity. Dashed line represents the median number of counts in each region. b, As in a, for spinal cord-projecting neuron counts. c, Number of transcriptionally active cells across the whole brain for each brain. Brains are colored by experimental group. Distribution reflects the variance across regions within each brain (n = 3 mice per group). Box plots reflect the median, with the limits of the box as the first and third quartile, and the range represented by the plot whiskers. d, As in c, for spinal cord-projecting neuron counts (n = 3 mice per group). Box plots reflect the median, with the limits of the box as the first and third quartile, and the range represented by the plot whiskers. e, Number of regions with transcriptionally active cells throughout the whole brain, for each brain. Brains are colored by experimental group. f, As in e, for spinal cord-projecting neuron counts. g, Number of transcriptionally active cells in each major region of the brain, for each brain. Brains are colored by experimental group (n = 3 mice per group). Box plots reflect the median, with the limits of the box as the first and third quartile, and the range represented by the plot whiskers. h, As in g, for spinal cord-projecting neuron counts (n = 3 mice per group). Box plots reflect the median, with the limits of the box as the first and third quartile, and the range represented by the plot whiskers. i, Spike plot indicating the number of transcriptionally active cells in each region of the brain (x-axis). Plots are faceted by major region of the brain (horizontal) and experimental group (vertical). Each spike represents the counts for a single region. j, As in i, for spinal cord-projecting neuron counts. k, Mean-variance relationship for count data from transcriptionally active cells and spinal cord-projecting neurons. l, Volcano plots indicate differential analysis of each comparison outlined in k. Colored dots represent those meeting statistical significance. FC: Fold Change.
Extended Data Fig. 4 Optogenetic stimulation of LHVglut2 neurons significantly augments walking after chronic SCI.
a, Schematic of the experimental scheme demonstrating injection of an AAV5-DIO-ChR2-YFP into the right-sided LH of Vglut2Cre mice with insertion of an optic fiber. Mice underwent a left lateral hemisection at T10, and behavioral recordings were performed at 8 weeks post-SCI. Representative photomicrograph demonstrating ChR2 expression in LHVglut2 neurons following injections of AAV5-DIO-ChR2-YFP. The tract of the optic fiber is also visible in the LH, magnified in the inset. 3 V: third ventricle.Photomicrographs demonstrating cFos activation in the LH in response to 10 minutes of blue light stimulation in a Vglut2Cre animal injected with AAV5-DIO-ChR2-YFP compared to a Vglut2Cre mice injected with control virus. Quantification of cFos-positive cells revealed significantly more cFosON cells in mice injected with AAV5-DIO-ChR2-YFP compared to control virus (n = 5 mice per group; independent samples two-tailed t-test; t = 7.068; p = 0.0001). Lesion reconstructions of animals included in kinematic analysis are provided. b, Kinematic analysis of mice following optogenetic activation of LHVglut2 neurons. Walking was quantified using principal component analysis as described in Fig. 1c and Extended Data Fig. 1c (n ≥ 10 gait cycles per mouse, n = 5 mice per group). Statistics are provided in Supplementary Data 1. c, As in b, for mice following different frequencies of optogenetic stimulation (n ≥ 10 gait cycles per mouse, n = 5 mice per group). d, As in b, for mice following photostimulation and injections of AAVs expressing only the reporter protein GFP, with no opsin expression (n ≥ 10 gait cycles per mouse, n = 5 mice per group). Statistics are provided in Supplementary Data 1.
Extended Data Fig. 5 Optogenetic stimulation of LHVgat neurons does not alter walking while optogenetic inhibition of LHVglut2 degrades walking after chronic SCI.
a, Schematic of the experimental scheme demonstrating injections of AAV5-DIO-ChR2-YFP into the right-sided LH of VgatCre mice with insertion of an optic fiber. Mice underwent a left lateral hemisection at T10, and behavioral recordings were performed at 8 weeks post-SCI. Representative photomicrograph demonstrating ChR2 expression in LHVgat neurons. The tract of the optic fiber is also visible in the LH, magnified in the inset. Spared tissue reconstructions for mice included in kinematic analyses are provided on the right. b, Kinematic analysis of mice following optogenetic activation of LHVgat neurons. Walking was quantified using principal component analysis as described in Fig. 1c and Extended Data Fig. 1c (n ≥ 10 gait cycles per mouse, n = 5 mice per group). Statistics are provided in Supplementary Data 1. c, Schematic of the experimental scheme demonstrating injection of an AAV5-DIO-eNpHR-YFP into the right-sided LH of Vglut2Cre mice with insertion of an optic fiber. Remaining experimental procedures mirror those outlined in a. Spared tissue reconstructions for mice included in kinematic analyses are provided on the right. d, As in b, for mice following optogenetic inhibition of LHVglut2 neurons with yellow light (n ≥ 10 gait cycles per mouse, n = 4 mice per group). Statistics are provided in Supplementary Data 1. e, As in b, for mice following photostimulation and injections of AAVs expressing only the reporter protein GFP, with no opsin expression (n ≥ 10 gait cycles per mouse, n = 5 mice per group). Statistics are provided in Supplementary Data 1.
Extended Data Fig. 6 Optogenetic stimulation of LHVglut2 neurons augments walking after contusion SCI via vGi neurons.
a, Schematic of the experimental scheme demonstrating contusion at T9 in the mouse. Cleared spinal cord demonstrating a representative lesion with cross-sectional histology of the lesion epicenter (preserved tissue stained with GFAP) and associated lesion reconstruction. b, Kinematic analysis of mice following contusion SCI. Walking was quantified using principal component analysis as described in Fig. 1c and Extended Data Fig. 1c (n ≥ 10 gait cycles per mouse, n = 5 mice in the uninjured group, n = 8 in the contusion SCI group). Statistics are provided in Supplementary Data 1. c, LH connections with the lumbar spinal cord are not reliably preserved after contusion SCI. Bilateral G-deleted rabies were injected into the lumbar spinal cord at 8 weeks post-SCI (n = 3 mice). After 4 days, mice were perfused and the brains extracted, cleared, imaged, and registered to the Allen Brain Atlas. Neurons were segmented and quantified. Left, Representative cleared whole mouse brain and spinal cord demonstrating injection site, contusion, and labeling of brain neurons. Middle, 3D brain representations demonstrating labeling in the vGi but not the lateral hypothalamus with associated optical sections (50 µm projection). Right, Bar plots demonstrating the percentage of all neurons with direct connections to the lumbar spinal cord after contusion for several regions with the highest proportion of neurons. vGi neurons (gigantocellular nucleus, magnocellular nucleus) with direct projections past the contusion SCI were prominent. We noted an absence of direct projections from the LH to the lumbar spinal cord. Lesion reconstructions and quantifications for the animals analyzed are also provided. Mean sparing = 29.0%. 3 V: third ventricle. d, Schematic of the experimental scheme to understand efferent projections from LHVglut2 neurons to various motor centers in the brainstem. Vglut2Cre mice underwent a left lateral thoracic hemisection and were allowed to spontaneously recover for 6 weeks (n = 3 mice). To understand the efferent projections of recovered mice, AAV5-DIO-mGFP was injected into the right LH of these mice, which was allowed to express for 4 weeks. Photomicrograph demonstrating a representative injection site with labeled LHVglut2 neurons. Representative images demonstrating axon projections from LHVglut2 neurons to various brainstem motor centers. Top panel demonstrates projections to the PAG (periaqueductal gray), CnF (cuneiform nucleus), and PTg (pedunculopontine tegmental nucleus). Middle panels demonstrate projections to the PnC (pontine reticular nucleus) and RMg (raphe magnus). Bottom panels demonstrate projections to the medullary reticular formation including the Gi (gigantocellular reticular nucleus) and vGi, and LPGi (lateral paragigantocellular reticular nucleus). Insets demonstrate magnified views of the axons. Density quantification of fibers to each of the brainstem motor centers divided into ipsilateral, contralateral, and midline (for RMg) quantifications. 3 V: third ventricle.
Extended Data Fig. 7 Characterization of neurons in the LH and motor cortex with projections to vGiVglut2 neurons.
a, Schematic of the experimental scheme to understand projection inputs on vGiVglut2 neurons with spared projections to the lumbar spinal cord. Vglut2Cre mice underwent a contusion SCI and were allowed to spontaneously recover for one month (n = 3 mice). To understand the inputs onto vGiVglut2 neurons with spared projections to the lumbar spinal cord, rAAV2-hSyn-GFP was injected into the lumbar spinal cord bilaterally. AAV-DIO-tdTomato was concomitantly injected into the right LH of these mice, which was allowed to express for 4 weeks. Photomicrographs were acquired with immunohistochemical staining for Homer and vGlut2, in addition to the viral labelling. b, Photomicrographs from each of the three tested mice, demonstrating synaptic-like appositions of LHVglut2 projections to vGiVglut2 with spared axonal projections to the lumbar spinal cord. Sections have been labelled with immunohistochemistry for the presynaptic marker vGlut2 and the postsynaptic marker Homer. c, Quantification of the number of vGiVglut2 neurons with at least one synaptic-like apposition from LHVglut2 neurons. d, Schematic of the experimental scheme to label neurons in the motor cortex and LH with monosynaptic projections to vGiVglut2 neurons. Vglut2Cre mice were injected with AAV8-DIO-TC66T-eGFP-oG in the vGi, followed by injections of EnvA G-deleted Rabies-mCherry two weeks later. Photomicrographs demonstrate neurons in the motor cortex and LH with monosynaptic projections to vGiVglut2 neurons. Right, quantification of the number of labelled neurons in each region. e, Schematic of the experimental scheme demonstrating injection of AAV5-Con/Fon-ChR2-YFP into the right LH with insertion of an optic fiber and of retro-AAV2-DIO-FLPo into the vGi of Vglut2Cre mice. Mice underwent a contusion injury at T9, and behavioral recordings were performed at 6 weeks post-SCI. Middle, Representative image demonstrating AAV5-Con/Fon-ChR2-YFP expression in the right LH and position of the optic fiber. Right, Contusion lesion epicenter reconstructions with percent spared tissue. Mean sparing = 25.5%. 3 V: third ventricle. f, As in Extended Data Fig. 6b, for mice following optogenetic activation of LHVglut2 neurons with direct projects to the vGi (n ≥ 10 gait cycles per mouse, n = 7 mice per group). Statistics are provided in Supplementary Data 1.
Extended Data Fig. 8 vGiVglut2 neuron activation is necessary for LH-mediated improvements in walking after SCI and these effects are phenocopied by electrical deep brain stimulation.
a, Schematic of the experimental scheme demonstrating injections of AAV5-DIO-ChR2-YFP into the right LH with insertion of an optic fiber and AAV5-hSyn-DIO-hM4D(Gi)-mCherry into the vGi of Vglut2Cre mice. Mice underwent a left lateral hemisection injury at T10, and behavioral recordings were performed at 8 weeks post-SCI after spontaneous recovery. Representative images demonstrating hM4D(Gi)-mCherry expression in the vGi. py: pyramidal tract; 4v: Fourth ventricle. b, Kinematic analysis of mice following optogenetic activation of LHVglut2 neurons and concomitant chemogenetic inactivation of vGiVglut2 neurons after SCI. Walking was quantified using principal component analysis as described in Fig. 1c and Extended Data Fig. 1c (n ≥ 10 gait cycles per mouse, n = 5 mice per group). Statistics are provided in Supplementary Data 1. c, Schematic of the experimental scheme demonstrating injections of AAV5-DIO-DTR into the right LH of Vglut2Cre mice, with electrical stimulation of the LH (DBSLH). Mice underwent a contusion injury at T9, and behavioral recordings were performed at 6 weeks post-SCI. Middle, Representative image demonstrating ablation of LHVglut2 neurons. Bar graph demonstrates significant reduction in LHVglut2 neurons (n = 4 per group; independent samples t-test; t = 0.2548; p-value = 0.0145). Right, Lesion reconstructions and quantifications for the animals analyzed are also provided. Mean sparing = 21.8 %. d, As in b, for mice with DBS followed by DBS and ablation of LHVglut2 neurons (n ≥ 10 gait cycles per mouse, n = 4 mice per group). Statistics are provided in Supplementary Data 1.
Extended Data Fig. 9 DBSLH in rats with contusion SCI leads to immediate and durable improvements in walking even when DBSLH is turned off.
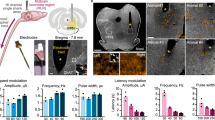
a, Schematic of the experimental scheme demonstrating electrical stimulation of the LH (DBSLH) in rats with contusion SCI at T9. Middle, Representative image demonstrating electrode ___location in the LH. Bar graph demonstrates significant increase in cFos positive cells in the LH with stimulation (n = 4 no DBS, n = 5 DBS; independent samples two-tailed t-test; t = 3.741; p-value = 0.0073). The stimulation was well-tolerated by all rats, as evidenced by no significant difference in grimace score (n = 6 per group; paired samples two-tailed t-test; t = 0.2548; p-value = 0.8090). b, Kinematic analysis of uninjured rats following increasing amplitudes of DBSLH. Walking was quantified using principal component analysis as described in Fig. 1c and Extended Data Fig. 1c (n ≥ 10 gait cycles per rat, n = 3 rats). c, As in b, for rats after contusion SCI (n ≥ 10 gait cycles per rat, n = 7 rats in the uninjured group, n = 6 rats in the contusion group). Statistics are provided in Supplementary Data 1. d, As in b, for rats after contusion SCI and acute activation of DBSLH at five weeks post-SCI (n ≥ 10 gait cycles per rat, n = 6 rats per group). Statistics are provided in Supplementary Data 1. e, As in b, for rats after contusion SCI and rehabilitation enabled by DBSLH (n ≥ 10 gait cycles per rat, n = 5 rats in SCI group, n = 4 rats in SCI + Rehab group). Statistics are provided in Supplementary Data 1. f, As in b, for rats after contusion SCI with rehabilitation, and acute activation of DBSLH after rehabilitation (n ≥ 10 gait cycles per rat, n = 4 rats per group). Statistics are provided in Supplementary Data 1.
Extended Data Fig. 10 DBSLH in two incomplete SCI individuals leads to immediate and durable improvements in walking.
a, Clinical trial timeline. b, Diffusion tensor imaging (DTI)-based tractography exploring LH neuronal projections in the first participant. c, Intra-operative spiking activity of the first participant while descending a micro-electrode within the LH exposes basal activity of neurons throughout the rostrocaudal extent of the LH. d, Coronal and axial sections of the preoperative MRI of the first participant showing the projection of the DBS leads. e, Computer simulations of volume of activated tissues of the most-used stimulation program of the first participant. The distribution of the proportion of activated regions shows the LH as the main activated region. P1 stimulation program: left & right DBS contacts 0 + 1-2 + , amplitude 7 mA. f, As in b, for the second participant. g, As in c, for the second participant. h, As in d, for the second participant. i, As in e, for the second participant. P2 stimulation program: left & right DBS contacts 1 + 2-3 + , amplitude 7 mA. Pulse width and frequency are not taken into account when calculating the activation volume. j, Kinematic and muscle analysis of participants P1 and P2 walking on parallel bars with and without DBSLH during the same calibration session. Walking was quantified using principal component analysis applied to gait parameters calculated from kinematic and muscle recordings. For both participants, the first principal component (PC1) distinguished steps enhanced by DBSLH from steps without DBSLH. Gait scores were thus quantified as the scores on PC1 (n ≥ 7 gait cycles). Statistics are provided in Supplementary Data 3. k, Attempts of participants P1 and P2 to perform 3 voluntary hip flexions with and without DBSLH, during the same calibration session. Associated recording of electromyographic activity from flexor muscle and its quantification (n = 3). l, Chronophotography of walking recorded in the second participant with and without DBSLH, together with the associated recording of electromyographic activity from extensor and flexor muscles and their quantification (n = 11 gait cycles, dots represent individual trials). Statistics are provided in Supplementary Data 3. m, Quantification of the walking improvements following 3 months of rehabilitation enhanced with DBS, for P1 and P2. Goal achievement score throughout rehabilitation for P1 and P2 (scale: 1, not achieved/with significant help; 2, very partially achieved / with light to moderate help; 3, partially achieved / under supervision; 4, achieved / independent). Proportion of weight support (BWS) provided to P1 during sessions. Quantification of number of stairs climbed and related speed of P2 throughout the whole study (PI: pre-implantation; Rehab: during rehabilitation, DBSON; M3: post-rehabilitation, DBSON; M6: end of main study, DBSON). Quantification of step height and step length while standing and walking on parallel bars respectively for P1 and P2, pre-implantation (PI) and at the end of main study (M6), DBSOFF. Statistics are provided in Supplementary Data 3.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Video 1
Space–time brain atlas.
Supplementary Video 2
LHVglut2 neurons augment walking after spinal cord injury.
Supplementary Video 3
Lateral hypothalamus augments walking through relays.
Supplementary Video 4
DBSLH immediately and durably improves walking.
Supplementary Video 5
DBSLH augments walking in humans.
Supplementary Data 1
Kinematic analyses and statistics for Figs. 1–4 and Extended Data Figs. 1 and 4–9.
Supplementary Data 2
Whole-brain analyses for Extended Data Fig. 3.
Supplementary Data 3
Human data and statistics for Fig. 5 and Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, N., Squair, J.W., Aureli, V. et al. Hypothalamic deep brain stimulation augments walking after spinal cord injury. Nat Med 30, 3676–3686 (2024). https://doi.org/10.1038/s41591-024-03306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03306-x
This article is cited by
-
Efficacy of deep brain stimulation in locomotor function recovery post-spinal cord injury: a systematic review and meta-analysis
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2025)
-
Brain stimulation aids walking after spinal injury
Nature Reviews Neurology (2025)
-
A hypothalamus–brainstem circuit governs the prioritization of safety over essential needs
Nature Neuroscience (2025)