Abstract
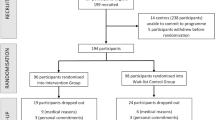
Effective, scalable dementia prevention interventions are needed to address modifiable risk factors given global burden of dementia and challenges in developing disease-modifying treatments. A single-blind randomized controlled trial assessed an online multidomain lifestyle intervention to prevent cognitive decline over 3 years. Participants were dementia-free community-dwelling Australians aged 55–77 years with modifiable dementia risk factors. Eligible participants (n = 6,104, 64% female) were randomized 1:1 to a personalized schedule of online coaching in two to four modules (targeting physical activity, nutrition, cognitive activity and depression or anxiety) or a control group that received module-eligible information only. At 3 years, the mean change in a global cognitive composite, the primary outcome, was met. The mean changes in z scores were 0.28 (95% confidence interval (CI): 0.25–0.32) for intervention, 0.10 (95% CI: 0.07–0.13) for control and 0.18 (95% CI: 0.13–0.23, P < 0.001) for the between-group difference. Trial-related adverse events occurred in 19 (0.60%) intervention and 1 (0.03%) control participant. Randomization of this internet-delivered lifestyle intervention tailored to individual dementia risk factors resulted in significantly better cognition in older adults over 3 years. This intervention is scalable with the potential for population-level rollout that may delay cognitive decline in the general community. Australian New Zealand ClinicalTrials.gov registration: ACTRN12618000851268.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Deidentified data collected for the trial, including individual participant data and data dictionaries, will be made available upon review and approval. Proposals need to be scientifically valid and methodologically sound. A signed data access agreement is needed to access data as per Ethics Committee requirements. Please contact MED CHeBA Data ([email protected]). The trial protocol is published16.
Code availability
The code for the data analysis is also available upon request to MED CHeBA Data ([email protected]).
References
World Health Organization. Global status report on the public health response to dementia. www.who.int/publications/i/item/9789240033245 (2021).
Kivipelto, M., Mangialasche, F. & Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666 (2018).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, 2013).
World Health Organization. International Classification of Diseases, 11th Revision (ICD-11) (World Health Organization, 2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Coley, N., Giulioli, C., Aisen, P. S., Vellas, B. & Andrieu, S. Randomised controlled trials for the prevention of cognitive decline or dementia: a systematic review. Ageing Res. Rev. 82, 101777 (2022).
Meng, X. et al. Multidomain lifestyle interventions for cognition and the risk of dementia: a systematic review and meta-analysis. Int. J. Nurs. Stud. 130, 104236 (2022).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Solomon, A. et al. Effect of a multidomain lifestyle intervention on estimated dementia risk. J. Alzheimers Dis. 82, 1461–1466 (2021).
Anstey, K. J. et al. A self-report risk index to predict occurrence of dementia in three independent cohorts of older adults: the ANU-ADRI. PLoS ONE 9, e86141 (2014).
Kim, S., Cherbuin, N. & Anstey, K. J. Assessing reliability of short and tick box forms of the ANU-ADRI: convenient alternatives of a self-report Alzheimer’s disease risk assessment. Alzheimers Dement. 2, 93–98 (2016).
Anstey, K. J. et al. Body brain life: a randomized controlled trial of an online dementia risk reduction intervention in middle-aged adults at risk of Alzheimer’s disease. Alzheimers Dement. 1, 72–80 (2015).
Anstey, K. J. et al. An internet-based intervention augmented with a diet and physical activity consultation to decrease the risk of dementia in at-risk adults in a primary care setting: pragmatic randomized controlled trial. J. Med. Internet Res. 22, e19431 (2020).
Wesselman, L. M. et al. Web-based multidomain lifestyle programs for brain health: comprehensive overview and meta-analysis. JMIR Ment. Health 6, e12104 (2019).
Richard, E. et al. Methodological challenges in designing dementia prevention trials—the European Dementia Prevention Initiative (EDPI). J. Neurol. Sci. 322, 64–70 (2012).
Heffernan, M. et al. Maintain your brain: protocol of a 3-year randomized controlled trial of a personalized multi-modal digital health intervention to prevent cognitive decline among community dwelling 55 to 77 year olds. J. Alzheimers Dis. 70, S221–S237 (2019).
Ginige, J. A. et al. Fully-online, interoperable clinical trial management system for multi-interventional RCT: maintain your brain digital platform. Stud. Health Technol. Inform. 268, 97–112 (2020).
Radd-Vagenas, S. et al. Validity of the Mediterranean Diet and Culinary Index (MediCul) for online assessment of adherence to the ‘traditional’ diet and aspects of cuisine in older adults. Nutrients 10, 1913 (2018).
Kessler, R. C. et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol. Med. 32, 959–976 (2002).
Hernandez, R. et al. Attrition from longitudinal ageing studies and performance across domains of cognitive functioning: an individual participant data meta-analysis. BMJ Open 14, e079241 (2024).
Rosenberg, A., Mangialasche, F., Ngandu, T., Solomon, A. & Kivipelto, M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to world-wide FINGERS. J. Prev. Alzheimers Dis. 7, 29–36 (2020).
Vellas, B. et al. MAPT study: a multidomain approach for prevention Alzheimer’s disease: design and baseline data. J. Prev. Alzheimers Dis. 1, 13–22 (2014).
Röhr, S., Kivipelto, M., Mangialasche, F., Ngandu, T. & Riedel-Heller, S. G. Multidomain interventions for risk reduction and prevention of cognitive decline and dementia: current developments. Curr. Opin. Psychiatry 35, 285–292 (2022).
Moll van Charante, E. P. et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388, 797–805 (2016).
Yaffe, K. et al. Effect of personalized risk-reduction strategies on cognition and dementia risk profile among older adults: the SMARRT randomized clinical trial. JAMA Intern. Med. 184, 54–62 (2024).
Lenze, E. J. et al. Effects of mindfulness training and exercise on cognitive function in older adults: a randomized clinical trial. JAMA 328, 2218–2229 (2022).
Eggink, E., Moll van Charante, E. P., van Gool, W. A. & Richard, E. A population perspective on prevention of dementia. J. Clin. Med. 8, 834 (2019).
Mao, H.-F., Tsai, A. Y.-J., Chang, L.-H. & Tsai, I.-L. Multi-component cognitive intervention for older adults with mixed cognitive levels: implementation and preliminary effectiveness in real-world settings. BMC Geriatr. 21, 543 (2021).
Jia, J. et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 380, e072691 (2023).
Hafdi, M., Hoevenaar-Blom, M. P. & Richard, E. Multi-___domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst. Rev. 11, CD013572 (2021).
Beintner, I. et al. Adherence reporting in randomized controlled trials examining manualized multisession online interventions: systematic review of practices and proposal for reporting standards. J. Med. Internet Res. 21, e14181 (2019).
Fleming, T. et al. Beyond the trial: systematic review of real-world uptake and engagement with digital self-help interventions for depression, low mood, or anxiety. J. Med. Internet Res. 20, e199 (2018).
Turunen, M. et al. Computer-based cognitive training for older adults: determinants of adherence. PLoS ONE 14, e0219541 (2019).
Belleville, S. et al. Is more always better? Dose effect in a multidomain intervention in older adults at risk of dementia. Alzheimers Dement. 18, 2140–2150 (2022).
Coley, N. et al. Older adults’ reasons for participating in an eHealth prevention trial: a cross-country, mixed-methods comparison. J. Am. Med. Dir. Assoc. 20, 843–849.e5 (2019).
Zuelke, A. E. et al. Gender-specific design and effectiveness of non-pharmacological interventions against cognitive decline and dementia-protocol for a systematic review and meta-analysis. PLoS ONE 16, e0256826 (2021).
Deeks, A., Lombard, C., Michelmore, J. & Teede, H. The effects of gender and age on health related behaviors. BMC Public Health 9, 213 (2009).
Licher, S. et al. Effects of eligibility criteria on patient selection and treatment implications from 10 multidomain dementia prevention trials: a population-based study. Neuroepidemiology 57, 14–24 (2023).
Welberry, H. J. et al. Factors associated with participation in a multidomain web-based dementia prevention trial: evidence from maintain your brain (MYB). J. Alzheimers Dis. 92, 959–974 (2023).
Harvey, P. D., McGurk, S. R., Mahncke, H. & Wykes, T. Controversies in computerized cognitive training. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 907–915 (2018).
Kochan, N. A. et al. Reliability, validity, and user-experience of remote unsupervised computerized neuropsychological assessments in community-living 55- to 75-year-olds. J. Alzheimers Dis. 90, 1629–1645 (2022).
Darby, D. G. et al. Reliability and usability of an internet-based computerized cognitive testing battery in community-dwelling older people. Comput. Hum. Behav. 30, 199–205 (2014).
Bleicher, K. et al. Cohort profile update: the 45 and Up Study. Int. J. Epidemiol. 52, e92–e101 (2023).
Kroenke, K., Spitzer, R. L. & Williams, J. B. W. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Altman, D. G. & Bland, J. M. Treatment allocation by minimisation. BMJ 330, 843 (2005).
Almendrales Rangel, C. et al. Nutrition module design in maintain your brain: an internet-based randomised controlled trial to prevent cognitive decline and dementia. Br. J. Nutr. 127, 1259–1268 (2022).
Walton, C. C. et al. Design and development of the brain training system for the digital ‘maintain your brain’ dementia prevention trial. JMIR Aging 2, e13135 (2019).
Newby, J. M. et al. Internet cognitive behavioural therapy for mixed anxiety and depression: a randomized controlled trial and evidence of effectiveness in primary care. Psychol. Med. 43, 2635–2648 (2013).
Marsh, A. P. et al. The virtual short physical performance battery. J. Gerontol. A 70, 1233–1241 (2015).
Australian Government Department of Health and Aged Care. Physical activity and exercise guidelines for all Australians. www.health.gov.au/topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians (2021).
Lim, Y. Y. et al. Use of the CogState Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J. Clin. Exp. Neuropsychol. 34, 345–358 (2012).
Owen, A. M. et al. Putting brain training to the test. Nature 465, 775–778 (2010).
Goldberg, T. E., Harvey, P. D., Wesnes, K. A., Snyder, P. J. & Schneider, L. S. Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer's disease randomized controlled trials. Alzheimers Dement. 1, 103–111 (2015).
Collie, A., Maruff, P., Darby, D. G. & McStephen, M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J. Int. Neuropsychol. Soc. 9, 419–428 (2003).
Van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Little, R. J. A. Pattern-mixture models for multivariate incomplete data. J. Am. Stat. Assoc. 88, 125–134 (1993).
Dunn, G., Maracy, M. & Tomenson, B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat. Methods Med. Res. 14, 369–395 (2005).
Acknowledgements
This trial was funded by a National Health and Medical Research Council (NHMRC) Dementia Research Team Grant (APP1095097). The funder had no role in the study design, data collection, data analysis, data interpretation or writing of the manuscript. This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW and partners the Heart Foundation and the NSW Ministry of Health. We thank the many thousands of people participating in the 45 and Up Study, particularly those who made the MYB trial possible. We acknowledge the contributions of the MYB Collaborative Team (N. Bosler, C. Boulamatsis, H. Christensen, I. Chuprov, L. Cobiac, K. Cox, J. Gunn, F. Harrison, A. Hayen, M. Hobbs, M. Kivipelto, H. Lapsley, J. Meiklejohn and S. Redman) and the MYB DSMB (J. Close, R. Day and M. Law). MYB used software developed by CogState, Creyos, NeuroNation and This Way Up. We also sought permission from Amsterdam Centrum to use the Amsterdam Instrumental Activity of Daily Living Questionnaire short version (A-IADL-Q-SV). MYB thanks Signs Publishing for a nonexclusive license to use traditional Mediterranean recipes from Food as Medicine: Cooking for Your Best Health by S.R.-V. and Adventist Media Network for the production and use of cooking videos. M.V. is supported by an NHMRC career development fellowship (APP1112813). K.J.A. is funded by ARC FL190100011. P.S.S. is supported by an NHMRC Investigator Grant.
Author information
Authors and Affiliations
Contributions
H.B., M.H. and T.C. wrote the first draft of the manuscript. H.B., M.V., M.F.S., P.S.S., K.J.A., N.T.L., J.J.M., L.J., G.A. and A.M. contributed to the design of the trial. M.F.S. led the physical activity and nutrition modules assisted by Y.M., S.R.-V., V.M.F., Y.N., F.O’.L. and C.A.R. M.V. led the brain training module assisted by P.B., C.C.W. and A.L. G.A. developed This Way Up, the peace of mind intervention module. T.C. led the control modules and curated the information for the control group. M.H. coordinated the trial. N.K. led the design of cognitive assessments and validation. N.E.B. and G.P. performed the statistical analyses. M.H. and H.W. contributed to statistical analyses. M.H., T.C. and J.C.S.J. had full access and managed all data in the trial. A.M. and J.A.G. led the development of the MYB digital platform, which was managed by J.C.S.J. All authors had final responsibility for the decision to submit for publication. They reviewed and contributed to drafts of the manuscript and approved the final manuscript. M.H., N.E.B. and G.P. directly accessed and verified the underlying data reported in the manuscript. H.B. is the chief investigator.
Corresponding author
Ethics declarations
Competing interests
H.B. has been an advisory board member or consultant to Biogen, Eisai, Eli Lilly, Medicines Australia, Roche, Skin2Neuron Pty, Cranbrook Care and Montefiore Homes. P.S.S. was a member of expert panels for Biogen and Roche in 2020 and 2021. M.V. has a financial interest in and is cofounder and CEO of Skin2Neuron Pty, unrelated to this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Tao Chen, Gill Livingston and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–9, Fig. 1 and Notes 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brodaty, H., Chau, T., Heffernan, M. et al. An online multidomain lifestyle intervention to prevent cognitive decline in at-risk older adults: a randomized controlled trial. Nat Med 31, 565–573 (2025). https://doi.org/10.1038/s41591-024-03351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03351-6
This article is cited by
-
Mechanisms of interventions targeting modifiable factors for dementia risk reduction
Molecular Neurodegeneration (2025)